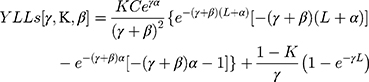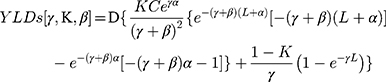Back to Journals » Infection and Drug Resistance » Volume 13
Economic Burden of Patients with Bloodstream Infections Caused by Extended-Spectrum β-Lactamase-Producing Escherichia coli
Authors Wang Y, Xiao T, Zhu Y, Ye J, Yang K, Luo Q, Shen P, Xiao Y
Received 8 July 2020
Accepted for publication 11 September 2020
Published 13 October 2020 Volume 2020:13 Pages 3583—3592
DOI https://doi.org/10.2147/IDR.S271230
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Yuan Wang,* Tingting Xiao,* Yunying Zhu, Jing Ye, Kai Yang, Qixia Luo, Ping Shen, Yonghong Xiao
State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, National Clinical Research Center for Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou 310003, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yonghong Xiao
State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou 310003, People’s Republic of China
Tel/Fax +86 571 87236421
Email [email protected]
Background: The prevalence of infections with extended-spectrum β-lactamase-producing Escherichia coli (ESBL-EC) is increasing worldwide, but the economic impact of ESBL-EC bloodstream infection (BSI) has not been comprehensively evaluated.
Patients and Methods: A retrospective cohort including patients hospitalized at a tertiary hospital between January 2013 and December 2016 who were confirmed with a BSI of ESBL-EC or non-ESBL-EC was set. Clinical data and medical costs were collected by chart review of electronic and paper medical records. The economic burden was evaluated with disability-adjusted life years (DALYs).
Results: A total of 580 patients with E. coli BSI, comprising 333 patients (57.4%) with ESBL-EC BSI and 247 patients (42.6%) with non-ESBL-EC BSI, were identified. There were no significant differences in comorbidity and severity of patients between ESBL-EC and non-ESBL-EC BSI. The median length of stay (LOS) after bacteremia was 12 days for ESBL-EC (interquartile range, 7 to 21) versus 11 days for non-ESBL-EC (interquartile range, 7 to 21) (P = 0.38), and appropriate empirical antimicrobial therapy occurred in 87.4% versus 89.9% (P = 0.353). The mortalities were 20.1% versus 17.4% (P = 0.41). Patients with ESBL-EC did not have significantly different in-hospital medical costs to those with non-ESBL-EC (median, $8048.68 vs $7476.84, respectively, with a difference of $571.84, P = 0.321). In the non-ESBL-EC group, 247 patients lost 531.05 DALYs in total, with an average of 2.15 DALYs per person, while in the ESBL-EC group, 333 patients lost 692.64 DALYs in total, with an average of 2.08 DALYs per person. There is no significant difference in average DALYs (P = 0.343).
Conclusion: In conclusion, patients with BSI due to ESBL-EC did not cost more than patients with BSI due to non-ESBL-EC. This phenomenon may be attributed to timely and effective antibiotic treatment, but the initial empiric therapy with second- or third-line antibiotics in non-ESBL-EC BSI should be corrected.
Keywords: Escherichia coli, extended-spectrum β-lactamase, ESBL, medical cost
Introduction
Escherichia coli, a member of the Enterobacterales, is a main pathogen responsible for community and nosocomial infections, and is the leading cause of Gram-negative bloodstream infections (BSIs).1 Extended-spectrum β-lactamase (ESBL) enzyme production is the common resistance mechanism to β-lactam antibiotics in Gram-negative bacteria. In addition, other resistance determinants, such as fluoroquinolones, aminoglycosides, trimethoprim-sulfamethoxazole resistance, are often associated with ESBLs.2 Thus, ESBL-producing organisms commonly exhibit a multidrug resistance phenotype. Infections due to ESBL-producing E. coli (ESBL-EC) have dramatically increased worldwide, presenting a great public concern. The prevalence of infections with ESBL-producing pathogens has steadily risen since 2000.3–5 Lately, a study showed that a high prevalence of community-acquired ESBL-producing Enterobacterales infections (46.5%, 256/550) had been reported in public county hospitals in China.6 Consistent herewith, the presence of faecal ESBL-producing Enterobacterales from healthy individuals are also very high in China ranging from 42.0% to 82.6%.7–9
Although the problem of ESBL resistance has attracted a great deal of attention from the public, the magnitude of the impact of drug-resistant bacteria on clinical and economic outcomes remains largely unknown. Therefore, we aimed to quantify the potential clinical and economic impact of ESBL production. In some studies, they showed increased mortality associated with ESBL-positive infection,10,11 while our previous study12 had come to the opposite conclusion that patients with BSI due to ESBL-EC did not show a higher mortality and a longer hospitalization than patients with BSI due to non-ESBL-EC.
