Back to Journals » Risk Management and Healthcare Policy » Volume 12
Economic Burden Of Inappropriate Empiric Antibiotic Therapy: A Report From Southern Iran
Authors Sadatsharifi A , Davarpanah MA, Namazi S, Mottaghi S, Mahmoudi L
Received 6 July 2019
Accepted for publication 8 November 2019
Published 12 December 2019 Volume 2019:12 Pages 339—348
DOI https://doi.org/10.2147/RMHP.S222200
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Marco Carotenuto
Arman Sadatsharifi,1 Mohammad-Ali Davarpanah,2 Soha Namazi,3 Shaghayegh Mottaghi,1 Laleh Mahmoudi1
1Department of Clinical Pharmacy, School of Pharmacy, Shiraz University of Medical Sciences, Shiraz, Iran; 2Shiraz HIV/AIDS Research Centre, Shiraz University of Medical Sciences, Shiraz, Iran; 3Department of Clinical Pharmacy, School of pharmacy, Tehran University of Medical Sciences, Tehran, Iran
Correspondence: Laleh Mahmoudi
Department of Clinical Pharmacy, School of Pharmacy, Shiraz University of Medical Sciences, PO Box: 7146864685, Shiraz, Iran
Tel/Fax + 98-713-2125400
Email [email protected]
Introduction: Widespread inappropriate antibiotic prescribing by healthcare professionals in the hospital setting is a great concern that may cause many undesirable consequences. Adherences to antibiotic guidelines have proven to be a simple and effective intervention to guide the choice of appropriate empiric antibiotic regimens and reduce the unnecessary variations in the practice among practitioners. The objective of this study was to evaluate the prescription patterns of empiric antibiotic therapy in relation to treatment guidelines and the economic burden of discordance with guidelines in a major referral Iranian university hospital.
Method: Hospital records of hospitalized patients with empiric antibiotic prescription, from September 2016 to February 2017 were reviewed. The process consisted of comparing empiric antimicrobial administration with institutional guidelines for each patient by a clinical pharmacist and an infectious disease specialist to evaluate the appropriate utilization of antibiotics. Adherence to guideline, the cost of antibiotics usage for each patient and the excess cost consequent from discordance with guideline was calculated.
Results: The most inappropriate prescribed antibiotics were carbapenems and aminoglycosides. Overall guideline adherence was 27.8%. Frequency of antibiotic usage incompatibility with the guidelines on the basis of dosing interval, duration of therapy and drug indication were 31.46%, 29.44% and 19.36%, respectively. General surgery and internal medicine wards had the least and the most inappropriate antibiotic administration, respectively. Totally antibiotic usage cost was 578,959.39 USD (24,316,294,800 Iranian Rials, IRR) for 6 months, which the excess costs of inappropriate antibiotic prescribing, was 471,319.69 USD (19,795,427,225 IRR). The estimated annual excess cost is 942,639.38 USD (39,590,854,450 IRR).
Conclusion: In this research, physicians’ adherence with guidelines for empiric antibiotic therapy was low which was led to 471,319.69 USD excess costs. These results urge institution policy makers to develop guidelines to ensure active dissemination and implementation of them to decrease inappropriate antibiotic usage.
Keywords: inappropriate antibiotic usage, consumption, hospital, economic burden
Introduction
Antibiotics are one of the most commonly prescribed drugs for the hospitalized patients. According to the previous studies, about one-third to one-half of all hospitalized patients receive antimicrobial therapy, which accounts for up to 30% of hospital drug budgets.1–3 Hospitals all over the world are faced with the rapid emergence and spread of antibiotic-resistant bacteria. Increasing rates of antibiotic resistance have contributed to the greater recognition of inappropriate antimicrobial treatment for hospital-acquired infections.4
To minimize inappropriate antimicrobial agent usage, clinicians must ensure that antibiotic administration follows certain minimal requirements, such as choosing an appropriate empiric therapy, considering accepted guidelines, proper dosing, optimal duration of treatment and changing treatment based on the subsequent culture results and antibacterial susceptibilities.5–8
Lack of adherence to these requirements based on antibiotic guidelines can lead to suboptimal or excessive antibiotic usage that increases the likelihood of antibiotic resistance, toxicity, and ineffective therapy.9,10 Several strategies have been suggested for controlling antibiotic usage including formulary restriction, review and feedback strategies, educational programs, implementation of clinical guidelines, antibiotic cycling and antibiotic order forms.11–13
Application of the clinical guidelines can contribute to an appropriate selection of empiric antibiotic regimens and also reduce the unnecessary variations in the treatment of different infectious diseases.14,15 According to the different patterns of antimicrobial sensitivity in hospitals, many hospitals have tried to develop their local guidelines based on the published international ones.16
Different studies have evaluated the overuse of antibiotics17–19 and only limited ones have studied the economic burden of inappropriate prophylactic antibiotic usage in Iran.19 As far as we know, there is no published research regarding the economic burden of inappropriate empiric antibiotic therapy from Iran. The objective of this study was to evaluate the prescription patterns of empiric antibiotic therapy in relation to treatment guidelines and the economic burden of discordance with guidelines in a major referral Iranian university hospital in southern Iran, Shiraz.
