Back to Journals » International Journal of Nanomedicine » Volume 16
Echogenic, Ultrasound-Sensitive Chitosan Nanodroplets for Spatiotemporally Controlled DKK-2 Gene Delivery to Prostate Cancer Cells
Authors Liu X, Shi D, Guo L, Zhou X , Shang M, Sun X, Meng D, Zhao Y, Li J
Received 16 October 2020
Accepted for publication 22 December 2020
Published 15 January 2021 Volume 2021:16 Pages 421—432
DOI https://doi.org/10.2147/IJN.S286474
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Thomas Webster
Xinxin Liu, Dandan Shi, Lu Guo, Xiaoying Zhou, Mengmeng Shang, Xiao Sun, Dong Meng, Yading Zhao, Jie Li
Department of Ultrasound, Qilu Hospital of Shandong University, Jinan, People’s Republic of China
Correspondence: Jie Li
Department of Ultrasound, Qilu Hospital of Shandong University, Wenhua Western Road, Jinan 107#, People’s Republic of China
Tel/Fax +86-531-82166101
Email [email protected]
Purpose: To synthesize echogenic chitosan/perfluorohexane nanodroplets (CNDs) for DKK-2 gene delivering in a spatiotemporally controlled manner in vitro.
Methods: The characteristics, contrast-enhanced ultrasound imaging, DNA binding and DNase protection capacity, DKK-2 gene transfection and effects on LNCaP cells of these CNDs were investigated.
Results: The obtained CNDs showed positive surface charges and could attract the genetic cargo with negative surface charges to form nanocomplexes. Agarose gel electrophoresis confirmed binding of the CNDs and pDNA. DKK-2 pDNA-loaded CNDs, in combination with ultrasound, ruptured and released DKK-2 pDNA, entering LNCaP cells through nano-scale pores in the cell membrane, which further reduced the proliferation of LNCaP cells.
Conclusion: These stable and safe CNDs may be a promising choice to achieve efficient ultrasound-mediated gene delivery to specific tissues in a spatiotemporally controlled manner.
Keywords: nanodroplets, chitosan, DKK-2, ultrasonic gene transfer, prostate cancer
Introduction
Gene therapy has been widely explored as a pharmacological approach, with great potential to treat a variety of diseases.1,2 Many diseases have definite lesion sites, especially tumors. This feature results in a great demand for the delivery of therapeutic genes to local lesion sites.3 In recent decades, viral vectors have predominated in gene therapy due to their remarkably efficient performance in gene delivery; however, they are still restricted by safety concerns.4 These limitations of viral vectors have expedited the hunt for alternative non-viral delivery systems. Among non-viral vectors, nanodroplets are promising gene transfer agents that have been successfully used for gene delivery both in vitro and in vivo.5
Meanwhile, ultrasound, in combination with nanodroplets, provides a promising platform for the delivery of genes in a spatiotemporally controlled manner.6 Ultrasound is a mechanical wave with an excellent and controlled transmission pathway.7 Ultrasound irradiation can easily be directed toward tumors using an external mobile transducer under imaging guidance. In addition, nanodroplets can serve as vehicles to carry genetic cargo for systemic delivery.8 More importantly, a sonoporation effect occurs when bubbles are exposed to the appropriate ultrasonic energy, producing transient, small holes in the adjacent cell membrane and thus increasing the vascular and cellular permeability.9 The bubbles are then destroyed, resulting in the local release of the genetic payload.10 In this way, ultrasound-targeted microbubble destruction (UTMD) can result in spatiotemporally controlled gene delivery with maximized therapeutic efficacy.