Furthermore, there are few assessments of the specific economic impact of ESBL production on patient outcomes.10,11,13 These articles mainly studied the direct economic burden of drug-resistant bacterial infections, and did not pay attention to the indirect economic burden caused by drug-resistant bacteria infections. Disability-adjusted life years (DALYs) is a widely used metric for estimating disease burden, which was developed and used by experts from Harvard University School of Public Health and the World Health Organization (WHO) in 1993. The Global Burden of Disease (GBD) study systematically compared the magnitude of health losses caused by different diseases worldwide. DALYs was successfully used by GBD to measure health losses quantitatively. It is a summary measure that combines the time lost due to premature mortality, expressed as years of life lost (YLL), with the time lived in states worse than full health, expressed as years lived with disability (YLD).14 One DALY can be thought of as one year of “healthy” life lost due to different diseases. The sum of these DALYs can be thought of as the gap between current health status and that of an ideal health situation, that is, a normative reference population that lives to an advanced age, free of disease and disability.15
To analyze the economic impact of ESBL-EC BSI, we conducted a retrospective cohort study to compare direct costs and indirect costs between inpatients with ESBL-EC and those with non-ESBL-EC.
Patients and Methods
Patients
A retrospective cohort study of adult inpatients with E. coli bacteremia at a 2500 bed teaching hospital (Zhejiang, China) from January 2013 to December 2016 was conducted. Inclusion criteria were as follows: (1) all patients had a positive blood culture for E. coli; (2) clinical manifestations of bloodstream infection; and (3) hospitalization with complete clinical microbiological and cost data for analysis. Patients were excluded if they had incomplete medical records or their age was younger than 16 years. For patients having multiple episodes of E. coli bacteremia during hospitalization or readmission within 6 months, data only from the first episode of E. coli bacteremia were included. The patients infected with ESBL-EC were referred as “cases” and patients infected with non-ESBL-EC were referred as “controls” in the study.
Microbiological Tests
These isolates were identified by the matrix-assisted laser desorption ionization-time-of-flight mass spectrometry (MALDI-TOF-MS) (Bruker, Bremen, German) and the VITEK 2 COMPACT automatic microbial identification system (bioMérieux, Marcy l’Etoile, France). Antimicrobial susceptibility of these strains was assessed by using the VITEK 2 system. According to the guidelines of the Clinical and Laboratory Standards Institute (CLSI) standards (2016),16 ESBL production was determined by the double-disk test using a combination of ceftazidime and ceftazidime-clavulanic acid or cefotaxime and cefotaxime-clavulanic acid.17
Definitions
Hospital-acquired bacteremia was defined as a positive blood culture obtained at greater than or equal to 48 h after admission. Community-acquired bacteremia was defined as a positive blood culture taken on admission or less than 48 h after admission. Severity of illness at the time of BSI onset was assessed by the Acute Physiology and Chronic Health Evaluation (APACHE) II scores and Pitt scores.18 The Charlson comorbidity index was calculated to determine the overall systemic health status of patients.19 Initial antibiotic treatment was defined as the drugs administered empirically in culture-negative situations. The initial antimicrobial therapy was considered effective if the empirical administration of at least one antibiotic being active against isolated microorganisms with in vitro susceptibility testing.20
Data Collection
For all patients enrolled in this study, clinical and laboratory data were collected from electronic medical records, including: patient demographics (sex; age), comorbid illnesses, Charlson comorbidity index, day of BSI onset, intensive care unit (ICU) stay before bacteremia, organ transplantation during hospitalization, severity of illness (APACHEII score and Pitt score), overall hospital length of stay (LOS) and LOS before/after the infection; microbiological data, susceptibility of empirical antimicrobials and targeted antimicrobial, and clinical outcome recorded as “death” or “discharged alive”. The study complies with the Declaration of Helsinki; Ethics Committee approval was received from the Hospital Ethics Committee.