Methods
Study Population
We conducted a prospective observational study within 6 months from September 2016 to February 2017, in Shahid Faghihi hospital which is a tertiary referral teaching hospital affiliated to Shiraz University of Medical Sciences, Shiraz, Iran with about 42,000 admissions per year and 376 beds capacity. All patients who were hospitalized in the wards of internal medicine, general surgery, internal intensive care unit (ICU) and surgical ICU with antibiotic prescription were included in this study. Patients admitted to neurology and cardiology units were excluded because of their infrequent use of antibiotics. All studied patients were followed until the end of their antibiotic treatment. Being confirmed to have no infectious disease after 48 hours, having more than one infection, receiving prophylactic antibiotic regimens, expiring in the first day of admission and absence of enough information for a complete evaluation of patients’ treatment course were exclusion criteria.20 For each patient, demographic variables, laboratory and clinical data were recorded. The high accurate hospital records of each patient were evaluated because each drug requested for each patient had been recorded in the hospital information system (HIS). All medical and pharmacological records and all inpatient records related to antibiotic administration and starting or stopping dates of each drug were evaluated during the study period.
Adhering Assessment
The primary outcome was the rate of inappropriate antibiotic prescribing. The process consisted of comparing empiric antimicrobial administration with institutional guidelines for each patient by a clinical pharmacist and an infectious disease specialist to evaluate the appropriate utilization of antibiotics. For this purpose, the quality of antibiotics prescription was assessed by using an algorithm (Figure 1) designed according to the method of van der Meer and Gyssens.21 The appropriateness of the criteria mentioned in this algorithm was evaluated for each antibiotic according to institutional guidelines provided under the guidelines of the Infectious Diseases Society of America (IDSA)22 and the US Centers for Disease Control and Prevention (CDC);23 by the side of local microbial susceptibility patterns and hospital drug formulary.
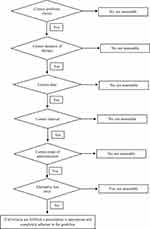 |
Figure 1 Assessment criteria for the evaluation of the quality of antibiotics prescription. |
Cost Evaluation
The total cost of antibiotics was calculated using the hospital pharmacy computer system and data was collected from the HIS. The costs evaluated in this study were pharmaceutical costs that included both the cost of the health system and the cost of the patient. The excess cost was computed according to the percentage of any referent inappropriate antibiotic usages. Costs are reported in Iranian Rials (IRR) and US dollars (USD), by a conversion rate of 1 USD = 42,000 IRR. This conversion factor is based on the currency index allocated by the Central Bank of the Islamic Republic of Iran for the supply of medicine and medical equipment. The amounts of antibiotics administration are documented in total doses and DDD/100 patients/day for each antibiotic.
Ethics Approval And Informed Consent
Institutional review board committee approval was obtained from the Shiraz University of Medical Sciences Ethics Committee (91-01-36-4872) and written informed consent was collected from patients. This study was conducted in accordance with the Declaration of Helsinki.
Data Analysis
Data analysis was performed by SPSS version 17 software package (SPSS Inc., Chicago, IL, USA). The quantitative data were reported as mean ± standard deviation (SD) and qualitative variables were presented by their frequencies and percentages. The odds ratios (ORs) and 95% confidence intervals (CIs) were used to evaluate the association between antibiotics and their appropriate use by employing a logistic regression model. Categorical variables were compared by using Chi‑square test. Binary logistic regression was conducted separately for the infections and wards evaluated. The wards and infections were compared regarding inappropriate antibiotic administrations using crosstab test. A P-value <0.05 was considered significant.