Prostate cancer (PCa) is the most common cancer affecting middle-aged and older men and represents the sixth leading cause of cancer-related mortality in the world.11 In the majority of cases, PCa progresses from prostatic intraepithelial neoplasia through locally invasive adenocarcinoma to castration-resistant prostate cancer (CRPC),12 which leads to a high mortality rate and few effective treatments. Ultrasound-sensitive nanodroplets can serve as vehicles to carry genetic cargo for systemic delivery and can allow the accurate and visualized treatment of tumors to be achieved. At the same time, the nanodroplet-mediated process can reduce the side effects of chemotherapeutics and reverse the drug resistance of CRPC.
Recent data point to a key role of the Wnt signaling pathway in the regulation of the generation and development of tumors. The Wnt signaling pathway is regulated by multiple families of secreted antagonists, including soluble frizzled-related receptors and dickkopfs (DKKs). The DKKs protein family consists of four members, including DKK-1 to 4.13,14 Since the Wnt pathway plays a crucial role in cancer,15 it is not surprising that DKKs are also involved. DKK-1 and DKK-3 are closely related to the development of PCa.16–18 No previous studies have evaluated the association of DKK-2 and PCa with ultrasound-sensitive nanodroplets.
Patients and Methods
Patients and Specimens
A total of 20 patients diagnosed with PCa and 20 patients diagnosed with benign prostatic hyperplasia (BPH) at the Department of Urological Surgery, Qilu Hospital of Shandong University from January 2017 to December 2018 were included. The diagnosis of each case was confirmed by two pathologists. None of the patients had received preoperative adjuvant therapy.
Histology and immunohistochemistry
Tissues were dissected and immediately fixed in 4% formalin. Tissues were paraffin-embedded. Successive transverse paraffin sections were cut at 5 μm thickness and were stained with haematoxylin and eosin. For immunohistochemical analysis, corresponding sections were incubated with the primary antibodies against DKK-2 (1:100, Abcam, UK) overnight, then appropriate secondary antibodies. Signals were amplified with the use of 3.3-diaminobenzidine, counterstained with hematoxylin and analyzed by use of Image-Pro Plus 6.0 (Media Cybernetics, USA).
Preparation of pDNA and Cell Culture
Homo-DKK2-EGFP-N1 vectors were used in our experiments. The Homo DKK-2 gene was cloned into the eukaryotic expression vector pEGFP-N1 (Clontech) with NheI and AgeI restriction enzymes (Biosune, Shanghai, China). LNCaP PCa cells (ATCC, Manassas, VA, USA) were grown in RPMI-1640 with 10% fetal bovine serum and streptomycin (100 g/mL). Cells were incubated in a humidified incubator at 37°C with 5% CO2.
Preparation and Characterization of Chitosan Nanodroplets (CNDs)
The CNDs were formulated by a nanoemulsion process.19 First, a fixed-ratio mixture of perfluorohexane (PFH), Tween-20 and lecithin was homogenized in deionized water for 1 min at 19,000 r.p.m. using an FJ2000-S homogenizer (Shanghai, China). Then, chitosan (0.15% w/v in 1% v/v acetic acid) was added dropwise to the emulsion under homogenization at 14,000 r.p.m. for 2 min. After centrifugation at 500 r.p.m. for 3 min, the upper solution was collected. The resulting CNDs were stored at 4°C for less than 2 h prior to use. After being diluted in deionized water, the particle size, polydispersity index (PDI) and ζ potential of the CNDs were analyzed using a DelsaNano C Particle Size and ζ Potential Analyzer (Beckman Coulter, CA, USA).
In vitro Ultrasound Imaging
Ultrasound imaging experiments with the CNDs were performed in vitro using a clinical ultrasound scanner (LOGIQ E9; GE, USA). The major ultrasound contrast parameters were as follows: mechanical index of 0.5; center frequency of 9.0 MHz; dynamic range of 60 dB. CNDs solution at the proper concentration was added to the plastic dropper fixed on an iron platform in a water bath at 37°C.
