Back to Journals » Clinical Interventions in Aging » Volume 18
Echocardiography E/A Abnormality is Associated with the Development of Primary Left Ventricle Remodeling in Middle-Aged and Elderly Women: A Longitudinal Study
Authors Wu J, Wang J , Wang Y, Fan W, Li H , Wu H
Received 8 December 2022
Accepted for publication 2 April 2023
Published 18 April 2023 Volume 2023:18 Pages 629—638
DOI https://doi.org/10.2147/CIA.S399996
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Zhi-Ying Wu
Jing Wu,1,* Jiaqi Wang,1,* Yiyan Wang,1 Wenjing Fan,1 Husheng Li,1 Hengjing Wu2
1School of Nursing, Shanghai University of Traditional Chinese Medicine, Shanghai, People’s Republic of China; 2Clinical Center for Intelligent Rehabilitation Research, Shanghai YangZhi Rehabilitation Hospital (Shanghai Sunshine Rehabilitation Center), School of Medicine, Tongji University, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hengjing Wu, Clinical Center for Intelligent Rehabilitation Research, Shanghai YangZhi Rehabilitation Hospital (Shanghai Sunshine Rehabilitation Center), School of Medicine, Tongji University, 2209 Xing Guang Road, Shanghai, 201613, People’s Republic of China, Tel +8615821525700, Email [email protected]
Background: Impaired left ventricular (LV) relaxation is indicative of grade I diastolic dysfunction, which is mainly assessed by late diastolic transmitral flow velocity (E/A ratio). Although the E/A ratio has important diagnostic and prognostic implications with cardiac outcomes, the causal link between abnormal E/A ratio and left ventricle remodeling (LV remodeling) remains unclear.
Methods: A longitudinal analysis of 869 eligible women aged ≥ 45 years, who had received echocardiography scans as well as 5-year follow-up assessments between 2015 and 2020. Women with pre-existing cardiac abnormalities including grade II/III diastolic dysfunction as diagnosed by echocardiography, or structural heart disease were excluded. E/A abnormality was defined as baseline E/A ratio < 0.8. The classification of LV remodeling was based on the measurements of left ventricular mass index (LVMI) and relative wall thickness (RWT). Logistic and linear regression models were used.
Results: Among the 869 women (60.71± 10.01 years), 164 (18.9%) had developed LV remodeling after the 5-year follow-up. The proportion of women with E/A abnormality versus non-abnormality was also significantly different (27.13% vs 16.59%, P=0.007). Multivariable-adjusted regression models showed that E/A abnormality (OR: 4.14, 95%Cl:1.80– 9.20, P=0.009) was significantly associated with higher risk of concentric hypertrophy (CH) after follow-up. No such association was found in either concentric remodeling (CR) or eccentric hypertrophy (EH). Higher baseline E/A ratio was correlated with lower ΔRWT during the 5-year follow-up (β=− 0.006 m/s, 95% CI: − 0.012 to − 0.002, P=0.025), which was independent of demographics and biological factors.
Conclusion: E/A abnormality is associated with a higher risk of CH. Higher baseline E/A ratio may be associated with decreased relative changes in RWT.
