Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 16
Eccentric Left Ventricular Hypertrophy and Left and Right Cardiac Function in Chronic Heart Failure with or without Coexisting COPD: Impact on Exercise Performance
Authors Dos Santos PB , Simões RP , Goulart CL , Roscani MG , Marinho RS , Camargo PF , Arbex RF, Casale G, Oliveira CR, Mendes RG , Arena R, Borghi-Silva A
Received 11 October 2020
Accepted for publication 21 December 2020
Published 3 February 2021 Volume 2021:16 Pages 203—214
DOI https://doi.org/10.2147/COPD.S285812
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Polliana B Dos Santos,1 Rodrigo P Simões,1,2 Cássia da L Goulart,1 Meliza G Roscani,3 Renan S Marinho,1 Patrícia Faria Camargo,1 Renata F Arbex,1 Guilherme Casale,3 Cláudio R Oliveira,3 Renata G Mendes,1 Ross Arena,4 Audrey Borghi-Silva1
1Cardiopulmonary Physical Therapy Laboratory, Federal University of Sao Carlos, Sao Carlos, Brazil; 2Postgraduate Program in Rehabilitation Sciences, Federal University of Alfenas, Minas Gerais, Brazil; 3Department of Medicine, Federal University of Sao Carlos, Sao Carlos, Brazil; 4Department of Physical Therapy, College of Applied Health Sciences, University of Illinois at Chicago, Chicago, IL, USA
Correspondence: Audrey Borghi-Silva
Cardiopulmonary Physiotherapy Laboratory, Federal University of São Carlos, UFSCar, São Carlos, São Paulo, Brazil
Tel +551633066704
Fax +551633612081
Email [email protected]
Aim: Our aim was to assess: 1) the impact of the eccentric left ventricular hypertrophy (ELVH) on exercise performance in patients diagnosed with chronic heart failure (CHF) alone and in patients with co-existing CHF and chronic obstructive pulmonary disease (COPD) and 2) the relationship between left and right cardiac function measurements obtained by doppler echocardiography, clinical characteristics and primary measures of cardiorespiratory fitness.
Methods: The current study included 46 patients (CHF:23 and CHF+COPD:23) that performed advanced pulmonary function tests, echocardiography and symptom-limited, incremental cardiopulmonary exercise testing (CPET) on a cycle ergometer.
Results: Patients with CHF+COPD demonstrated a lower work rate, peak oxygen uptake (VO2), oxygen pulse, rate pressure product (RPP), circulatory power (CP) and ventilatory power (VP) compared to those only diagnosed with CHF. In addition, significant correlations were observed between VP and relative wall thickness (r: 0.45 p: 0.03),VE/VCO2 intercept and Mitral E/e’ ratio (r: 0.70 p: 0.003) in the CHF group. Significant correlations were found between indexed left ventricle mass and RPP (r: − 0.47; p: 0.02) and relative VO2 and right ventricle diameter (r: − 0.62; p: 0.001) in the CHF+COPD group.
Conclusion: Compared to a diagnosis of CHF alone, a combined diagnosis of CHF+COPD induced further impairments in cardiorespiratory fitness. Moreover, echocardiographic measures of cardiac function are related to cardiopulmonary exercise performance and therefore appear to be an important therapeutic target when attempting to improve exercise performance and functional capacity.
Keywords: heart failure, chronic obstructive pulmonary disease, eccentric hypertrophy, ventricular dysfunction, cardiopulmonary exercise testing, echocardiography
Introduction
Chronic heart failure (CHF) is defined as an inability of the heart to maintain systemic perfusion at a rate compatible with the needs of a body without the need for high filling pressures and is the result of common triggers of disease, including hypertension, myocardial infarction or ischemia associated with coronary artery disease, dilated cardiomyopathies, diabetic cardiomyopathy, and others.1–4 The initial phase of this process is marked by adaptative mechanisms that lead to left ventricle (LV) hypertrophy.5–7 However, maintaining this stimulus leads to the development of a unique pattern of geometry, which is characterized as an eccentric left ventricular remodeling resulting from volume overload.8 LV hypertrophy is characterized by an increase of chamber diameter, but a normal relationship between wall thickness and LV radius. Eccentric left ventricle hypertrophy (ELVH) is associated with increased death of cardiomyocytes and fibrotic remodeling that culminates in reduced diastolic and systolic cardiac function as well as increased risk for a higher cardiovascular morbidity and mortality.9
Chronic obstructive pulmonary disease (COPD) is a chronic condition characterized by progressive airflow limitation that is not fully reversible, airways inflammation, and a host of systemic effects that contribute to a poor prognosis.10 The prevalence of COPD GOLD stages II–IV is approximately 5–10% of the adult population and epidemiologic studies show that it is currently the third leading cause of death in the world, producing a significant economic burden, with a high hospitalization rate, absence from work, and disability.11,12