DNA Binding and DNAase Protection Capacity of CNDs
The gel retardation assay was used to confirm the pDNA binding capability of the CNDs. CND/pDNA (1 µg of pDNA) complexes were prepared at different weight ratios, ranging from 1 to 20, by incubating pDNA with CNDs for 30 min at room temperature. The complexes were then electrophoresed through a 1% (w/v) agarose gel containing GelRed. Electrophoresis was performed using 1× TAE buffer at 100 V for 30 min. Then, the gel was visualized using a UV transilluminator. To monitor the protection of pDNA against DNase I degradation, the CND/pDNA complexes were incubated with 0.5 U of DNase I at 37°C for 30 min, followed by 1% (w/v) agarose gel electrophoresis.
Gene Transfection of CNDs with Ultrasound Exposure
LNCaP cells were seeded in 6-well plates and cultured overnight at 37°C in a humidified 5% CO2 atmosphere. Immediately prior to transfection, the medium was removed and replaced with 1 mL of the CND/pDNA complex solution diluted in serum-free medium. The pDNA was incubated with the CNDs at the optimal weight ratio for 30 min at room temperature to produce the CND/pDNA complex. Then, the complexes were added to the LNCaP cells and incubated for 60 min at 37°C before ultrasound treatment. Ultrasound irradiation was applied at 1 MHz, 1 W/cm2 and a 50% duty cycle for 3 min using a planar ultrasound apparatus (WELLD). All experiments were performed in triplicate. After the ultrasound treatment, the serum-free medium was replaced by normal culture medium. At 48 h after the treatment, the in vitro transfection efficiency was qualitatively assessed by fluorescence microscopy and analyzed using ImageJ.
Cell Proliferation Analysis
Cell proliferation was determined by cell counting and EdU incorporation (RiboBio, Guangzhou, China). After LNCaP cells were treated under different conditions, the medium was removed, and cells were washed with cold PBS twice. The EdU incorporation assay was performed according to the manufacturer’s instructions, or cells were harvested by trypsinization and counted.
Statistical Analysis
All data are expressed as the mean ± standard deviation. Statistical comparisons between groups were analyzed using Student’s t test. Statistical significance was assigned at P < 0.05.
Results
DKK-2 Content Was Decreased in PCa Tissues
To determine the role of DKK-2 in PCa, human specimens were collected from the Qilu Hospital of Shandong University. First, H&E staining was applied to observe the tissue morphology (Figure 1A). Second, the expression level of DKK-2 was compared between PCa and BPH tissues. The DKK-2 level was lower in PCa tissues than in BPH tissues (Figure 1B).
 |
Figure 1 Expression of DKK-2 in human PCa and BPH tissues. (A) H&E staining of the tissues. (B) Immunostaining for DKK-2 and quantitative analysis (P<0.01 versus BPH). |
Characterization of CNDs
CNDs consisting mainly of chitosan shell and PFH core were successfully synthesized. The resulting solution was translucent, pale blue liquid. A diagram of the CNDs is presented in Figure 2A. Bright-field microscopy, SEM and TEM images of CNDs are shown in Figure 2B–D; the CNDs exhibited a spherical morphology with a well-defined core-shell structure. The typical mean CNDs diameters were 387.85 ± 36.87 nm, with a narrow dispersion (PDI ranged from 0.121 to 0.198) (Figure 2E). The ζ potential of the CNDs was +44.98 mV. Next, we measured the size distribution and ζ potential of the CND/pDNA complexes, which are presented in Table 1. Notably, when using a decreased CND/pDNA weight ratio, an increased average size and a decreased ζ potential were observed.
 |
Table 1 Size Distribution and ζ Potential of CNDs Incubated with pDNA at Different Weight Ratios (CND/pDNA, w/w) |
 |
Figure 2 Characteristics of CNDs. (A) Diagram of CNDs. (B) Bright-field microscopy images of CNDs. (C) SEM images of CNDs. (D) TEM images of CNDs. (E) Size distribution of CNDs. |
DNA Binding and DNase Protection Capacity of CNDs
As shown in Table 1, the ζ potential shift demonstrated the successful binding of pDNA to the CNDs. To further verify the DNA binding capacity of the CNDs, the cationic CNDs were mixed with pDNA at different weight ratios ranging from 1 to 20. After incubation at room temperature for 30 min, the agarose gel retardation assay was performed (Figure 3). The electrophoretic mobility of the pDNA was completely retarded when the CND/pDNA weight ratio was ≥20. These results indicate that the positively charged CNDs bound the negatively charged pDNA strongly; thus, CNDs can serve as an efficient vehicle for DNA transport.