Keywords: diastolic dysfunction, middle-aged women, left ventricular remodeling, echocardiography, heart failure
Introduction
Left ventricular diastolic dysfunction (LVDD) describes abnormal diastolic properties of the left ventricle and is characterized by delayed myocardial relaxation and incomplete ventricular filling. Studies have reported that patients with impaired left ventricle (LV) relaxation have an increased risk of sudden cardiac death.1,2 Based on the current literature, the early to late diastolic transmitral flow velocity (E/A ratio) was the key marker for the assessment of LV relaxation impairment.3,4 E/A ratio <0.8 has been recognized as a simple but strong indicator of LVDD and related to the poor prognosis of heart failure (HF).5–7 Among middle-aged and elderly community dwellers, 20–25% can develop E/A abnormality, with prognostic implications and higher adverse cardiovascular outcome.8
It is well established that adverse left ventricle remodeling (LV remodeling) and diastolic dysfunction are the main pathophysiological markers of patients with heart failure with preserved ejection fraction.9,10 Although the major risk factors of LV remodeling and diastolic dysfunction have been separately reported in many studies, the interactive role of diastolic dysfunction and LV remodeling in the general population is still unclear. In the conventional paradigm of the natural history of HF, the left ventricle may first develop structural abnormalities with a subsequent change in diastolic function impairment. Nevertheless, evidence from experimental animal models sheds light on the interactive relationship between LVDD and ventricular remodeling. By using a spontaneous hypertensive rat model, Dupont et al11 suggests that impaired LV relaxation is compromised before the onset of hypertension and left ventricular hypertrophy (LVH). These results have been subsequently confirmed by Di Bello et al.12 However, it is still difficult to consider impaired LV relaxation as an early event in the cascade of the development of LVH, due to the limited data in humans.
In this study, we hypothesized that an E/A ratio abnormality in middle-aged and elderly women may precede LV remodeling. The association between the E/A abnormality and the development of LV remodeling may vary across the subtypes of LV remodeling. This longitudinal study sought to determine, in a community-based population, whether a baseline E/A abnormality determined by the results of an echocardiographic test was associated with abnormal left ventricular structural changes over time in the absence of interval myocardial infarction.
Materials and Methods
Study Population and Data Collection
The data in this study were obtained from a 5-year cohort in the Shanghai Zhangjiang community. At the baseline examination, all participants received a structured interview, physical examination, blood biochemical indexes’ tests and echocardiography scan at the baseline survey in 2015. Among 1,438 middle-aged and elderly women with no history of myocardial infarction (MI), HF, valvular heart disease (VHD) and echocardiography-based LV structural abnormality, 934 participants had repeated echocardiography assessment in 2020. 869 participants who had echocardiography measures at both time-points in outpatient clinic and had no missing values in any covariates were included in the present study (Figure 1). Informed consent for this study was given by all participants and the Ethics Committee of Shanghai University of Traditional Chinese Medicine approved the study.
 |
Figure 1 Flowchart. Abbreviations: CAD, coronary artery disease; HF, heart failure; VHD, valvular heart disease. |
Demographic and medical information including age, body mass index (BMI), and history of diabetes/hypertension was obtained from medical records or the structured interview. Subjects with fasting blood glucose (FBG) ≥7.0 mmol/L were diagnosed with diabetes.13 Subjects with either systolic blood pressure (SBP) ≥140 mmHg or diastolic blood pressure (DBP)≥90 mmHg were considered as having hypertension.14
Blood samples were obtained after an overnight fast, and the brain natriuretic peptide (BNP), serum high-sensitivity C-reactive protein (hsCRP), and homocysteine (HCY) were obtained measured in a laboratory. The value of the brachial-ankle pulse wave analysis (BaPWV) was obtained from an automatic radial arterial pulse wave monitor (Colin Medical Technology, Komaki, Japan). Additionally, SBP and DBP were determined with a physical examination. Details regarding the process of blood sample collection and other data collection have been reported elsewhere.8,15
Echocardiographic Analysis
A two-dimensional (2D) echocardiographic evaluation was completed using a color Doppler ultrasonic device equipped with a 1.0–5.0 MHz transducer (GE Vivid 7; General Motors Corporation, NY, USA). One of two trained sonographers performed all echocardiograms. An experienced cardiologist, without prior knowledge of the patients, interpreted the results. Recorded measurements included: the left atrium diameter (LAD), left ventricular ejection fraction (LVEF), left ventricular internal diameter at end-diastole (LVIDd), left ventricular at end-systolic diastole (LVISd), posterior left ventricular wall thickness (PVWT), and interventricular septal thickness (IVST). Furthermore, the calculation of the left ventricular mass (LVM) was performed using the following formula:16 LVM (g) = 0.8 × {1.04 × [(LVI Dd + PVWT + IVST)3 - (LV I Dd)3 + 0.6 (g). The LVM index (LVMI) was generated by indexed body surface area. E (peak early diastolic flow velocity) and A (late diastolic transmitral flow velocity) were recorded by the transmittal inflow velocity signals at the mitral tip by color Doppler echocardiography in the apical way.