Cost Analysis
The cost was obtained from the hospital information system. The patient’s direct costs and indirect costs (i.e. productivity losses due to absenteeism and mortality) were analyzed. All the costs were converted into US dollars ($) with an exchange rate (average: $1 = 6.33 Renminbi).
Direct Cost
The total direct costs comprised of room and board, nursing, medicines (including antibacterial agents, traditional chinese medicines), oxygen inhalation, mechanical ventilation, blood transfusion, operation, laboratory tests and images.
Indirect Economic Burden
The indirect economic burden of E. coli bacteremia was analyzed through DALYs and human capital methods, which are equal to DALYs multiplied by Gross Domestic Product (GDP) per capita multiplied by productivity weight. And the GDP per capita in China in 2013 was $6944.99, in 2014 was $7682.90, in 2015 was $8063.72 and in 2016 was $8153.46.21 Productivity weights are different for different age groups.22 Children aged from 0 to 14 years old did not participate in social wealth creation, with a weight of 0.15; 15–44 years old and 45–59 years old created more wealth, with a value of 0.75 and 0.80 respectively; over 60 years old, it dropped to 0.1.22
DALYs for E.coli BSI are calculated by summing the YLLs for all deaths caused by this disease and the YLDs for people living in states of less than good health caused by this disease.15 These factors, such as life expectancy, age, future time and disability, were included in the YLLs and YLDs calculation.
The formula for YLLs is described below. The calculation YLLs [γ,K,β] is used to signify key factors (age weight and discount rate). Values were recommended and used by Murray and Lopez,23,24 i.e. γ = 0.03, K = 1 and β = 0.04.
K = age weighting modulation factor; C = constant; γ = discount rate; a = age of onset of disability; β = parameter from the age weighting function; L = standard expectation of life at age a.
The difference with the formula for YLLs is that D (the disability weight) was added in the formula for YLDs [γ,K,β], as follows:
K = age weighting modulation factor; C = constant; γ = discount rate; a = age of onset of disability; β = parameter from the age weighting function; L = duration of disability; D = disability weight.
This formula uses the values recommended by the WHO,25 constant value is 0.1658.25 The value of disability weight (D) ranges from 0 to 1 according to the GBD template provided by the WHO.14,26,27 Since the recent GBD studies did not mention the standard for BSI, we decided that the evaluation of D is based on the acute infection, which is divided into 0.006, 0.051 and 0.133 according to severity of the diseases in mild, moderate and severe conditons.14 In order to calculate L (the years lost by death and discounted by disability), we used “standard expected years of life lost” (SEYLL) as a good approximation of life expectancy.25
Statistical Methods
Dates were expressed as mean standard deviation or median (interquartile range [IQR]) for continuous variables and percentage n (%) for categorical variables. For statistical analysis, inter-group differences were tested using Student’s t-test (for variables with normal distribution) or the Mann–Whitney U-test (for variables with non-normal distribution) for continuous variables and χ2 or two-tailed Fisher exact test for Categorical variables. P values less than 0.05 were considered statistically significant. Statistical analyses were performed using SPSS version 23.0 package (SPSS, Chicago, IL, USA).