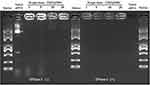 |
Figure 3 Gel retardation assay to evaluate the pDNA binding efficiency and DNase I protection ability of CND/pDNA complexes at various weight ratios. Naked pDNA was used as a control. |
To protect pDNA from degradation by extracellular enzymes during gene delivery, it is essential to condense pDNA into stable particles.20 To verify that the synthesized micelles provide sufficient protection for pDNA from endonucleases, DNase I protection assay was performed by agarose gel electrophoresis. Figure 3 shows that the naked pDNA was completely degraded by DNase I, while pDNA did not show significant degradation when mixed with CNDs. These results clearly demonstrate that CNDs may protect pDNA from degradation by DNase I under physiological conditions.
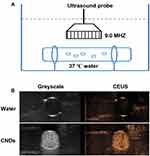
In vitro Contrast-Enhanced Ultrasound Imaging of CNDs
The Contrast-Enhanced Ultrasound (CEUS) imaging ability was one critical function of CNDs. To be a potential clinically applied gene-delivery system, one essential ability of CNDs was that they would be visualized whether CNDs arrived at the desired position or not. The in vitro ultrasound enhancement ability of the CNDs was examined by experiments carried out in a water bath at 37°C (Figure 4) using a clinical ultrasound scanner (LOGIQ E9). The CNDs showed an excellent ultrasound enhancement ability, indicating the feasibility of using CNDs in ultrasound imaging to monitor gene delivery in cancer therapy, as shown in Figure 4B.
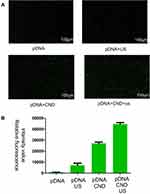
DKK-2 Gene Transfection of CNDs with Ultrasound
Ultrasound has potential as a driving force for enhancing gene delivery.5 To evaluate the effect of ultrasound on DKK-2 gene transfection, we transfected Homo-DKK2-EGFP-N1 pDNA encoding green fluorescence protein (EGFP) into LNCaP cells with or without ultrasound. At the same time, naked pDNA without CNDs and with or without ultrasound was used as a control. The naked pDNA and pDNA with ultrasound could not produce sufficient EGFP signals in the LNCaP cells. However, much more green fluorescence could be detected in cells treated with pDNA-loaded CNDs. Notably, cells treated with a combination of pDNA-loaded CNDs and ultrasound exposure exhibited stronger fluorescence intensity than those treated with pDNA-loaded CNDs (Figure 5A). The relative green fluorescence intensity was measured using ImageJ (Figure 5B) and indicated that CNDs and ultrasound can enhance gene delivery.
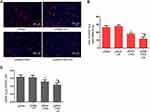
DKK-2 Expression is Involved in Decreased LNCaP Cells Proliferation
Uncontrolled and accelerated proliferation is one of the most fundamental biological behaviors of cancer cells. We found that the DKK-2 level was decreased in PCa tissues. Meanwhile, CNDs, as a safe and non-toxic gene carrier, were used to carry plasmids in this experiment. To identify the role of DKK-2 in regulating LNCaP cells proliferation, cells were transfected with a combination of pDNA-loaded CNDs and ultrasound exposure, and the effect of DKK-2 on LNCaP cells proliferation was determined by EdU incorporation assay and cell counting. The number of EdU-positive cells was decreased significantly after treatment with pDNA-loaded CNDs with or without ultrasound exposure compared with naked pDNA. Meanwhile, ultrasound exposure aggravated the inhibitory response after pDNA-loaded CNDs treatment (Figure 6A and B). The cell counting data is consistent with the EdU incorporation results (Figure 6C).