Results
Among the evaluated patients, 229 patients met the inclusion criteria; 115 males (50.2%) and 114 females (49.8%). The average of the patients’ age was 56.42 ± 19.54, ranging from 18 to 99 years. Demographic and clinical data of the patients are shown in Table 1. Cardiovascular diseases (81 patients, 35.3%) and diabetes mellitus (69 patients, 30.1%) were the most frequent pre-existing diseases.
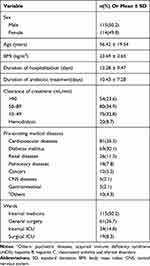 |
Table 1 Demographic And Clinical Data Of The Patients (n=229) |
A total of 493 antibiotics were prescribed for the evaluated patients during the study. The most frequently prescribed antibiotics were cephalosporins, carbapenems and glycopeptides which comprised 116 (23.5%), 78 (15.8%) and 66 (13.4%) of total prescriptions, respectively. Among these, aminoglycosides (100%) and carbapenems (97.43%), had the most and miscellaneous antibiotic group (42.10%) had the least proportions of inappropriate administrations (Figure 2, Table 2).
 |
Table 2 The Appropriateness Of Antimicrobial Therapy In Different Groups Of Antibiotics |
 |
Figure 2 The appropriateness of antimicrobial therapy in different groups of antibiotics. Note: *Miscellaneous included metronidazole.Abbreviation: IA, inappropriate administration. |
Among prescribed antibiotics, azithromycin (53.8%) and metronidazole (42.1%) were the most compatible antibiotics with the guidelines, whereas imipenem, amikacin and piperacillin-tazobactam were the most incompatible ones (all of them 100%). The best adherence to the guideline was in peritonitis and surgical site infections, while pyelonephritis and meningitis had the lowest compatibility with the guidelines (Both 0%) (Table 3).
 |
Table 3 Adherence To The Guidelines For Antibiotic Therapy In Different Infections |
Antibiotics were used least inappropriately in general surgery wards (52.8%), whereas the internal medicine wards had the most inappropriate usage of antibiotics (80.9%) (Table 4).
 |
Table 4 The Appropriateness Of Antimicrobial Therapy In The Evaluated Wards |
Comparison of antibiotics administrations appropriateness, regarding various aspects is shown in Table 5. Most of the prescription appropriate cases were related to route of administration, while the least appropriateness was due to dosing intervals.
 |
Table 5 Comparison Of Antibiotics Administrations Appropriateness, Regarding Various Aspects |
In this study, the overall adherence of antibiotics regimen to the guidelines was 27.8%. The main aspects of inappropriate antibiotic usage were dosing interval, duration of therapy, and drug indication which were incorrect in 156 (31.46%), 146 (29.44%) and 96 (19.36%) of prescribed ones, respectively.
Based on the patients’ renal function, dosing interval of 108 (21.9%) and dose of 38 (7.7%) antibiotics must be adjusted, but just 37 (34.2%) and 10 (26.3%) of these antibiotics met the correct adjustment for dosing interval and dose, respectively.
According to the culture results, the antibiotic regimen was de-escalated in 41 cases out of 85 positive cultures, whereas in 44 cases the modification was not suitable (Figure 3).
 |
Figure 3 Performing culture and modifying antibiotic regimens according to the culture results. |
As it is shown in Table 6, total antibiotic use was 248.56 DDD/100 patients/day, while the most frequent-used antibiotic was ceftriaxone, 98.692 DDD/100 patients/day.
 |
Table 6 Antibiotic administrations in DDD/100 patients/Day |
The total cost of antibiotics during our study was 578,959.39 USD (24,316,294,800 IRR) and the excess cost due to inappropriate antibiotic administration was 471,319.69 USD (19,795,427,225 IRR), equal to 81.41% of the total cost. The estimated annual excess cost is 942,639.38 USD (39,590,854,450 IRR) (Table 7).
 |
Table 7 Total Costs And Waste Costs For Each Antibiotic |
Discussion
To the best of our knowledge, this is the first study from Iran to the economic consequence of inappropriate empiric antibiotic prescription at the hospital level as well as at the ward level. This hospital receives patients from a variety of healthcare settings and hospitals in south-west of Iran. Thus, we believe that our results are largely representative of tertiary care facilities of our country.
Despite guidelines which lead clinicians to prescribe appropriate empiric antimicrobial therapy to the hospitalized patients, growing literature show the inappropriate initial antimicrobial treatment.9,10 A major concern is the increased use of broad-spectrum or new antibiotics and the prolonged use of antibiotics for empiric therapy. Physicians’ adherence to local or hospital guideline is very important for the effective and appropriate use of medications.5–8 In our study, the total adherence (appropriateness of antimicrobial agent, de-escalation based on culture results, duration of treatment, route of antibiotic administration) with guidelines in empirical antibiotic treatment was found in 27.8% of patients.