The calculation of E/A ratio was the value of early diastolic flow velocity (E velocity) divided by late diastolic transmitral flow velocity (A velocity). The E/e’ ratio was calculated by the value of E velocity and mitral annulus early diastolic velocity (e’).
Definition of an E/A Abnormality
The American Society of Echocardiography17 suggested that patients with an E/A ratio ≤0.8 should be considered as having an early stage of LVDD. Recently, Jeong et al18 proposed a diagnosis of normal LV diastolic function including 0.75 < E/A ratio < 1.5. Therefore, we defined E/A ratio abnormality as E/A ratio < 0.8, and normal E/A ratio as 0.8 ≤ E/A ratio < 1.5. The E/A ratio was calculated based on the data obtained using Doppler tissue imaging from a four-chamber view.
Classification of Left Ventricular Remodeling
Different LV remodeling types were determined according to the guidelines from the American Heart Association (AHA) and recent studies.16,19 The calculation of RWT was based on the following formula: (RWT) = (2 × PVWT)/ LVIDd. As shown in Figure S1, we classified patients into four types of LV remodeling: 1) normal geometry, 2) concentric remodeling (CR), 3) concentric hypertrophy (CH), and 4) eccentric hypertrophy (EH). Echocardiography measurements in different patterns of LV remodeling are presented in Table S1.
Statistical Analysis
All data in this study were analyzed by SPSS software (SPSS 26.0, Chicago, IL, USA). Continuous data were expressed as mean ± standard deviation (SD). Independent sample t-test was used for normally distributed variables. Variables with a skewed distribution were analyzed by the Mann Whitney U-test. Categorical variables were assessed using a Chi-square test. The Wilcoxon rank sum test was utilized to compare the proportions of the E/A abnormality in four types of LV remodeling. Moreover, we analyzed whether the E/A abnormality is independently associated with LV remodeling by using multivariate logistic regression before and after adjusting for age, BMI, hsCRP, BNP, history of hypertension, and history of diabetes. In the consideration of non-independence of these repeated data, linear regression models were used to measure the effect of baseline E/A ratio on 5-year longitudinal change of LVMI and RWT respectively. We also adjusted for baseline RWT or baseline LVMI in all models. LVMI change, as well as RWT change, was represented by the difference between the two timepoint repeated measures. A P value < 0.05 was considered as being statistically significant.
Results
Baseline Characteristics
This study included a total of 869 subjects in total. Among them, 188 (21.6%) were diagnosed with an E/A abnormality at baseline. The average age of the E/A abnormality group was 62.19 ± 8.42 years. Table 1 summarized the demographic information and clinical features. The comparison between groups showed that the levels of BMI, peripheral blood pressure, HbA1C (Hemoglobin A1C), HCY and baPWV were significantly higher in subjects with E/A abnormality than in non-E/A abnormality group (all P < 0.05). The echocardiographic parameters of all subjects at baseline are shown in Table 2.
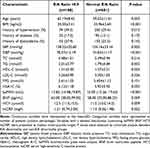 |
Table 1 Baseline Clinical Characteristics of Study Population According to E/A Ratio Abnormality |
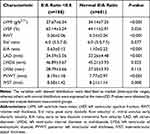 |
Table 2 Baseline Echocardiographic Characteristics of Study Population According to E/A Ratio Abnormality |
5-Year Longitudinal Incidence of LV Remodeling and Its Subtypes Based on Baseline E/A State
Over the five years follow-up period, a total of 164 (18.9%) subjects developed LV remodeling. According to the classification of LV remodeling, the most frequent subtype was EH (46.3%), followed by CR (41.5%) after 5 years’ follow-up (Table S1). During 5 years’ follow-up, participants with EH or CH had significant differences in multiple echocardiographic measures. LVMI, RWT and PVWT were significantly different in participants with CH. Significant changes in E/e’ ratio, LVIDd and LAD were found in EH group. Only the E/A ratio was significantly different in both the EH and CH groups after 5 years’ follow-up. (Table S2). The proportion of LV remodeling in group of E/A abnormality was significantly higher than that in normal E/A group (27.13% vs 16.59%, P=0.007) (Table 3, Figure 2). Among all subjects with different types of LV remodeling, subjects with an E/A abnormality had the highest proportion of CH (P < 0.001) (Table 3, Figure 2).