Discussion
Ultrasound-mediated delivery systems have been widely used in targeted gene delivery to realize the temporal and spatial regulation of gene expression. Meanwhile, studies have demonstrated that the microscale size of microbubbles restricts them from extravasating from blood vessels into tissues, resulting in failed interactions with targeted cells.21,22
In this study, nanobubbles with chitosan shell and PFH core (CNDs) were developed for gene transfection. Compared to microbubbles, CNDs can more easily reach pathological cells by overcoming the vascular endothelial barrier. The same phenomenon also exists in tumor tissues, where nanobubbles but not microbubbles can gather due to the EPR effect.23
In our previous study, we developed DOX-loaded, C3F8-encapsulated nanobubbles coated with chitosan, which showed excellent biosafety.24 In contrast, our nanodroplets with PFH as the core and chitosan as the shell have a distinct nanocapsule structure. Meanwhile, they manifest several distinct advantages, such as greater stability. These CNDs have a strong positive charge and can successfully carry pDNA.
In our study, CNDs were synthesized via the nanoemulsion method under high shear stress.19 The precise mean size of CNDs was 387.85 ± 36.87 nm, with a narrow size distribution (PDI ranged from 0.121 to 0.198), which can reach the tumor sites for easily leaking through vascular endothelial cell barriers of tumor tissue and accumulate locally with EPR effect. The CNDs presented positive surface charges (+44.98 mV). CNDs with positive charges and pDNA with negative charges were mixed and incubated directly, and then CND/pDNA complexes formed by electrostatic adsorption. And an increased average size and a decreased ζ potential were observed when using a decreased CND/pDNA weight ratio (Table 1). Additionally, the formation of CND/pDNA complexes protected pDNA from degradation by nucleases, as shown in Figure 3. Compared to viral vectors, CNDs is one of the chitosan-based gene-delivery systems, which have been designed for gene transfection, with excellent biocompatibility and low immunogenicity.22,25–28 Meanwhile, ultrasound, in combination with CNDs, provides a promising platform for the delivery of genes in a spatiotemporally controlled manner.6
CNDs could be used for ultrasound imaging and ultrasound-mediated treatment and are thus superior to common nanosized delivery systems. Figure 4 shows that our nanodroplets showed an ability to enhance good ultrasound imaging. The nanodroplets in this study with a liquid core overcome the drawbacks of traditional gas-centered ultrasound contrast agents, which have a relatively large diameter and dissatisfactory structural stability in circulation. The PFH-core nanodroplets underwent an instant phase transition into gas bubbles under low-frequency ultrasound stimulation, resulting in large microbubble formation. Compared with the original small nanodroplets, these large microbubbles were very echogenic and could generate high contrast with greater acoustic impedance.29,30 The CNDs showed a good ultrasound imaging ability at 37°C, which is far below the boiling temperature of PFH. These results further confirm that the mechanical effect of ultrasound plays a predominant role in droplet vaporization.19
The dickkopfs protein family, as an inhibitor of the Wnt signaling pathway, consists of four members, including DKK-1 to 4.13,14 Since the Wnt pathway plays a crucial role in cancer,15 it is not surprising that DKKs are also involved. DKK‐1 expression increases during PCa development but significantly decreases as the disease progresses to bone metastasis.16,17 It has been reported that DKK‐3 expression is downregulated in primary PCa cells and is associated with the progression of the cancer.18 However, the biological role and function of DKK-2 remains to be elucidated. In Ewing’s sarcoma, DKK-2 increases the proliferation and invasion of the sarcoma cells. In addition, it regulates the expression of different genes which are important for invasion into bone and osteolysis.31 Overexpression of DKK-2 suppressed malignant cell growth and invasion in SKOV3 and ES-2 cell lines.32 Treatment of B16F10 melanoma-bearing mice with adenovirus expressing DKK-2 increased tumor growth compared with controls.33 These findings suggested that DKK-2 plays different roles in different tumors. The present study shows that DKK-2 expression is decreased in human PCa tissues (Figure 1). These findings indicate that DKK-2 is involved in PCa.