Both COPD and CHF are highly prevalent worldwide.13,14 In addition, CHF+COPD frequently coexists in combination, characterized as an overlap syndrome that increases both morbidity and mortality.15 Both conditions share common etiologies (eg, chronic tobacco use, systemic inflammation, etc.), have significant systemic effects (eg, dyspnea, fatigue, exercise intolerance and impaired functional capacity) and a chronic progressive evolution.11,16 The prevalence of CHF+COPD coexistence is estimated to be ≈25% among adults smoking is considered the common risk factor for both diseases, and both increased systemic inflammation and hyperoxidative stress are common.16,17
Interestingly, differences in cardiac function and geometry patterns could influence cardiorespiratory responses to exercise, in this context, cardiopulmonary exercise testing (CPET) allows for the most refined assessment of cardiorespiratory fitness.18,19 In addition, CPET allows for the comprehensive assessment of physiological responses to physical exertion and identify the impact of pathophysiologic processes that are not always readily apparent at rest.20 In addition to quantifying exercise capacity [ie, peak oxygen uptake (VO2)], the minute ventilation/carbon dioxide production (VE/VCO2) slope and ventilatory power (VP = systolic blood pressure/VE/VCO2 slope) have emerged as important physiologic markers of cardiovascular events in both CHF and COPD.21 However, resting variables obtained by doppler echocardiography could help to understand the relationship between the structure and cardiac function at rest and their relationship to primary markers of cardiorespiratory fitness obtained through CPET.22
In the present study, we hypothesized that CHF+COPD would further deteriorate cardiorespiratory fitness compared to patients diagnosed with CHF in isolation. We additionally hypothesized that there are relationships between measures of cardiac function obtained by doppler echocardiography, clinical characteristics and primary CPET measures of cardiorespiratory fitness.
Methods
Study Design and Subjects
This cross-sectional study followed recommendations of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.23 Two-hundred and twenty-two patients were screened from 3 cardiology and pneumology outpatient clinics at our University, from 01 June 2017 to 30 August 2019. All patients who attended during this period with the diagnosis of CHF with reduced or borderline ejection fraction (EF) and/or COPD were contacted by phone and was asked questions regarding diagnosis, clinical conditions, disease stability, drug optimization, and functional mobility. For all patients, eligibility criteria were: 1) age range of 40–85 years; 2) clinically stable for at least 3 months (no worsening of symptoms, exacerbation or decompensation of diseases; 3) no change in dose or change in medication for at least 3 months; 4) no hospitalizations for any cause for at least 3 months; and 5) absence of any condition that may affect exercise performance (ie, anemia, neuromuscular disorders, or malignancies). Exclusion criteria included: 1) long-term O2 therapy; 2) musculoskeletal disease that would impact exercise performance (eg, osteoarthritis, osteonecrosis, trauma, etc.); and 3) peripheral arterial disease associated with claudication. Moreover, CHF or COPD exacerbation or hospitalization during the study was a criterion for study drop-out. All patients who met the eligibility criteria were invited for an initial assessment and tests to confirm the diagnosis of one (CHF) or both (CHF+COPD) diseases being assessed in the current study.
Disease treatment was optimized before study entry and patients underwent CPET only after an agreement had been reached between pneumologists and cardiologists regarding disease stability. As shown in Figure 1, 46 patients with a confirmed reduced or borderline ejection fraction of left ventricle by echocardiography in combination with symptoms consistent with heart failure were included. Twenty-three of these patients also had a coexisting diagnosis of COPD. The Study followed the resolution no. 466 of the National Health Council (current guideline in Brazil) and The Declaration of Helsinki and was approved by the Ethics and Research Committee of the Federal University of São Carlos, São Paulo, Brazil (CAAE nº 91088318.7.1001.5504). All participants were informed about the objectives, experimental procedures and potential risks involved in this study and gave written informed consent statement prior to participation.