 |
Table 3 The Longitudinal Observed Incidence of LV Remodeling Based on Baseline E/A States |
 |
Figure 2 The subtypes of LV remodeling based on baseline E/A states. Abbreviations: LVR, left ventricle remodeling; CR, concentric remodeling; EH, eccentric hypertrophy; CH, concentric hypertrophy. |
Effect of Baseline E/A Ratio on the Development of LV Remodeling
In multi-variable adjusted models, no independent correlation was observed between E/A abnormality and EH (OR:1.24, 95%Cl: 0.99–1.78, P=0.177) (Table 4). However, E/A abnormality was independently predicted CH (OR:4.14, 95%Cl: 1.80–9.20, P=0.009) (Table 5). BMI and BNP>100pg/mL were correlated with higher risks of both EH and CH (Table 4, Table 5). Linear regression models were used to measure the effect of E/A on 5-year longitudinal change of LVMI and RWT respectively. As shown in Table 6, there was significant association between baseline E/A and RWT difference (β=−0.006 m/s, 95% CI:−0.012 to −0.002, P=0.025) (Table 6), independent of age, gender, BMI, hypertension, diabetes, and levels of BNP and hsCRP. However, no such association was found in LVMI change (β=−0.268 g/m2, 95% CI: −1.013 to 0.478, P=0.482) (Table 6). In addition, stratified analyses were performed to assess the risk of LV remodeling in participants with different grade degree of E/A abnormality. As shown in Table S3, the group of E/A ratio ≤0.6 had the highest risk of LV remodeling (OR:2.113, 95%Cl: 1.500–5.986, P=0.036).
 |
Table 4 Predictors of 5-Year Incidence of Eccentric Hypertrophy |
 |
Table 5 Predictors of 5-Year Incidence of Concentric Hypertrophy |
 |
Table 6 Association of the 5-Year Differences of LV Remodeling Indicators with Baseline E/A Ratio |
Discussion
In this study, we presented insights into the contribution of E/A abnormality to the development of LV remodeling in population level. By analyzing the 5 years longitudinal data of middle-aged and elderly women, we demonstrated that E/A abnormality independently predicted the incidence of concentric hypertrophy. Higher baseline E/A ratio may correlate with subsequent change in RWT.
Our results contribute to ongoing efforts to unravel the causal link between impaired LV relaxation and cardiac remodeling.
In this study, LV remodeling was divided into three subtypes based on the diagnostic criteria from AHA.16,19 After five years’ follow-up, CR had the highest proportion in three types of LV remodeling, followed by ER. Moreover, we found that the proportion of LV remodeling was significantly higher in patients with E/A abnormality. In order to further confirm which types of LV remodeling could be better predicted by E/A abnormality, we performed a regression analysis on the three pathological subtypes of LV remodeling. The regression models showed that the baseline E/A abnormality significantly increased the risk of developing CH. However, E/A abnormality was not significantly associated with EH and CR after multivariable adjustment. Masugata20 reported that CH is more associated with LVDD than EH. In line with this, our study found E/A abnormality, as the key marker of LVDD diagnosis, is also correlated with higher risk of CH. CH commonly occurs with increased left ventricular afterload. This remodeling process maintains the systolic function, but yields a substrate for LVDD. Iyengar21 reported that subjects with CH had more adverse cardiovascular events and higher mortality than subjects with other types of LV remodeling. A recent study22 found that not all cardiac hypertrophy leads to adverse cardiac events. Actually, cardiac wall stress and cardiac output can be normalized and preserved at certain condition called physiological cardiac hypertrophy. Therefore, some EH might appear during the process of physiological cardiac hypertrophy. But there is still no evidence proving that E/A ratio is not related to eccentric hypertrophy. Combined with our findings in this study, we assume that E/A abnormality is one of the early clinical features of CH. However, our findings warrant further studies based on a larger sample size needed to explore the associations between them.