As is shown in Table 6, antibiotic consumption in our report is relatively high, comparing to studies in developed countries. This may underscore the need of adherence to guidelines in developing countries. In addition, high use of some antibiotics may be due to differences in the epidemiological status of antimicrobial resistance. Carbapenems administration in Turkish hospitals with bed capacity < 500, was reported to be 32.8 DDD/1000 patient-days.24 In another study in French hospitals, this rate of use was stated to be 3.7 DDD/1000 patient-days.25 This rate was much higher in our study than reported in these studies (Table 6, 30.77 DDD/100 patient-day). Surprisingly, piperacillin-tazobactam consumption in Turkish report was 22.6 DDD/1000 patient-day,24 while this rate was 1.373 DDD/100 patient-day in our research (Table 6).
Glycopeptides usages in 530 French hospitals were 6.7 DDD/1000 patient-day,25 however vancomycin, as the only glycopeptide in Iran, was administered 34.22 DDD/100 patient-day in our research. The high proportion of vancomycin use in the present study may be explained by the epidemiological status of antimicrobial resistance. The high rate of resistance to methicillin in Staphylococcus aureus is reported in our country.26
As it is mentioned, some antibiotics have more frequent consumption comparing to developed countries, while some are administered less. World Health Organization (WHO) published a report, expressing antibiotic use in Iran as 38.78 DDD/1000 inhabitant day in 2015.27 However, total antibiotic consumption in our study was 248.56 DDD/100 patient-day. Then, antibiotic use in our hospital did seem to be higher than some reports in other countries or also from the average of our own country.
Considering the pattern of use, the present study showed a high consumption of total antibiotics, which can be due to different reasons for this inappropriate use.
We found a significant overuse of antimicrobials that were clearly not indicated in more than 49% of cases. In the present study, 72.2% of total antibiotic prescriptions were incompatible with guidelines (Tables 2–4). As shown in some reports, the lack of indication is a popular reason for inappropriate use of antibiotics.20,28,29
Carbapenems and aminoglycosides were reported to be used the most inappropriately in the present study (Table 2). The most inappropriate prescribed antibiotic groups reported in various studies are different based on the country and centers in which each study has been done.29–31
Use of broad-spectrum antimicrobials for empiric management of infections may be initially necessary. However, antimicrobial de-escalation is a key approach to balance the need of broad-spectrum initial treatment while limiting the emergence of antimicrobial resistance.32 De-escalation requires that the initial regimen is subsequently narrowed after the pathogen and its antibiotic susceptibility are known. Besides, antimicrobial treatment is needed to be discontinued when the signs and symptoms of infection resolve. The present research showed that in 48.23% of cases the culture results were incorporated in antibiotic selection mostly in internal ICU and the least attention was paid in general surgery ward (Table 8). The notable point is that antibiotic choice according to culture results was inappropriate in 51.76% of positive cultures (Figure 3).
 |
Table 8 Culture Practice Rates In Different Wards |
In a report from 323 hospitals of USA received in 2010, the common deviations from accepted protocols were missing of de-escalation, administering longer than needed, antibiotic prescription for nonbacterial infections and treating contaminations or colonizations.33
Medical records of patients with positive blood culture in a tertiary university hospital in Norway affirmed that 97% received empirical treatment at the time of culture report. Among these patients, 26% continued it, as being appropriate and 73% needed an adjustment, of which 12.05% did not meet this need.5
Erbay et al stated that 45.3% of empiric regimens were inappropriate and generally, empirically antibiotic orders were less appropriate than those administered after culture tests.29
Antibiotics overuse, consequent from non-adherence to antibiotic guidelines, may cause an increase in direct cost of treatment. Furthermore, inappropriate use of antibiotics can lead to an increase in bacterial resistance and length of hospital stay. Our findings showed that the excess cost because of inappropriate antibiotic usage was 471,319.69 USD (19,795,427,225 IRR, Table 7) among 229 patients. In this study, 81.41% of total costs were due to the misprescription of antibiotics, which is a considerable rate. The increasing cost of antibiotics due to inappropriate usage has been addressed in number of studies.34–36
Such hospital-level analysis is useful for feedback to physicians in order to target key areas for improved performance. However, this research had some limitations, as there was not a feasible method to evaluate the clinical outcomes. Therefore, some conclusions about the inappropriateness of antibiotic therapy duration might not be drawn correctly. Also, there are different factors that affect an appropriate prescription, including the presence of clinical pharmacists, educational factors, and availability of antibiotics. The influence of these factors was not examined in our study, and prophylactic regimens were not evaluated.