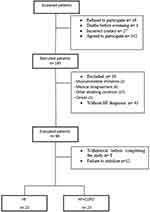 |
Figure 1 Study flow chart (screened patients n=222). |
Cardiac and Lung Function Assessments
All patients underwent a transthoracic two-dimensional and Doppler echocardiographic examination at baseline (HD11 XE, Philips, Amsterdam, Netherlands) to confirm the CHF diagnosis, stratify the degree of systolic dysfunction and obtain the necessary measures for the left and right cardiac function and calculation of eccentric hypertrophy. For inclusion in the study, the left ventricular ejection fraction had to be ≤50%.24 Eccentric hypertrophy of the left ventricle was calculated using the values of relative wall thickness (RWT) at end-diastole and LV mass indexed for body surface area. A cut-off value adopted to classify an eccentric hypertrophy geometric pattern was RWT ≤ 42 and LV mass greater than or equal to 115 g/m2 for men and greater than or equal to 95 g/m2 for women.25,26 Advanced pulmonary function assessment (Masterscreen Body, Mijnhardt/Jäger, Würzburg, German) was performed to obtain dynamic and static lung volumes and capacities pre- and post-bronchodilator therapy. The GOLD criteria [post-bronchodilator forced expiratory volume in the 1 second (VEF1)/forced vital capacity (FVC) ratio <0.70] was used to confirm a COPD diagnosis.27
Cardiopulmonary Exercise Testing
All patients underwent a symptom-limited incremental CPET on an electronically braked cycle ergometer (Corival Recumbent, Lode, Groningen, Netherlands) using the Oxycon Mobile System (Mijnhardt/Jäger, Würzburg, German). The exercise protocol started with an initial 5 min of rest, followed by unloaded cycling for 1 min with a subsequent increment of 5–10 watts each minute (ramp protocol). Patients were instructed to pedal at the speed of 60 rotation per minute and the work rate (WR) increment was individually selected according to reported exercise tolerance. Breath-by-breath VO2 (L/min), VCO2 (L/min), and VE (L/min) were recorded. The CPET variables were reported as 20-second averaged data. During the exercise test, heart rate (HR) twelve-lead electrocardiogram (ECG), blood pressure, and arterial oxygen saturation were monitored. Arterial oxygen saturation was measured non-invasively by pulse oximetry (SpO2, %). Breathlessness and leg effort scores were rated according to the 10-point Borg category ratio.28 Established exercise test termination criteria were followed and included Angina (score above 2 on a scale of 0–10), life-threatening arrhythmias, electrocardiographic evidence of ischemia, a drop in systolic blood pressure, or arterial oxygen saturation ≤84% were considered to interrupt the test.29 Key CPET variables were calculated for all patients as previously described. The VE/VCO2 slope was obtained through linear regression analysis.30 Additionally, the linear relationship between oxygen uptake and the log transformation of VE (OUES) was calculated using the following equation: VO2= a log VE+ b, with the constant “a” referring to the rate of increase of VO2.31 Circulatory power (CP) was obtained through the product of peak VO2 and peak systolic blood pressure and Ventilatory Power (VP) was calculated by dividing peak systolic blood pressure by the VE/VCO2 slope.32,33
Statistical Analysis
A sample calculation was performed (GPower 3.1- Universidade de Kiel, Kiel, Alemanha) using the peak VO2 obtained in pilot studies previously performed in our laboratory with individuals who were diagnosed with CHF. From this sample calculation, 52 subjects, 26 for each group, were needed to reach sufficient statistical power (1-β err prob) of 0.80. The Shapiro–Wilk test was used to verify the data distribution. Descriptive variables were expressed as mean ± standard deviation (when normal distribution) or median and interquartile (when non-normal distribution). Categorical variables are expressed as frequencies and percentages and compared using the chi-square test. The unpaired t-test was used to compare anthropometric measures, cardiac and pulmonary function measures and CPET measures. Relationships between measures of cardiac function and other measures collected in the current study were assessed by the Pearson Correlation coefficient (variables with non-normal distribution were log-transformed to reduce non-uniformity of error) for CHF and CHF+COPD groups separately. A p-value <0.05 was considered as statistically significant for all tests. All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) 20.0 (IBM, Armonk, New York).
Results
Clinical and Resting Characteristics
The characteristics of the patients are reported in Table 1. Groups were homogeneous in relation to clinical characteristics, anthropometric data and the presence of comorbidities. Most patients in both groups were male while patients in the CHF+COPD group were older. In relation to the left cardiac function, differences were found only in the Mitral e’. There were no between-group differences with respect to systolic dysfunction assessed by EF, LV mass and relative LV wall thickness. In relation to the right cardiac function, differences were found between right ventricle diameter (RVD). Interestingly, the CHF group obtained higher values; however, both groups were within the established normal value range for this variable (<42mm).34 The Tricuspid E/A ratio and Tricuspid E/e’ ratio was different between groups. The Tricuspid E/A in both groups suggests the presence of impaired RV relaxation. As expected, CHF+COPD patients showed evidence of an obstructive ventilatory disorder. The frequency of patients in stage 2 according to the GOLD guidelines was greater in CHF+COPD group. CHF patients showed preserved spirometry but similar static lung volumes when compared to the CHF+COPD group; differences between-group were found only in Total lung capacity (TLC) and lung diffusing factor for carbon monoxide (DLCO). In relation to medications, patients in the CHF group used diuretics with greater frequency compared to the CHF+COPD group.
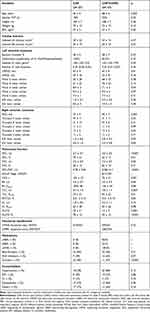 |
Table 1 Anthropometric and Clinical Characteristics of Studied Subjects |
Metabolic, Cardiovascular and Ventilatory Responses to Exercise
Table 2 lists the CPET responses in both groups and the comparison between them. WR and absolute peak VO2 were significantly lower in CHF+COPD when compared to CHF (p<0.05). As expected from pharmacological β-blocking use, both groups similarly presented with lower peak HR. Systolic and diastolic blood pressure was higher in the CHF group when compared to the CHF+COPD group (p<0.05). The CHF+COPD group demonstrated a significantly lower peak O2 pulse and rate-pressure product (RPP) compared to the CHF+COPD. In addition, a significant difference in CP was found, again being significantly lower in the CHF+COPD group (p<0.05).
 |
Table 2 Comparison Between Group Responses to Incremental CPET |
Similar ventilatory responses were found between groups; however, minute-ventilation was higher in the CHF group. The VE/MVV ratio was 0.49 ± 0.16 and 0.53 ± 0.17 in the CHF and CHF+COPD, respectively, and both groups presented with a higher VE/VCO2 slope. A low OUES was also observed in both groups. Ventilatory power was significantly higher in the CHF+COPD group when compared with CHF group (p<0.05). No significant difference was found in peak SpO2 between groups; however, dyspnea and leg fatigue were higher in CHF+COPD group (p<0.05). In both groups, the main reason for test termination was dyspnea.