However, Wei et al found that DKK-2 was overexpressed in both PCa tissues and cells.34 In this study, the mRNA expression level of DKK-2 was compared between PCa and adjacent normal prostate tissues by PCR. We do not consider this method to be accurate because the PCa tissues were indistinguishable from the normal prostate tissues. The prostate tissues needed to be examined pathologically for the most accurate diagnosis. Therefore, we used immunohistochemistry to detect the expression of DKK-2 in human prostate tissues in this study.
We used CNDs as gene carriers for the transportation of Homo-DKK2-EGFP-N1 plasmids. As shown in Figure 5, cells treated with pDNA-loaded CNDs showed a more pronounced transfection efficiency than cells treated with naked plasmids, with or without ultrasound irradiation. In particular, the combination of ultrasound with pDNA-loaded CNDs achieved the highest gene transfection efficiency in cells compared with the other three treatments. Under ultrasound irradiation, a proportion of CNDs change from liquid to gas, which causes the disassembly of the micellar structure and results in the release of the genetic cargo.
The proliferation, migration and apoptosis of cancer cells are closely related to the development of tumors. We found that the effects of DKK-2 on migration and apoptosis in cultured LNCaP cells were minimal (data not shown). As shown in Figure 6, the proliferation of cells treated with DKK-2 pDNA-loaded CNDs was inhibited compared with that of cells treated with naked plasmids, with or without ultrasound irradiation. Furthermore, the combination of ultrasound with DKK-2 pDNA-loaded CNDs aggravated the inhibitory response. The general schematic diagram of the article (Figure 7) showed that DKK-2 pDNA-loaded CNDs ruptured and released DKK-2 pDNA under ultrasound irradiation. Meanwhile, DKK-2 pDNA entered LNCaP cells through nano-scale pores in the cell membrane, which further reduced the proliferation of LNCaP cells.
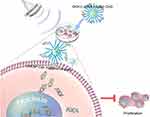 |
Figure 7 Schematic depiction of ultrasound-assisted DKK-2 gene delivery into LNCaP cells. |
This study aimed to produce an improved safe and visual gene-delivery system for the efficient delivery of DKK-2. The research results will provide new ideas for the application of ultrasound-sensitive nanodroplets in the clinical treatment of drug-resistant CRPC.
Ethics Approval and Consent to Participate
All human specimens were obtained during therapeutic surgery. This study was performed using the samples after the pathological diagnosis. This study was specifically approved by the Qilu Hospital Committee of Shandong University for human experiments. The study complied with the Declaration of Helsinki. All patients signed informed consent for the research and use of their specimens for research.
Acknowledgments
This work was supported by the Natural Science Foundation of Shandong Province of China (No. ZR2019PH117), the National Natural Science Foundation of China (No. 81771843) and the Science and Technology Developing Program of Shandong Provincial Government of China (No. 2017GSF18107) .
Disclosure
Xinxin Liu and Jie Li report a patent (Application of ultrasound-sensitive chitosan nanodroplets combined Dickkopf-2 Gene for the treatment of prostate cancer). The authors report no other potential conflicts of interest in this work.