Conclusion
Antibiotic therapy inappropriateness is a widespread matter which is intended to be decreased by designing different guidelines. Though this ongoing issue is responsible for increasing antibiotic resistance all over the world yet. In this research, the high rate of incompatibility with the guidelines confirms the necessity of a closer monitoring of antibiotics administration, culture tests and appropriate sampling. The authors believe that there is a crucial need in incorporating evidence-based guidelines according to local conditions and cultural background in routine practice to improve patient safety and decrease direct costs in our hospital.
Disclosure
The authors report no conflicts of interest in this work.
References
1. John JF
2. Knox K, Lawson W, Dean B, Holmes A. Multidisciplinary antimicrobial management and the role of the infectious diseases pharmacist – a UK perspective. J Hosp Infect. 2003;53(2):85–90. doi:10.1053/jhin.2002.1350
3. Pestotnik SL, Classen DC, Evans RS, Burke JP. Implementing antibiotic practice guidelines through computer-assisted decision support: clinical and financial outcomes. Ann Intern Med. 1996;124(10):884–890. doi:10.7326/0003-4819-124-10-199605150-00004
4. Del Fio FS, de Sá F, Lopes LC, Barberato-Filho S, Motta CD. Evaluation of the prescription and use of antibiotics in Brazilian children. Braz J Infect Dis. 2013;17(3):332–337. doi:10.1016/j.bjid.2012.10.025
5. Berild D, Mohseni A, Diep LM, Jensenius M, Ringertz SH. Adjustment of antibiotic treatment according to the results of blood cultures leads to decreased antibiotic use and costs. J Antimicrob Chemother. 2006;57(2):326–330. doi:10.1093/jac/dki463
6. Firouzabadi D, Mahmoudi L. Knowledge, attitude, and practice of health care workers towards antibiotic resistance and antimicrobial stewardship programmes: a cross-sectional study. J Eval Clin Pract. 2019;21:1–7.
7. Kollef M. Appropriate empirical antibacterial therapy for nosocomial infections: getting it right the first time. Drugs. 2003;63(20):2157–2168. doi:10.2165/00003495-200363200-00001
8. Quintiliani R, Cooper BW, Briceland LL, Nightingale CH. Economic impact of streamlining antibiotic administration. Am J Med. 1987;82(4A):391–394.
9. Cosgrove SE. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infect Dis. 2006;42 Suppl 2:S82–S89. doi:10.1086/499406
10. Galayduyk N, Colodner R, Chazan B, et al. Adherence to guidelines on empiric use of antibiotics in the emergency room. Infection. 2008;36(5):408–414. doi:10.1007/s15010-008-6306-1
11. Doron S, Davidson LE. Antimicrobial stewardship. Mayo Clin Proc. 2011;86(11):1113–1123.
12. Drew RH. Antimicrobial stewardship programs: how to start and steer a successful program. J Manag Care Pharm. 2009;15(2 Suppl):S18–23. doi:10.18553/jmcp.2009.15.s2.18
13. MacDougall C, Polk RE. Antimicrobial stewardship programs in health care systems. Clin Microbiol Rev. 2005;18(4):638–656. doi:10.1128/CMR.18.4.638-656.2005
14. Bodi M, Rodriguez A, Sole-Violan J, et al. Antibiotic prescription for community-acquired pneumonia in the intensive care unit: impact of adherence to infectious diseases society of America guidelines on survival. Clin Infect Dis. 2005;41(12):1709–1716. doi:10.1086/498119
15. Di Giammarino L, Bihl F, Bissig M, et al. Evaluation of prescription practices of antibiotics in a medium-sized Swiss hospital. Swiss Med Wkly. 2005;135(47–48):710–714. doi:2005/47/smw-11174
16. Paterson DL. The role of antimicrobial management programs in optimizing antibiotic prescribing within hospitals. Clin Infect Dis. 2006;42 Suppl 2:S90–5. doi:10.1086/499407
17. Alavi SM, Roozbeh F, Behmanesh F, Alavi L. Antibiotics use patterns for surgical prophylaxis site infection in different surgical wards of a teaching hospital in ahvaz, iran. Jundishapur J Microbiol. 2014;7(11):e12251. doi:10.5812/jjm.12251.