Results
Patient Demographics and Clinical Outcomes
We identified a total of 1115 E. coli isolates from blood from 647 patients between January 2013 and December 2016, and excluded 468 duplicate isolates from the same patient and then 67 patients without complete information about their hospitalization. Finally, 580 non-replicate clinical isolates of E. coli isolated from blood from 580 patients were included in the analysis. The 580 patients comprised 333 patients (57.4%) with ESBL-EC BSI and 247 patients (42.6%) with non-ESBL-EC BSI. Half of the 580 patients were male, and the average ages were 61 and 60 years old for those with ESBL-EC BSI and non-ESBL-EC BSI, respectively. In the ESBL-EC group, the most common comorbidities were hepatobiliary disease (27.9%), hypertension (27.3%) and malignant tumor (20.4%) while being hepatobiliary disease (29.6%), hypertension (27.5%) and hematological diseases (20.6%) in the non-ESBL-EC group. There was no significant difference in the comorbidities and Charlson comorbidity index between patients with ESBL-EC and those with non-ESBL-EC. The severity of BSI (APACHEII score and Pitt score) of the ESBL-EC group showed no significant difference compared with the non-ESBL-EC group (Table 1).
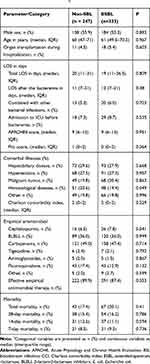 |
Table 1 Characteristics of Patients with Escherichia coli Bloodstream Infection (BSI) Stratified by Extended-Spectrum-Lactamase (ESBL) Productiona |
For the initial therapy, β-lactam-β-lactamase inhibitors (BLBLI) and carbapenems were administered empirically in 85% of patients with non-ESBL-EC infection while in 83.4% of patients with ESBL-EC infection. Notably, 87.4% of patients with ESBL-EC BSI received effective initial antibiotic treatment, compared with 89.9% of those with non-ESBL-EC BSI (P = 0.975).
The total LOS of patients with ESBL-EC BSI and non-ESBL-EC BSI was 19 (IQR, 11–36.5) and 20 (IQR, 11–31) days, respectively (P = 0.809). The LOS after bacteremia with ESBL-EC were longer (but without statistical significance) than those with non-ESBL-EC (median, 12 days vs 11 days, respectively, with a difference of 1 day, P > 0.05). 67 patients (20.1%) with ESBL-EC BSI and 43 patients (17.4%) with non-ESBL-EC BSI died (P = 0.41) during hospitalization. No statistical differences were observed in the 28-day mortality in patients with BSI caused by ESBL-EC or non-ESBL-EC (16.2% vs. 15.4%; P = 0.786).
Cost Analysis
Direct Costs
The median cost for patients with ESBL-EC was $8048.68 and for patients with non-ESBL-EC was $7476.84, respectively (P > 0.05) (Table 2). The direct cost after E. coli BSI also did not show significant difference between the two groups.
 |
Table 2 Costs of Patients with Escherichia coli Bloodstream Infection (BSI) Stratified by Extended-Spectrum-Lactamase (ESBL) Productiona |
In both groups, the cost of antibiotics accounted for 19.79% and 19.13% of the medicine, respectively. The median cost of antibiotics for patients with ESBL-EC BSI during hospitalization was $1592.50, which was higher than that for patients with non-ESBL-EC BSI (median, $1430.06, P = 0.252). To exclude the effects of antibiotic costs before infection, we also compared the cost of antibiotics after infection between the two groups, no significant difference between the two groups was evident (Table 1). BLBLI and carbapenems were the main antibiotics used in the two groups.
Indirect Loss
In the non-ESBL-EC group, 247 patients lost 531.05 DALYs in total, with an average of 2.15 DALYs per person. While in the ESBL-EC group, 333 patients lost 692.64 DALYs in total, with an average of 2.08 DALYs per person (P>0.05). The total indirect economic loss was $3349.32 for 247 patients with non-ESBL-EC and $6616.71 for 333 patients with ESBL-EC. No significant increase of the average indirect loss in the ESBL-EC group was found compared with the non-ESBL-EC group (median, $19.87 vs. $13.56, P=0.361) (Table 2). Among all patients with E. coli bloodstream, the heaviest total DALYs and indirect economic loss were in the age group 45–69, and the least total DALYs and indirect economic loss were in the age group >74 years (Figure 1).