Relationships Between Measures of Left Function, Right Function, ELVH Pattern, Clinical Characteristics and Exercise Responses
We found associations between key components of left and right cardiac function and CPET variables. For the CHF group (Figure 2), significant correlations were observed between VP and RWT (r: 0.45 p: 0.03) and VE/VCO2 intercept and Mitral E/e’ ratio (r: 0.70 p: 0.003). In the CHF+COPD group (Figure 3), significant correlations were found between Indexed LV mass and RPP (r: −0.47; p: 0.02) and relative VO2 and RVD (r: −0.62; p: 0.001).
Discussion
This is the first study that investigated the exercise responses of CHF patients with confirmed eccentric left ventricular hypertrophy and, in a group, the coexistence of COPD. The main original findings of the present investigation is that CHF+COPD led to a significantly poorer CPET response, including a lower work rate, peak VO2, O2 pulse, RPP, CP, and VP. In addition, we found correlations between VP and RWT and VE/VCO2 intercept and Mitral E/e’ ratio, in the CHF group. In contrast, we found the CHF+COPD group presented with correlations between Indexed LV mass and RPP, relative VO2 and RVD. These distinct correlations between groups indicate cardiac function and pattern of remodelling differently impacts the exercise response when CHF is present on isolation in comparison to the coexistence of CHF+COPD patients.
In relation to clinical and anthropometric variables, we can see that the groups were homogeneous; however, the CHF+COPD group was older than the CHF group and comprised of male subjects exclusively. Factors that can influence the VO2 and maximal WR are age, sex, weight and height. In the Brazilian population, age had an important role in the decline of VO2 peak and WR. Moreover, a greater reduction in males with aging may have influenced the differences found between groups with respect to VO2 peak, maximal predicted VO2, WR and predicted WR.35 However, compared to the clinical condition, association of other comorbidities and level of physical activity, we believe age and sex were not the main influencing factors for the significant limitations observed in exercise capacity (see study limitations section)
According to Weber rating, in both groups peak VO2 presented with a compatible worse prognosis.36 Eccentric hypertrophy and higher left ventricular end diastolic diameter in both groups was indicative of advanced CHF.37 Given the influence of cardiac output on aerobic capacity, eccentric hypertrophy remodeling and associated reduced LV function certainly contributes to an impaired CPET response, as observed in the present study.38 In addition, the heart’s incapacity to deliver O2 may also arise from secondary complications, such as vascular or pulmonary complications. Another important factor that may have contributed to worsening peak VO2 values in patients in the current study is potential hyperinflation that raises intrathoracic pressure, which is associated with reduced left ventricular end-diastolic volume, stroke volume and cardiac output.39 The CHF+COPD group obtained worse values of absolute peak VO2, which is also potentially associated with a reduced pulmonary diffusing capacity, as indicated by the DLCO response. COPD leads to pulmonary alveolar and vascular destruction, promoting to increased inefficiency between ventilation - perfusion, thus decreasing lung diffusion capacity.40
In one study with 644 patients followed for 48 months, Myers et al determined cut-off points for risk stratification through peak VO2, showing that patients that reach a cut-off between 10 and 18 mL.kg.min−1 have a worse diagnosis.41 In our study, both groups presented average values of peak VO2 below 18 mL.kg−1.min−1, reflecting a worse prognosis. Hemodynamic abnormalities arise in patients with CHF+COPD, due to increased intrathoracic pressure resulting from hyperinflation leading to a reduced gradient for venous return. Moreover, an increase in right ventricular afterload may occur due to pulmonary vasoconstriction caused by hypoxemia and posteriorly hypercapnia.42,43 Patients in the group CHF+COPD presented with worse values when compared to the CHF group in the variables that reflect hemodynamic function. Peak RPP and O2 pulse were significantly lower in individuals with overlap, showing poor cardiac function. Both variables are heavily dependent on VO2 and stroke volume, while arteriovenous O2 difference does not change significantly. The association of the two conditions (CHF leading to direct changes in the myocardial structure and the change in lung volumes resulting from COPD directly affecting the ventricular diastolic function) leads to a greater impairment of systolic volume and function, thus changing the volume of blood ejected and consequently the supply of systemic O2.39,44