It has been established that the enlargement of LVMI and RWT indicate geometric remodeling, that can predict cardiac events in the population. By using linear regression models, we found that women with an E/A measure lower than 0.8 have a greater risk of LV enlargement, which was indicated by RWT. Of note, changes of this marker persisted to be statistically significant after adjustment for demographic and clinical factors. It is reported that LV relaxation impairment was preserved in a number of middle-aged and elderly women, especially in newly diagnosed untreated hypertension. De Marchi et al23 found a correlation between RWT and E/A ratio in both hypertrophic cardiomyopathy and hypertensive heart disease. There was limited evidence of this association in a general population. Our findings corroborate, from different population perspectives, the link between regional extent of hypertrophy and abnormalities of relaxation.
The predictors of LV remodeling in this population included BNP and BMI, which is consistent with the result of a previous study.8 The BMI is independently associated with EH according to our study. This finding is consistent with the outcome described in Cuspidi et al’s report,24 which pointed out that EH was more frequent among obese patients than CH. Previous studies25,26 reported that BNP is closely associated with the cardiac pump function and LV remodeling. The current study further found that BNP is more related to EH. Age is one of the major risk factors associated with cardiovascular disease;27 however, our regression model showed that age is not related to either kind of LV remodeling. One reason may be that the ages of participants in our study were too close to identify the effect of age. Patients with different demographic information are needed in future studies to explore the influence of other factors.
This was a community-based longitudinal study, based on widespread, well-defined and validated echocardiographic acquisition techniques. A potential limitation may derive from the use of M-mode and 2D echo, when gold standard (three-dimensional echocardiography; magnetic resonance imaging) have been introduced and validated. Although we excluded subjects with clinically confirmed cardiac disease at baseline, there might be a subset of individuals with underlying cardiac structural and functional abnormalities. Additionally, data on assessing the effect of E/A abnormality on different period of LV remodeling in this 5 years’ longitudinal study were not available. Therefore, more studies aiming to define which point is the best timing to prevent LV remodeling on E/A abnormality patients are needed. This study only included middle-aged women in People’s Republic of China, so the outcome of this study may not be the same with different groups such as male or younger female groups. A recent study28 indicated that estrogen plays a critical role in the process of LV remodeling development, especially in postmenopausal women, but we were unable to analyze these female-specific variables. Future studies will be needed to determine how these potential factors affect the process of LV remodeling. Other values such as LA volume, septal and lateral e’ values and pulmonary venous flow assessment, Valsalva maneuver, tricuspid regurgitation velocity should also be reported because it is valuable in assessing LVDD, further studies are needed to clarify the association between these values and LV remodeling.
The results from this longitudinal study suggested that E/A abnormality is associated with the higher risk of concentric hypertrophy (CH) in a population of middle-aged women. In a community setting, the incidence of LV remodeling was 18.9% during 5 years of follow-up. An E/A abnormality indicated by cardiac ultrasound was independently related to CH incidence. Future studies with more comprehensive assessments are warranted to confirm our findings.
Abbreviations
HF, heart failure; LVEF, left ventricle ejection fraction; LV remodeling, left ventricle remodeling; LVDD, left ventricular diastolic dysfunction; LVEF, left ventricular ejection fraction; AHA, American Heart Association; E/A ratio, early to late diastolic transmitral flow velocity; LVH, left ventricular hypertrophy; BMI, body mass index; FBG, fasting blood glucose; SBP, systolic blood pressure; DBP, diastolic blood pressure; BNP, brain natriuretic peptide; hsCRP, serum high, sensitivity C, reactive protein; HCY, homocysteine; BaPWV, brachial, ankle pulse wave analysis; LAD, left atrium diameter; LVIDd, left ventricular internal diameter at end, diastole; LVISd, left ventricular at end, systolic diastole; PVWT, posterior left ventricular wall thickness; IVST, interventricular septal thickness; LV Mass, left ventricular mass; LVMI, left ventricle mass index; E/ e’, E:peak early diastolic flow velocity/ e’, mitral annulus early diastolic velocity; LVMI, left ventricle mass index; RWT, relative wall thickness; CR, concentric remodeling; CH, concentric hypertrophy; EH, eccentric hypertrophy; HbA1C, Hemoglobin A1C.