 |
Figure 1 Total DALYs (A), average DALYs (B), total indirect loss (C) and average indirect loss (D) of patients with E. coli BSI by age group and sex. |
Discussions
The information on the economic burden of infections caused by antibiotic-resistant pathogens can only be obtained from observational cohort studies, which are highly susceptible to bias and confoundings.28 So, in the process of assessing the economic impact of the production of ESBL, the first thing we need to do is to eliminate the effects of intergroup differences on patients’ characteristics. Therefore, it is crucial to adjust for the relevant confounders when investigating the link between drug resistance and economic burden.29 In our study, no significant differences in the baseline characteristics of patients (including patient demographics, comorbidity status, severity of illness, overall LOS and LOS before/after the infection, susceptibility of empirical antimicrobials demographics, and mortality) between the ESBL-EC and non-ESBL-EC groups were found. Therefore, we can directly compare the economic burden of the two groups without considering the confounding.
Our study found that the production of ESBL did not lead to a significant increase in direct costs. The direct cost of an ESBL-EC infection was $8048.68 and a non-ESBL-EC infection was $7476.84, with a difference of $571.84. However, Schwaber et al.11 found that the average medic cost due to the production of ESBL was $9,620. Tumbarello et al.10 found that ESBL BSIs were associated with more cost (+EUR 5,026.00). This difference may be caused by the following reasons: first, in order to control the confounding factors, the previous studies may not have included a large enough sample size to evaluate the economic impact of ESBL infection. Next, there are differences in the inpatient environment, treatment level and test devices, and price of drugs in different regions and hospitals. Last but not least, whether the patient infected by E. coli bacteremia had received rapid and effective empirical antibiotic treatment. In our study, before in vitro susceptibility test results were available, BLBLI and carbapenems were administered empirically in 85% of patients with non-ESBL-EC infection while in 83.4% of patients with ESBL-EC infection. It shows that whether ESBL or not, BLBLI and carbapenems are mainly used in clinical patients with E. coli bloodstream infection, which makes no significant difference in the direct economic burden between the two groups. While other studies10,11 showed that higher hospital costs and longer hospitalization for patients infected with E. coli BSIs are associated with ESBL production and delay in appropriate therapy.
Clinicians should take the risk factor for ESBL production into consideration when choosing empirical antimicrobials before in vitro susceptibility test results were available. For patients with low risk of ESBL-EC infection, the third cephalosporins, fluoroquinolones and aminoglycosides were recommended as the treatment choice. And in some studies BLBLI30,31 and carbapenem32 were the most powerful antibiotics for the treatment of patients with high risk of ESBL-EC infection. However, in our study, for non-ESBL-EC infection group, only 26% of patients used recommended antimicrobials (cephalosporins, fluoroquinolones and aminoglycosides), 85% of patients used BLBLI and carbapenems. The reason why second-line and third-line antibiotics are chosen as empirical treatment drugs can be explained in our previous study,12 older age, the presence of comorbidities, ICU stay, recurrent urinary tract infections (UTI), previous use of antibiotics, previous colonization with ESBL-producing commensal bacteria, and higher ESBL prevalence were identified as risk factors for acquisition of BSI caused by ESBL-producing Enterobacterales.33–36 There are many risk factors for ESBL infection in patients with non-ESBL-EC BSI, so clinicians tend to choose antimicrobial agents covering ESBL-producing bacteria as empirical antibiotic therapy.37 In the short term, the use of second- and third-line antibiotics can improve the cure rate, reduce the direct and indirect costs for individual and society, but in the long term, the use of antibiotics across the ladder will lead to the increase and spread of resistance of advanced antibiotics, so for the rational application of antibiotics, clinics need to balance individual and societal needs.38
In our study, the per case burden of ESBL-EC BSI and non-ESBL-EC BSI was 2.15 DALYs and 2.08 DALYs respectively. And E. coli bacteremia had a higher burden in women. Initially, DALYs was often used to calculate the indirect burden of chronic disease. However, in the current study, DALYs was a powerful tool to provide quantitative information on communicable diseases. In 2018, in order to evaluate the burden of communicable diseases, Alessandro Cassini et al.39 used DALYs to calculate the burden of several selected infectious diseases. For viral infections, the per case burden of influenza, human immunodeficiency virus infection, and hepatitis B was 0.01 DALYs, 6.03 DALYs and 2.79 DALYs respectively. For bacterial infections, the per case burden of invasive pneumococcal disease, tuberculosis, and invasive Haemophilus influenzae disease was 2.74 DALYs, 3.58 DALYs, 3.43 DALYs respectively. Rabies had the highest per case burden (52.1 DALYs) compared to other infectious illness discussed in this study.39 In our study, E. coli bloodstream had a significantly lower burden compared to the infectious diseases discussed above (except influenza). We can also infer from these data that the duration of E. coli bloodstream is not as long as that of pneumococcal disease, hepatitis B, Haemophilus influenzae disease, etc. For E. coli bloodstream, 0.07 DALYs were produced due to the production of ESBL, which is seven times the burden of influenza. Hence using DALYs can quantify and compare the burden of different diseases. especially using DALYs to evaluate the burden of antimicrobial resistant pathogens can help decision makers to measure how much resources and energy should be invested to track and control the spread of antimicrobial-resistant organisms.