For CP, Goulart et al recently suggested cut-off values for predicting adverse events in patients with CHF+COPD, being that individuals with values lower than 2383 mmHg.mL/kg−1 min−1, are more likely to be hospitalized.45 Patients with CHF+COPD, in the current study, presented with average values below this threshold; 65% of the CHF+COPD patients were below the cut-off values whereas, in the CHF group, only 40% of the patients were below the threshold. This reflects worse central and peripheral cardiac function, representing the relationship between the blood flow generated by the heart and peripheral perfusion pressure.46 In patients with CHF+COPD, the presence of low cardiac output due to negative cardiopulmonary interactions associated with impaired central hemodynamics and abnormalities in peripheral vascular control and sympathetic overexcitation may also contribute to a decreased CP.47
Patients with overlap syndrome present with impaired exercise capacity due to an increased ventilatory response to metabolic demand.48 The physiological determinants are already widely studied - the dynamic hyperinflation, inspiratory constraints and lower tidal volume contribute to ventilatory inefficiency.49,50 Interestingly, with respect to ventilatory inefficiency, both groups showed similar responses, except for VP, which was reduced in the CHF-COPD group. Similar responses between groups are possible because patients with advanced CHF have lower changes in lung volume compared to variations in transpulmonary pressure and other factors such as: 1) accumulation of extravascular water in the lung; 2) pulmonary congestion; 3) septal thickening; 4) pulmonary compression due to heart enlargement; and; 5) in some patients, weakness of the inspiratory musculature leading to increased dead-space, low tidal volume, increased ventilation, hypocapnia and inefficient gas exchange.51–53 Another important factor that may have contributed to similar ventilatory responses between groups is the fact that the CHF+COPD group was mostly comprised of individuals in GOLD I–II stages, indicative of less severe ventilatory limitations (as observed in the variables VE/MMV, VE/VCO2 slope, VE/VCO2 intercept and OUES). As such, exercise limitations may have been more reliant on cardiovascular and skeletal muscle dysfunction. The VP in our study was less in the CHF+COPD group as expected in this population. This variable reflects peak cardiac output, alveolar perfusion, peripheral perfusion, and skeletal muscle chemo- and afferent-reflexes, which in the presence of COPD in moderate-to-very severe stages is more impaired. There are currently no established threshold values for patients with CHF+COPD; however, CHF patients with values below 3.5 mmHg indicate a worse prognosis. In our study, only 4% of our sample of patients with CHF were below this value, whereas in the CHF+COPD group, 30% of the individuals had a VP less than 3.5.33
Systemic cardiocirculatory maladjustments were apparent in both groups, with CHF+COPD patients demonstrating a more prominent phenotype. Muscle weakness is the most common systemic effect, in which is a result of the chronic processes in both conditions where there is an imbalance between the synthesis and degradation of muscle protein leading to sarcopenia and subsequent cachexia. These peripheral impairments produce loss of muscle strength and endurance that directly impacts in respiratory function, exercise intolerance, health status and mortality.54–56 Our patients with CHF+COPD presented with a worse perception of symptoms, being that dyspnea and fatigue were the main causes for test termination.
Eccentric left ventricular hypertrophy is an adaptation that involves a complex process of modifications in cardiac structure, signaling, transcriptional, electrophysiological, metabolic, and functional events within the growing cardiac cell.57 The hypertrophic growth of the myocardium is a compensatory mechanism that helps maintain cardiac contractility; however, some authors believe that this adaptation leads to an increase in mortality due to cardiovascular causes.58 We found that left and right cardiac function and eccentric left ventricular hypertrophy appear influence exercise responses differently, depending on whether or not a patient with CHF has coexisting COPD. For the CHF cohort, our results indicate that global cardiac function has an effect on exercise responses. However, in the CHF+COPD group, the exercises responses are influenced by cardiac function differently.