Data Sharing Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Ethics and Approval
Informed consent for this study was given by all participants and Ethics Committee of Shanghai University of Traditional Chinese Medicine approved the study. Also, we confirmed that this study complies with the Declaration of Helsinki.
Acknowledgments
We thank all the members of this study for their contributions.
Author Contributions
Jing Wu and Jiaqi Wang share first authorship. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The study was funded by the Nature Science Foundation of China (No. 71904127). The authors express their sincere gratitude to all the staffs in this survey for their important contribution.
Disclosure
The authors declared that they have no conflicts of interest to this work.
References
1. Aljaroudi W, Alraies MC, Halley C, et al. Impact of progression of diastolic dysfunction on mortality in patients with normal ejection fraction. Circulation. 2012;125(6):782–788. doi:10.1161/circulationaha.111.066423
2. Saeed S, Rajani R, Tadic M, Parkin D, Chambers JB. Left atrial volume index predicts adverse events in asymptomatic moderate or severe aortic stenosis. Echocardiography. 2021;38(11):1893–1899. doi:10.1111/echo.15225
3. Mitter SS, Shah SJ, Thomas JD. A Test in Context: e/A and E/e’ to Assess Diastolic Dysfunction and LV Filling Pressure. J Am Coll Cardiol. 2017;69(11):1451–1464. doi:10.1016/j.jacc.2016.12.037
4. Chang D, Xu TT, Zhang SJ, et al. Telmisartan ameliorates cardiac fibrosis and diastolic function in cardiorenal heart failure with preserved ejection fraction. Exp Biol Med (Maywood). 2021;246(23):2511–2521. doi:10.1177/15353702211035058
5. Johansson B, Lundin F, Tegerback R, Bojö L. E/a´ ratio a simple detector of left ventricular dysfunction in patients with decreased ejection fraction. Scand Cardiovasc J. 2018;52(1):20–27. doi:10.1080/14017431.2017.1414954
6. Rajyaguru C, Kalra A, Samim A, Abejie B, Wessel R, Vempilly JJ. Increased E/A Ratio is a Risk Factor for the Formation of Pleural Effusion in Heart Failure. Lung. 2020;198(1):229–233. doi:10.1007/s00408-019-00308-2
7. Dokainish H. Left ventricular diastolic function and dysfunction: central role of echocardiography. Glob Cardiol Sci Pract. 2015;2015:3. doi:10.5339/gcsp.2015.3
8. Wu J, Yu SY, Wo D, Zhao MM, Zhang LJ, Li J. Risks and predictors of mild diastolic dysfunction among middle-aged and aged women: a population-based cohort study. J Hum Hypertens. 2016;30(5):335–340. doi:10.1038/jhh.2015.85
9. Cohen JB, Schrauben SJ, Zhao L, et al. Clinical Phenogroups in Heart Failure With Preserved Ejection Fraction: detailed Phenotypes, Prognosis, and Response to Spironolactone. JACC Heart Fail. 2020;8(3):172–184. doi:10.1016/j.jchf.2019.09.009
10. Chamsi-Pasha MA, Zhan Y, Debs D, Shah DJ. CMR in the Evaluation of Diastolic Dysfunction and Phenotyping of HFpEF: current Role and Future Perspectives. JACC Cardiovasc Imaging. 2020;13(1):283–296. doi:10.1016/j.jcmg.2019.02.031
11. Dupont S, Maizel J, Mentaverri R, et al. The onset of left ventricular diastolic dysfunction in SHR rats is not related to hypertrophy or hypertension. Am J Physiol Heart Circ Physiol. 2012;302(7):H1524–32. doi:10.1152/ajpheart.00955.2010