References
1. Hill AB, Chen M, Chen CK, Pfeifer BA, Jones CH. Overcoming Gene-Delivery Hurdles: physiological Considerations for Nonviral Vectors. Trends Biotechnol. 2016;34(2):91–105. doi:10.1016/j.tibtech.2015.11.004
2. Xia Y, Xu T, Wang C, et al. Novel functionalized nanoparticles for tumor-targeting co-delivery of doxorubicin and siRNA to enhance cancer therapy. Int J Nanomedicine. 2018;13:143–159. doi:10.2147/IJN.S148960
3. Hu D, Chen L, Qu Y, et al. Oxygen-generating Hybrid Polymeric Nanoparticles with Encapsulated Doxorubicin and Chlorin e6 for Trimodal Imaging-Guided Combined Chemo-Photodynamic Therapy. Theranostics. 2018;8(6):1558–1574. doi:10.7150/thno.22989
4. Lukashev AN, Zamyatnin AA. Viral vectors for gene therapy: current state and clinical perspectives. Biochemistry. 2016;81(7):700–708. doi:10.1134/S0006297916070063
5. Yu J, Chen Z, Li Y, Du M, Yan F, Zheng H. Echogenic Chitosan Nanodroplets for Spatiotemporally Controlled Gene Delivery. J Biomed Nanotechnol. 2018;14(7):1287–1297. doi:10.1166/jbn.2018.2575
6. Whitman GJ, Hortobagyi GN. Ultrasound Molecular Imaging: A Good Start. J Clin Oncol. 2017;35(19):2101–2102. doi:10.1200/JCO.2016.71.9997
7. Zhou M, Wen K, Bi Y, et al. The Application of Stimuli-responsive Nanocarriers for Targeted Drug Delivery. Curr Top Med Chem. 2017;17(20):2319–2334. doi:10.2174/1568026617666170224121008
8. Zhou QL, Chen ZY, Wang YX, Yang F, Lin Y, Liao YY. Ultrasound-mediated local drug and gene delivery using nanocarriers. Biomed Res Int. 2014;2014:963891.
9. Mullick Chowdhury S, Lee T, Willmann JK. Ultrasound-guided drug delivery in cancer. Ultrasonography. 2017;36(3):171–184. doi:10.14366/usg.17021
10. Chen ZY, Yang F, Lin Y. New development and application of ultrasound targeted microbubble destruction in gene therapy and drug delivery. Curr Gene Ther. 2013;13(4):250. doi:10.2174/15665232113139990003
11. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi:10.3322/caac.21262
12. Isaacs JT. The biology of hormone refractory prostate cancer. Why does it develop? Urol Clin North Am. 1999;26(2):263–273. doi:10.1016/S0094-0143(05)70066-5
13. Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006;25(57):7469–7481. doi:10.1038/sj.onc.1210054
14. Bafico A, Yaniv LG, Yaniv A, Gazit A, Aaronson SA. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat Cell Biol. 2001;3(7):683–686. doi:10.1038/35083081
15. Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14(15):1837–1851.
16. Hall CL, Bafico A, Dai J, Aaronson SA, Keller ET. Prostate Cancer Cells Promote Osteoblastic Bone Metastases through Wnts. Cancer Res. 2005;65(17):7554–7560. doi:10.1158/0008-5472.CAN-05-1317
17. C L H, Shah DSD, Shah RB, Pienta KJ, Keller ET. Dickkopf-1 expression increases early in prostate cancer development and decreases during progression from primary tumor to metastasis. The Prostate. 2008;68(13):1396–1404. doi:10.1002/pros.20805
18. Kawano Y, Kitaoka M, Hamada Y, Walker MM, Waxman J, Kypta RM. Regulation of prostate cell growth and morphogenesis by Dickkopf-3. Oncogene. 2006;25(49):6528–6537. doi:10.1038/sj.onc.1209661
19. Baghbani F, Mohandesi MF, Mohandesi JA, Yazdian F, Mokhtari-Dizaji M, Hamedi S. Formulation design, preparation and characterization of multifunctional alginate stabilized nanodroplets. Int J Biol Macromol. 2016;89:550–558. doi:10.1016/j.ijbiomac.2016.05.033
20. Layek B, Singh J. Cell penetrating peptide conjugated polymeric micelles as a high performance versatile nonviral gene carrier. Biomacromolecules. 2013;14(11):4071–4081. doi:10.1021/bm401204n
21. Fan CH, Lin CY, Liu HL, Yeh CK. Ultrasound targeted CNS gene delivery for Parkinson’s disease treatment. J Control Release. 2017;261:246–262. doi:10.1016/j.jconrel.2017.07.004
22. Ramesan RM, Sharma CP. Modification of chitosan nanoparticles for improved gene delivery. Nanomedicine. 2012;7(1):5–8. doi:10.2217/nnm.11.170.