However, there are still some limitations in our study. First, this was a retrospective single-center study with inherent biases. The disease prevalence and treatment options in this hospital might have had an impact on the results. The situation in other medical institutions or healthcare systems might be different. Secondly, this was an observational study, not a randomized controlled trial, so our findings are susceptible to unmeasured confounding at both the hospital and patient levels. Thirdly, due to difficulties about statistic measurement and calculation, our study ignored direct non-medical costs and intangible economic burden including transportation costs, food costs and others, which may lead the results to not fully represent the economic burden of disease.
In conclusion, patients with BSI due to ESBL-EC did not cost more than patients with BSI due to non-ESBL-EC. This phenomenon may be attributed to timely and effective antibiotic treatment. But the antimicrobial stewardship should be implemented to avoid overaggressive use of second- and third-line antibiotics in sensitive bacterial infections.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request. All patient data accessed complied with relevant data protection and privacy regulations.
Ethics Approval and Consent to Participate
Ethics approval for this study was submitted and approved through Research Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University (Reference number 2,016,605). The consent to participate was waived by our institutional review board since this study was retrospective data collection.
Acknowledgments
We would like to thank Professor Hengjin Dong, Zhejiang University, for directing economic data analysis. Thanks also to Jinru Ji and Chaoqun Ying, the First Affiliated Hospital, School of Medicine, Zhejiang University for providing bacterial identification help.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed on the journal to which the article will be submitted; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This work was partially supported by grants from The National Key Research and Development Program of China (2017YFC1200203) and National Natural Science Foundation of China (81,971,984).
Disclosure
None of the authors report conflicts of interest for this work.
References
1. Uslan DZ, Crane SJ, Steckelberg JM, et al. Age- and sex-associated trends in bloodstream infection: a population-based study in Olmsted County, Minnesota. Arch Intern Med. 2007;167:834–839. doi:10.1001/archinte.167.8.834
2. Schwaber MJ, Navon-Venezia S, Schwartz D, Carmeli Y. High levels of antimicrobial coresistance among extended-spectrum-β-lactamase-producing enterobacteriaceae. Antimicrob Agents Chemother. 2005;49:2137.
3. Meyer E, Ziegler R, Mattner F, Schwab F, Gastmeier P, Martin M. Increase of patients co-colonised or co-infected with methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus faecium or extended-spectrum β-lactamase-producing Enterobacteriaceae. Infection. 2011;39(6):501–506. doi:10.1007/s15010-011-0154-0
4. Gajdács M, Ábrók M, Lázár A, Burián K. Comparative epidemiology and resistance trends of common urinary pathogens in a Tertiary-Care Hospital: a 10-year Surveillance Study. Medicina. 2019;55:356.
5. Gajdács M, Ábrók M, Lázár A, Burián K. Revival of older antibiotics for the therapy of urinary tract infections: old, but gold Part 1: antimicrobial susceptibility of extended-spectrum β-lactamase-producing and AmpC β-lactamase-producing: Escherichia coli: isolates. Rev Med Microbiol. 2020.