In CHF group, we observed that RWT positively influenced VP. A lower RWT associated with an increase in LV mass suggests greater CHF severity. In these patients, reduced RWT associated with increased LV mass results in loss of myocardial contractility, so the heart is unable to maintain its normal pumping function, thereby decreasing cardiac output.37 During high-intensity exercise, reduced cardiac function can lead to pulmonary congestion, which, associated with higher pulmonary arterial pressure and pulmonary vascular resistance, increases ventilation-perfusion mismatch, producing ventilatory inefficiency and contributing to low values of PV.59 We found a strong correlation between the VE/VCO2 intercept and Mitral E/e’ ratio in the CHF group. These variables, respectively, reflect the VE versus VCO2 relationship (representing one index of ventilatory efficiency during exercise) and LV filling pressures. Individuals with increased filling pressures may present with changes in LV relaxation and diastolic dysfunction, which can lead to changes in pulmonary capillary wedge pressure compromising the ventilation at the beginning of the exercise.50,60
For CHF+COPD group, we found one important correlation between RVD and relative VO2. The increased RV diameter suggests dilation, which is the first indicator of increased pulmonary resistance. An increased pulmonary vascular resistance leads to pulmonary hypertension and consequent impairment of systemic oxygen supply.61 Burgess et al conducted a study with 87 COPD patients, with the aim of verifying whether echocardiographic markers of RV function could determine prognosis. Findings indicated that RV dimensions, RV area, and Doppler indices were all strongly associated with survival.62,63 For individuals with CHF+COPD, a higher LV mass may negatively influence RPP because when maladaptive eccentric left ventricular hypertrophy occurs, myocardial oxygen uptake increases, leading to the exhaustion of coronary blood flow reserve. In this context, changes in coronary microcirculation may lead to a loss of myocytes and myocardial fibrosis in addition to myocardial perfusion impairment.64–66
Study Limitations
The main limitation in our study is the sample size in both groups; however, we managed to reach 88% of the a priori sample calculation. However, it is important to consider that in the present study, all patients presented with similar conditions of CHF, including reduced or borderline EF and left ventricular shape and cardiac geometry (ie, eccentric hypertrophy), which contrasts other published studies that did not consider these measures to assess patients with CHF+COPD. In addition, we consider it important to note that our findings are limited to men with CHF+COPD. However, in order to mitigate this bias, knowing that some variables could be influenced by sex, we performed a Spearman Correlation coefficient analysis and found no correlation with the variables analyzed (WR, absolute VO2, RPP, O2 pulse, CP, VP). Another important factor that is known to influence variables is age that was different between groups; however, we performed a linear regression analysis to verify the influence of age on CPET variables that differed. We verified that age had a weak but significant influence on absolute VO2 (R2:0.16 p: 0.006) and O2 pulse (R2:0.15 p: 0.006).
In conclusion, the coexistence of CHF+COPD induced marked impairment of exercise capacity as well as ventilatory and circulatory inefficiency, all negatively impacting functional capacity, exertional symptoms and quality of life. Moreover, given our findings related to cardiac function and geometry and exercise responses, left and right cardiac function, LV mass and RWT measured at rest may provide important information to guide treatment decisions for these patients with the goal of improving exercise performance and functional capacity.
Acknowledgment
Acknowledgement of grant support: We would like to thank the Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP) and the Coordenação de Apoio a Pessoal do Ensino Superior (CAPES) for providing financial support, allowing this work to be carried out. We extend our gratitude to the University Hospital of the Federal University of São Carlos for supporting patient recruitment for this study. Borghi-Silva A is an Established Investigator (level IB) of the Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil. We are also grateful to all physical therapy team for technical support. More importantly, however, we are indebted to the patients for their effort and enthusiastic cooperation throughout the study.
Authorship Contribution Statement
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This study was funded by FAPESP process numbers: 2015/26501-1 and 2018/03314-0; PROEX-CAPES Finance Code 001.
Disclosure
Polliana Dos Santos reports grants from Fundação de Amparo a Pesquisa do Estado de São Paulo, outside the submitted work. The authors report no other potential conflicts of interest for this work and no relationships that could be construed as a conflict of interest.
References
1. Verbrugge FH, Guazzi M, Testani JM, Borlaug BA. Altered hemodynamics and end-organ damage in heart failure: impact on the lung and kidney. Circulation. 2020;142(10):998–1012.
2. Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007;93:1137–1146.
3. Levy D, Kenchaiah S, Larson MG, et al. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397–1402.
4. Zannad F, Briancon S, Juilliere Y, et al. Incidence, clinical and etiologic features, and outcomes of advanced chronic heart failure: the EPICAL study. Epidemiologie de l’Insuffisance Cardiaque Avancee en Lorraine. J Am Coll Cardiol. 1999;33:734–742.
5. Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nature Rev Mol Cell Biol. 2006;7:589–600.
6. Lips DJ, deWindt LJ, Van Kraaij DJ, Doevendans PA. Molecular determinants of myocardial hypertrophy and failure: alternative pathways for beneficial and maladaptive hypertrophy. Eur Heart J. 2003;24:883–896.
7. Berenji K, Drazner MH, Rothermel BA, Hill JA. Does load-induced ventricular hypertrophy progress to systolic heart failure? Am. J Physiol Heart Circ Physiol. 2005;289:H8–H16.
8. Grant C, Greene DG, Bunnell IL. Left ventricular enlargement and hypertrophy. A clinical angiocardiographic study. Am J Med. 1965;39:895–904.
9. Lyon RC, Zanella F, Omens JH, Sheikh F. Mechanotransduction in cardiac hypertrophy and failure. Circ Res. 2015;116:1462–1476.
10. Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med. 2000;343:269–280.
11. Hawkins NM, Virani S, Ceconi C. Heart failure and chronic obstructive pulmonary disease: the challenges facing physicians and health services. Eur Heart J. 2013;34:2795–2803.
12. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128.
13. Dharmarajan K, Strait KM, Tinetti ME, et al. Treatment for multiple acute cardiopulmonary conditions in older adults hospitalized with pneumonia, chronic obstructive pulmonary disease, or heart failure. J Am Geriatr Soc. 2016;64(8):1574–1582.
14. Carter P, Lagan J, Fortune C, et al. Association of cardiovascular disease with respiratory disease. J Am Coll Cardiol. 2019;73(17):2166–2177.