23. Miller DLPS, Sonoporation GJ, Greenleaf JF. mechanical DNA delivery by ultrasonic cavitation. Somat Cell Mol Genet. 2002;27(1–6):115–134. doi:10.1023/A:1022983907223
24. Zhou X, Guo L, Shi D, Duan S, Li J. Biocompatible Chitosan Nanobubbles for Ultrasound-Mediated Targeted Delivery of Doxorubicin. Nanoscale Res Lett. 2019;14:1. doi:10.1186/s11671-019-2853-x
25. Tan L, Han S, Ding S, et al. Chitosan nanoparticle-based delivery of fused NKG2D-IL-21 gene suppresses colon cancer growth in mice. Int J Nanomedicine. 2017;12:3095–3107. doi:10.2147/IJN.S128032
26. Duan S, Song M, He J. Folate-Modified Chitosan Nanoparticles Coated Interferon-Inducible Protein-10 Gene Enhance Cytotoxic T Lymphocytes’ Responses to Hepatocellular Carcinoma. J Biomed Nanotechnol. 2016;12(4):700–709. doi:10.1166/jbn.2016.2216
27. Fu SZ, Xia J, Wu J. Functional chitosan nanoparticles in cancer treatment. J Biomed Nanotechnol. 2016;12(1585):2016. doi:10.1166/jbn.2016.2228
28. Bonferoni MC, Sandri G, Dellera E. Palmitoyl glycol chitosan micelles for corneal delivery of cyclosporine. J Biomed Nanotechnol. 2016;12:231. doi:10.1166/jbn.2016.2140
29. Baghbani F, Hadian MF, Moztarzadeh F, Hadian-Ghazvini S, Raz M. Novel ultrasound-responsive chitosan/perfluorohexane nanodroplets for image-guided smart delivery of an anticancer agent: curcumin. Mater Sci Eng C. 2016;74:186. doi:10.1016/j.msec.2016.11.107
30. C H W, Lee KST, Lee Y-H, Luo Y-L, Huang Y-F, Yeh C-K. Aptamer-conjugated and drug-loaded acoustic droplets for ultrasound theranosis. Biomaterials. 2012;33(6):1939–1947. doi:10.1016/j.biomaterials.2011.11.036
31. Hauer K, Calzada-Wack J, Steiger K, et al. DKK2 mediates osteolysis, invasiveness, and metastatic spread in Ewing sarcoma. Cancer Res. 2013;73(2):967–977. doi:10.1158/0008-5472.CAN-12-1492
32. Zhu J, Gu ZS, Gu L, Di W. Epigenetic silencing of DKK2 and Wnt signal pathway components in human ovarian carcinoma. Carcinogenesis. 2012;33(12):2334–2343. doi:10.1093/carcin/bgs278
33. Park H, Jung HY, Choi H-J, et al. Distinct roles of DKK1 and DKK2 in tumor angiogenesis. Angiogenesis. 2013;17(1):221–234. doi:10.1007/s10456-013-9390-5
34. Xu W, Pang K, Zhou ZG, et al. Dickkopf 2 promotes proliferation and invasion via Wnt signaling in prostate cancer. Mol Med Rep. 2016;14(3):2283–2288. doi:10.3892/mmr.2016.5502
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.