6. Zhang J, Zheng B, Zhao L, et al. Nationwide high prevalence of CTX-M and an increase of CTX-M-55 in Escherichia coli isolated from patients with community-onset infections in Chinese county hospitals. BMC Infect Dis. 2014;14(1):659. doi:10.1186/s12879-014-0659-0
7. Sun Q, Tärnberg M, Zhao L, et al. Varying high levels of faecal carriage of extended-spectrum beta-lactamase producing Enterobacteriaceae in rural villages in Shandong, China: implications for global health. PLoS One. 2014;9:e113121. doi:10.1371/journal.pone.0113121
8. Zhang H, Zhou Y, Guo S, Chang W. High prevalence and risk factors of fecal carriage of CTX-M type extended-spectrum beta-lactamase-producing Enterobacteriaceae from healthy rural residents of Taian, China. Front Microbiol. 2015;6:239.
9. Zhong Y-M, Liu W-E, Liang X-H, Li Y-M, Jian Z-J, Hawkey PM. Emergence and spread of O16-ST131 and O25b-ST131 clones among faecal CTX-M-producing Escherichia coli in healthy individuals in Hunan Province, China. J Antimicrob Chemother. 2015;70(8):2223–2227. doi:10.1093/jac/dkv114
10. Tumbarello M, Spanu T, Di Bidino R, et al. Costs of bloodstream infections caused by Escherichia coli and influence of extended-spectrum-β-lactamase production and inadequate initial antibiotic therapy. Antimicrob Agents Chemother. 2010;54(10):4085–4091. doi:10.1128/AAC.00143-10
11. Schwaber MJ, Navon-Venezia S, Kaye KS, Ben-Ami R, Schwartz D, Carmeli Y. Clinical and Economic Impact of bacteremia with extended- spectrum-β-lactamase-producing Enterobacteriaceae. Antimicrob Agents Chemother. 2006;50:1257.
12. Xiao T, Wu Z, Shi Q, et al. A retrospective analysis of risk factors and outcomes in patients with extended-spectrum beta-lactamase-producing Escherichia coli bloodstream infections. J Glob Antimicrob Resist. 2019;17:147–156.
13. Stewardson A, Fankhauser C, Augelis GD, et al. Burden of bloodstream infection caused by extended-spectrum β-lactamase–producing enterobacteriaceae determined using multistate modeling at a Swiss University Hospital and a Nationwide Predictive Model. Infect Control Hosp Epidemiol. 2013;34(2):133–143. doi:10.1086/669086
14. Salomon JA, Haagsma JA, Davis A, et al. Disability weights for the Global Burden of Disease 2013 study. Lancet Glob Health. 2015;3:e712–e723.
15. WHO methods and data sources for global burden of disease estimates 2000–2016. Available from: http://www.who.int/gho/mortality_burden_disease/en/index.html.
16. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing;
17. Gajdács M, Urbán E. Resistance Trends and Epidemiology of Citrobacter-Enterobacter-Serratia in Urinary Tract Infections of Inpatients and Outpatients (RECESUTI): a 10-year survey. Medicina. 2019;55:285.
18. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829.
19. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251. doi:10.1016/0895-4356(94)90129-5
20. Hill PC, Birch M, Chambers S, et al. Prospective study of 424 cases of Staphylococcus aureus bacteraemia: determination of factors affecting incidence and mortality. Intern Med J. 2001;31(2):97–103. doi:10.1111/j.1444-0903.2001.00029.x
21. China’s Gross Domestic Product (GDP). Available from: http://data.stats.gov.cn/search.htm?s=GDP.
22. Barnum H. Evaluating healthy days of life gained from health projects. Soc Sci Med. 1987;24(10):833–841. doi:10.1016/0277-9536(87)90184-5
23. Murray CJL, Lopez AD. Global Health Statistics: A Com-Pendium of Incidence, Prevalence and Mortality Estimates Forover 200 Conditions. Cambridge, MA: Harvard University Press; 1996a.
24. Murray CJL, Lopez AD, eds. The Global Burden of Disease: Acomprehensive Assessment of Mortality and Disability from Dis-Eases, Injuries, and Risk Factors in 1990 and Projected to 2020. TheGlobal Burden of Disease and Injury Series. Vol. 1. Boston, MA: Harvard University Press, for Harvard School of PublicHealth on behalf of the World Health Organisation and TheWorld Bank; 1996b.