15. Rutten FH, Cramer M-JM, Lammers J-WJ, Grobbee DE, Hoes AW. Heart failure and chronic obstructive pulmonary disease: an ignored combination? Eur J Heart Fail. 2006;8:706–711.
16. Sin DD, Hogg J. Chronic obstructive pulmonary disease as a risk factor for cardiovascular morbidity and mortality. Proc Am Thorac Soc. 2005;2:8–11.
17. Van Mourik Y, Bertens LCM, Cramer MJM, et al. Unrecognized heart failure and chronic obstructive pulmonary disease (COPD) in frail elderly detected through a near-home targeted screening strategy. J Am Board Fam Med. 2014;27(6):811–821.
18. Mayer B, Holmer SR, Hengstenberg C, Lieb W, Pfeifer M, Schunkert H. Functional improvement in heart failure patients treated with beta-blockers is associated with a decline of cytokine levels. Int J Cardiol. 2005;103(2):182–186.
19. Guazzi M, Myers J, Vicenzi M, et al. Cardiopulmonary exercise testing characteristics in heart failure patients with and without concomitant chronic obstructive pulmonary disease. Am Heart J. 2010;160(5):900–905.
20. Ingle L. Theoretical rationale and practical recommendations for cardiopulmonary exercise testing in patients with chronic heart failure. Heart Fail Rev. 2007;12:12–22.
21. Borghi-Silva A, Labate V, Arena R, et al. Exercise ventilatory power in heart failure patients: functional phenotypes definition by combining cardiopulmonary exercise testing with stress echocardiography. Int J Cardiol. 2014;176(3):1348–1349.
22. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108.
23. Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–1499.
24. Lang RM, Bierig M, Devereux RB, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiography. 2015;28(1):1–39.
25. Lurbe E, Agabiti-Rosei E, Cruickshank JK, et al. European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens. 2016;34(10):1887–1920.
26. Konstam MA, Kramer DG, Patel AR, Maron MS, Udelson JE. Left ventricular remodeling in heart failure: current concepts in clinical significance and assessment. JACC Cardiovasc Imaging. 2011;4(1):98–108.
27. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Chronic Respir Dis. 2020;1:1–141.
28. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):371–388.
29. Balady GJ, Arena R, Sietsema K. on behalf of the American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; and Interdisciplinary Council on Quality of Care and Outcomes Research. Clinician’s Guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122:191–225.
30. Arena R, Myers J, Aslam S, Varughese EB, Peberdy MA. Technical considerations related to the minute ventilation/carbon dioxide output slope in patients with heart failure. Chest. 2003;124(2):720–727.
31. Baba R, Nagashima M, Goto M, et al. Oxygen uptake efficiency slope: a new index of cardiorespiratory functional reserve derived from the relation between oxygen uptake and minute ventilation during incremental exercise. J Am Col Cardiol. 1996;28:1567–1612.
32. Cohen-Solal A, Tabet JY, Logeart D, Bourgoin P, Tokmakova M, Dahan M. A noninvasively determined surrogate of cardiac power (‘circulatory power’) at peak exercise is a powerful prognostic factor in chronic heart failure. Eur Heart J. 2002;23(10):806–814.
33. Forman DE, Guazzi M, Myers J, et al. A novel index that enhances prognostic assessment of patients with heart failure. Circ Heart Fail. 2012;5(5):621–626.
34. Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the Echocardiographic Assessment of the Right Heart in Adults: a report from the American Society of Echocardiography Endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713.
35. Neder JA, Nery LE, Castelo A, et al. Prediction of metabolic and cardiopulmonary responses to maximum cycle ergometry: a randomized study. Eur Respir J. 1999;14:1304–1313.
36. Weber KT, Janicki JS. Cardiopulmonary exercise testing for evaluation of chronic cardiac failure. Am J Cardiol. 1985;55:22A–31A.
37. Hein S, Arnon E, Kostin S, et al. Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: structural deterioration and compensatory mechanisms. Circulation. 2003;107(7):984–991.
38. Jørgensen ME, Andersson C, Vasan RS, Køber L, Abdulla J. Characteristics and prognosis of heart failure with improved compared with persistently reduced ejection fraction: a systematic review and meta-analyses. Eur J Prev Cardiol. 2018;25:366–376.
39. Jörgensen K, Müller MF, Nel J, Upton RN, Houltz E, Ricksten SE. Reduced intrathoracic blood volume and left and right ventricular dimensions in patients with severe emphysema. Chest. 2007;131:1050–1057.
40. Burger CD. Pulmonary hypertension in COPD: a review and consideration of the role of arterial vasodilators. COPD. 2009;6:137–144.
41. Myers J, Gullestad L, Vagelos R, et al. Cardiopulmonary exercise testing and prognosis in severe heart failure: 14 mL/kg/min revisited. Am Hear J. 2000;139:78–84.
42. Boerrigter B, Trip P, Bogaard HJ, et al. Right atrial pressure affects the interaction between lung mechanics and right ventricular function in spontaneously breathing COPD patients. PLoS One. 2012;7(1):e30208.