12. Di Bello V, Talini E, Dell’Omo G, et al. Early left ventricular mechanics abnormalities in prehypertension: a two-dimensional strain echocardiography study. Am J Hypertens. 2010;23(4):405–412. doi:10.1038/ajh.2009.258
13. Care D. 2. Classification and Diagnosis of Diabetes: standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42(Suppl1):S13–s28. doi:10.2337/dc19-S002
14. Jones NR, McCormack T, Constanti M, McManus RJ. Diagnosis and management of hypertension in adults: NICE guideline update 2019. Br J Gen Pract. 2020;70(691):90–91. doi:10.3399/bjgp20X708053
15. Kang S, Fan HM, Li J, et al. Relationship of arterial stiffness and early mild diastolic heart failure in general middle and aged population. Eur Heart J. 2010;31(22):2799–2807. doi:10.1093/eurheartj/ehq296
16. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–1463. doi:10.1016/j.echo.2005.10.005
17. Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: an Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29(4):277–314. doi:10.1016/j.echo.2016.01.011
18. Jeong EM, Dudley SC. Diastolic dysfunction. Circ J. 2015;79(3):470–477. doi:10.1253/circj.CJ-15-0064
19. Gaasch WH, Zile MR. Left ventricular structural remodeling in health and disease: with special emphasis on volume, mass, and geometry. J Am Coll Cardiol. 2011;58(17):1733–1740. doi:10.1016/j.jacc.2011.07.022
20. Masugata H, Senda S, Inukai M, et al. Differences in left ventricular diastolic dysfunction between eccentric and concentric left ventricular hypertrophy in hypertensive patients with preserved systolic function. J Int Med Res. 2011;39(3):772–779. doi:10.1177/147323001103900309
21. Iyengar SS, Ram CVS. Concentric vs. Eccentric Left Ventricular Hypertrophy: does It Matter? It Is All “Blood Pressure Centered”. Am J Hypertens. 2021;34(6):581–582. doi:10.1093/ajh/hpab037
22. Gallo S, Vitacolonna A, Bonzano A, Comoglio P, Crepaldi T. ERK: a Key Player in the Pathophysiology of Cardiac Hypertrophy. Int J Mol Sci. 2019;20(9):2164. doi:10.3390/ijms20092164
23. De Marchi SF, Allemann Y, Seiler C. Relaxation in hypertrophic cardiomyopathy and hypertensive heart disease: relations between hypertrophy and diastolic function. Heart. 2000;83(6):678–684. doi:10.1136/heart.83.6.678
24. Cuspidi C, Rescaldani M, Sala C, Grassi G. Left-ventricular hypertrophy and obesity: a systematic review and meta-analysis of echocardiographic studies. J Hypertens. 2014;32(1):16–25. doi:10.1097/HJH.0b013e328364fb58
25. Qin L, Liu X, Li Y. Correlation of serum BNP and ET-1 levels with cardiac pump function and ventricular remodeling in patients with heart failure. Cell Mol Biol (Noisy-le-Grand). 2020;66(3):125–131. doi:10.14715/cmb/2020.66.3.19
26. Wu L, Zhang L, Ai Z, et al. Association between risk factors and left ventricular remodeling in middle-aged and aged population: a community-based study. J Hypertens. 2012;30(9):1862–1873. doi:10.1097/HJH.0b013e3283563418
27. Costantino S, Paneni F, Cosentino F. Ageing, metabolism and cardiovascular disease. J Physiol. 2016;594(8):2061–2073. doi:10.1113/jp270538
28. Oneglia A, Nelson MD, Merz CNB. Sex Differences in Cardiovascular Aging and Heart Failure. Curr Heart Fail Rep. 2020;17(6):409–423. doi:10.1007/s11897-020-00487-7
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