18. Rahbarimanesh A, Mojtahedi SY, Sadeghi P, et al. Antimicrobial stewardship program (ASP): an effective implementing technique for the therapy efficiency of meropenem and vancomycin antibiotics in Iranian pediatric patients. Ann Clin Microbiol Antimicrob. 2019;18(1):6. doi:10.1186/s12941-019-0305-1
19. Mahmoudi L, Ghouchani M, Mahi-Birjand M, Bananzadeh A, Akbari A. Optimizing compliance with surgical antimicrobial prophylaxis guidelines in patients undergoing gastrointestinal surgery at a referral teaching hospital in southern Iran: clinical and economic impact. Infect Drug Resist. 2019;12:2437–2444. doi:10.2147/IDR.S212728
20. Baktygul K, Marat B, Ashirali Z, Harun-Or-rashid M, Sakamoto J. An assessment of antibiotics prescribed at the secondary health-care level in the Kyrgyz Republic. Nagoya J Med Sci. 2011;73(3–4):157–168.
21. van der Meer JW, Gyssens IC. Quality of antimicrobial drug prescription in hospital. Clin Microbiol Infect. 2001;7 Suppl 6:12–15. doi:10.1046/j.1469-0691.2001.00079.x
22. Deresinski S. Principles of antibiotic therapy in severe infections: optimizing the therapeutic approach by use of laboratory and clinical data. Clin Infect Dis. 2007;45(Suppl 3):S177–83. doi:10.1086/519472
23. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309–332. doi:10.1016/j.ajic.2008.03.002
24. Guclu E, Ogutlu A, Karabay O, et al. Antibiotic consumption in Turkish hospitals; a multi-centre point prevalence study. J Chemother. 2017;29(1):19–24. doi:10.1080/1120009X.2016.1156893
25. Dumartin C, L’Hériteau F, Péfau M, et al. Antibiotic use in 530 French hospitals: results from a surveillance network at hospital and ward levels in 2007. J Antimicrob Chemother. 2010;65(9):2028–2036. doi:10.1093/jac/dkq228
26. Dadashi M, Nasiri MJ, Fallah F, et al. Methicillin-resistant Staphylococcus aureus (MRSA) in Iran: a systematic review and meta-analysis. J Glob Antimicrob Resist. 2018;12:96–103. doi:10.1016/j.jgar.2017.09.006
27. WHO Report on Surveillance of Antibiotic Consumption, November 2018, ISBN: ISBN 978-92-4-151488–0.
28. Bugnon-Reber A, de Torrente A, Troillet N, Genne D. Antibiotic misuse in medium-sized Swiss hospitals. Swiss Med Wkly. 2004;134(33–34):481–485. doi:2004/33/smw-10599
29. Erbay A, Bodur H, Akıncı E, Çolpan A. Evaluation of antibiotic use in intensive care units of a tertiary care hospital in Turkey. J Hosp Infect. 2005;59(1):53–61. doi:10.1016/j.jhin.2004.07.026
30. Ceyhan M, Yildirim I, Ecevit C, et al. Inappropriate antimicrobial use in Turkish pediatric hospitals: a multicenter point prevalence survey. Int J Infect Dis. 2010;14(1):e55–61. doi:10.1016/j.ijid.2009.03.013
31. Ingram PR, Seet JM, Budgeon CA, Murray R. Point-prevalence study of inappropriate antibiotic use at a tertiary Australian hospital. Intern Med J. 2012;42(6):719–721. doi:10.1111/j.1445-5994.2012.02809.x
32. Masterton RG. Antibiotic de-escalation. Crit Care Clin. 2011;27(1):149–162. doi:10.1016/j.ccc.2010.09.009
33. Ashraf M, Cook P. Antibiotic misuse in hospital, outpatient, and long-term care settings. N C Med J. 2016;77(5):346–349. doi:10.18043/ncm.77.5.346
34. Fukuda T, Watanabe H, Ido S, Shiragami M. Contribution of antimicrobial stewardship programs to reduction of antimicrobial therapy costs in community hospital with 429 beds –before-after comparative two-year trial in Japan. J Pharm Policy Pract. 2014;7(1):10. doi:10.1186/2052-3211-7-10
35. Meyer E, Buttler J, Schneider C, et al. Modified guidelines impact on antibiotic use and costs: duration of treatment for pneumonia in a neurosurgical ICU is reduced. J Antimicrob Chemother. 2007;59(6):1148–1154. doi:10.1093/jac/dkm088
36. Fraser GL, Stogsdill P, Dickens JD
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