43. Ranieri VM, Dambrosio M, Brienza N. Intrinsic PEEP and cardiopulmonary interaction in patients with COPD and acute ventilatory failure. Eur Respir J. 1996;9(6):1283–1292.
44. Gobel FL, Nordstron LA, Nelson RR, Jorgensen CR, Wang Y. The rate-pressure product as an index of myocardial oxygen consumption during exercise in patients with angina pectoris. Circulation. 1978;57:549–556.
45. Goulart CL, Dos Santos PB, Caruso FR, et al. The value of cardiopulmonary exercise testing in determining severity in patients with both systolic heart failure and COPD. Sci Rep. 2020;10:4309.
46. Giardini A, Specchia S, Berton E, et al. Strong and independent prognostic value of peak circulatory power in adults with congenital heart disease. Am Heart J. 2007;154(3):441–447.
47. Oliveira MF, Arbex FF, Alencar MC, et al. Heart failure impairs muscle blood flow and endurance exercise tolerance in COPD. COPD. 2016;13(4):407–415.
48. Apostolo A, Laveneziana P, Palange P, et al. Impact of chronic obstructive pulmonary disease on exercise ventilatory efficiency in heart failure. Int J Cardiol. 2015;189:134–140.
49. Neder JA, Berton DC, Arbex FF, et al. Physiological and clinical relevance of exercise ventilatory efficiency in COPD. Eur Respir J. 2017;49(3):1602036.
50. Clark AL, Chua TP, Coats AJ. Anatomical dead space, ventilatory pattern, and exercise capacity in chronic heart failure. Br Heart J. 1995;74:377–380.
51. Wensel R, Georgiadou P, Francis DP, et al. Differential contribution of dead space ventilation and low arterial pCO2 to exercise hyperpnea in patients with chronic heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 2004;93:318–323.
52. Chase SC, Taylor BJ, Cross TJ, Coffman KE, Olson LJ, Johnson BD. Influence of thoracic fluid compartments on pulmonary congestion in chronic heart failure. J Card Fail. 2017;23(9):690–696.
53. Hosenpud JD, Stibolt TA, Atwal K, Shelley D. Abnormal pulmonary function specifically related to congestive heart failure: comparison of patients before and after cardiac transplantation. Am J Med. 1990;88(5):493–496.
54. Gosker HR, Kubat B, Schaart G, van der Vusse GJ, Wouters EF, Schols AM. Myopathological features in skeletal muscle of patients with chronic obstructive pulmonary disease. Eur Respir J. 2003;22:280–285.
55. Montes de Oca M, Torres SH, Gonzalez Y, et al. Peripheral muscle composition and health status in patients with COPD. Respir Med. 2006;100:1800–1806.
56. Buller NP, Jones D, Poole-Wilson PA. Direct measurements of skeletal muscle fatigue in patients with chronic heart failure. Br Heart J. 1991;65:20–24.
57. Burchfield JS, Xie M, Hill JA. Pathological ventricular remodeling: mechanisms: part 1 of 2. Circulation. 2013;128:388–400.
58. Frey N, Katus HA, Olson EN, Hill JA. Hypertrophy of the heart: a new therapeutic target? Circulation. 2004;109:1580–1589.
59. Witte KK, Clark AL. Why does chronic heart failure cause breathlessness and fatigue? Prog Cardiovasc Dis. 2007;49:366–384.
60. Rohde LE, Palombini DV, Polanczyk CA, Goldraich LA, Clausell N. hemodynamically oriented echocardiography-based strategy in the treatment of congestive heart failure. J Card Fail. 2007;13:618–625.
61. Davis JA, Sorrentino KM, Soriano AC, Pham PH, Dorado S. Is ventilatory efficiency dependent on the speed of the exercise test protocol in healthy men and women? Clin Physiol Funct Imaging. 2006;26(2):67–71.
62. McConnell MV, Solomon SD, Rayan ME, Come PC, Goldhaber SZ, Lee RT. Regional right ventricular dysfunction detected by echocardiography in acute pulmonary embolism. Am J Cardiol. 1996;78:469–473.
63. Ferlinz J. Right ventricular function in adult cardiovascular disease. Prog Cardiovasc Dis. 1982;25:225–267.
64. Burgess MI, Mogulkoc N, Bright-Thomas RJ, Bishop P, Egan JJ, Ray SG. Comparison of echocardiographic markers of right ventricular function in determining prognosis in chronic pulmonary disease. J Am Soc Echocardiogr. 2002;15:633–639.
65. Dini FL, Ghiadoni L, Conti U, Buralli S, Taddei S, De Tomassi SM. Coronary flow reserve in idiopathic dilated cardiomyopathy: relation with left ventricular wall stress, natriuretic peptides, and endothelial dysfunction. J Am Soc Echocardiogr. 2009;22(4):354–360.
66. Dini FL, Galeotti GG, Terlizzese G, Fabiani L, Pugliese NR, Rovai I. Left ventricular mass and thickness: why does it matter? Heart Failure Clin. 2019;15:159–166.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