25. Murray CJ, Salomon JA, Mathers C. A critical examination of summary measures of population health. Bull World Health Organ. 2000;78:981–994.
26. Disease, G. B. D., Injury, Incidence, and Prevalence, Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1545–1602.
27. WHO methods and data sources for global burden of disease estimates 2000–2015. Available from: http://www.who.int/healthinfo/global_burden_disease/GlobalDALYmethods_2000_2015.pdf?ua=1.
28. Rottier WC, Ammerlaan HSM, Bonten MJM. Effects of confounders and intermediates on the association of bacteraemia caused by extended-spectrum β-lactamase-producing enterobacteriaceae and patient outcome: a meta-analysis. J Antimicrob Chemother. 2012;67:1311–1320.
29. Zhen X, Lundborg CS, Sun X, Hu X, Dong H. Economic burden of antibiotic resistance in ESKAPE organisms: a systematic review. Antimicrob Resist Infect Control. 2019;8:137.
30. Vardakas KZ, Tansarli GS, Rafailidis PI, Falagas ME. Carbapenems versus alternative antibiotics for the treatment of bacteraemia due to Enterobacteriaceae producing extended-spectrum β-lactamases: a systematic review and meta-analysis. J Antimicrob Chemother. 2012;67(12):2793–2803. doi:10.1093/jac/dks301
31. Rodríguez-Baño J, Navarro MD, Retamar P, Picón E, Pascual Á. β-Lactam/β-lactam inhibitor combinations for the treatment of bacteremia due to extended-spectrum β-lactamase-producing Escherichia coli: a post hoc analysis of prospective cohorts. Clin Infect Dis. 2012;54:167–174.
32. Pitout JD. Infections with extended-spectrum beta-lactamase-producing enterobacteriaceae: changing epidemiology and drug treatment choices. Drugs. 2010;70:313–333. doi:10.2165/11533040-000000000-00000
33. Rodríguez-Baño J, Navarro MD, Romero L, Muniain MA. Risk-factors for emerging bloodstream infections caused by extended-spectrum beta-lactamase-producing Escherichia coli. Clin Microbiol Infect. 2008;14:180–183. doi:10.1111/j.1469-0691.2007.01884.x
34. Bilavsky E, Temkin E, Lerman Y, et al. Risk factors for colonization with extended-spectrum beta-lactamase-producing enterobacteriaceae on admission to rehabilitation centres. Clin Microbiol Infect. 2014;20(11):O804–10. doi:10.1111/1469-0691.12633
35. Wu UI, Yang CS, Chen WC, Chen YC, Chang SC. Risk factors for bloodstream infections due to extended-spectrum beta-lactamase-producing Escherichia coli. J Microbiol Immunol Infect. 2010;43:310–316. doi:10.1016/S1684-1182(10)60048-5
36. Gudiol C, Calatayud L, Garcia-Vidal C, et al. Bacteraemia due to extended-spectrum beta-lactamase-producing Escherichia coli (ESBL-EC) in cancer patients: clinical features, risk factors, molecular epidemiology and outcome. J Antimicrob Chemother. 2010;65:333–341. doi:10.1093/jac/dkp411
37. Guan X, He L, Hu B, et al. Laboratory diagnosis, clinical management and infection control of the infections caused by extensively drug-resistant Gram-negative bacilli: a Chinese consensus statement. Clin Microbiol Infect. 2016;22(Suppl 1):S15–S25. doi:10.1016/j.cmi.2015.11.004
38. Gajdács M, Albericio F. Antibiotic resistance: from the bench to patients. Antibiotics. 2019;8.
39. Cassini A, Colzani E, Pini A, et al; On Behalf Of The, BCoDE Consortium. Impact of infectious diseases on population health using incidence-based disability-adjusted life years (DALYs): results from the Burden of Communicable Diseases in Europe study, European Union and European Economic Area countries, 2009 to 2013. Euro Surveill. 2018;23(16). doi:10.2807/1560-7917.ES.2018.23.16.17-00454
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.