Back to Journals » Journal of Pain Research » Volume 13
Eastern Pain Association Annual Meeting 2019 Abstract Session Award Winners
Authors Gharibo C, Nicholas D, Ohara K, Cao L , Saint-Preux F, Mendoza J, Portugal S, Gupta A , Sze-Tu R, Ibim SE, Levy HJ, El-Amin III SF
Received 15 January 2020
Accepted for publication 15 February 2020
Published 20 April 2020 Volume 2020:13 Pages 755—760
DOI https://doi.org/10.2147/JPR.S246073
Checked for plagiarism Yes
Editor who approved publication: Professor Robert B. Raffa
Christopher Gharibo
NYU Langone Health, New York, NY, USA
Correspondence: Christopher Gharibo NYU Langone Health, 333 East 38th Street, New York, NY 10016, USA Tel +1-212-201-1004 Email [email protected]
Introduction
The 2019 Annual Meeting of the Eastern Pain Association provided a clear foundation for the future of pain medicine as it heralded the year 2020. The best way to optimally manage chronic pain patients of the future is for pain professionals to go back to our past as a multidisciplinary discipline. The multimechanistic, multimodal and multidisciplinary approach lies at the heart of Eastern Pain Association’s mission as a multidisciplinary society that is dedicated to disseminating scientific knowledge as it relates to pain medicine through improved pathophysiological understanding, discovery of new therapeutic targets and interventions to improve patient outcomes.
Moderation and a combination approach are key. We are not allowing the field of pain to be reduced to pro vs anti-opioid or pro vs anti-interventional pain wars. The focus is to gain a deeper understanding of our patients’ pain through improved knowledge, and the abstracts presented at the Eastern Pain Association’s 2019 Annual Meeting are a small step in that direction.
The three award-winning abstracts that are presented here give us a glimpse into what the future holds, from improved understanding of chronic pain mechanisms to further progress in the field of regenerative medicine. Enjoy.
NYU Langone Health, New York, NY, USA
Correspondence: Christopher Gharibo NYU Langone Health, 333 East 38th Street, New York, NY 10016, USA Tel +1-212-201-1004 Email [email protected]
Introduction
The 2019 Annual Meeting of the Eastern Pain Association provided a clear foundation for the future of pain medicine as it heralded the year 2020. The best way to optimally manage chronic pain patients of the future is for pain professionals to go back to our past as a multidisciplinary discipline. The multimechanistic, multimodal and multidisciplinary approach lies at the heart of Eastern Pain Association’s mission as a multidisciplinary society that is dedicated to disseminating scientific knowledge as it relates to pain medicine through improved pathophysiological understanding, discovery of new therapeutic targets and interventions to improve patient outcomes.
Moderation and a combination approach are key. We are not allowing the field of pain to be reduced to pro vs anti-opioid or pro vs anti-interventional pain wars. The focus is to gain a deeper understanding of our patients’ pain through improved knowledge, and the abstracts presented at the Eastern Pain Association’s 2019 Annual Meeting are a small step in that direction.
The three award-winning abstracts that are presented here give us a glimpse into what the future holds, from improved understanding of chronic pain mechanisms to further progress in the field of regenerative medicine. Enjoy.
Spinal Cord Microglial Phenotypic Changes Following Sciatic Nerve Crush in CD137LKO Mice
1University of New England College of Osteopathic Medicine, Biddeford, Maine, USA; 2Department of Endodontics, Nihon University School of Dentistry, Tokyo 101-8310, Japan
Correspondence: University of New England College of Osteopathic Medicine, 11 Hills Beach Road Biddeford, Maine, 04005, USA Tel +1 207 602 2213 Fax +1 207 602 5931 Email [email protected]
Purpose: Neuropathic pain is defined as pain caused by a lesion or disease of the somatosensory system. This debilitating illness affects roughly 7 percent of the population in the United States. Microglia, important immune cells in the central nervous system, are shown to play a critical role in the development of neuropathic pain. Peripheral nerve injury can activate spinal cord microglia to become pro-inflammatory versus anti-inflammatory phenotypes. CD137 ligand is a receptor on microglia that binds to the CD137 receptor on T lymphocytes. We have shown that CD137L knockout (KO) mice display reduced sensory sensitivity and faster functional recovery following sciatic nerve crush (SNC). We hypothesize that CD137L depletion induces this change through promoting microglia to preferentially differentiate into an anti-inflammatory subtype after SNC.
Methods: To test this hypothesis, qRT-PCR and Flow Cytometry were used to measure the expression of markers specific to pro- vs anti-inflammatory microglia at various times following SNC in both wild-type (WT) and CD137L KO mice.
Results: Data from qRT-PCR showed that CD137L KO microglia displayed a more pronounced increase of IRF3 and earlier induction of downstream chemokines CCL3, CCL4 and CCL5 compared to WT microglia. The CD137L KO microglia also showed delayed or lack of upregulation of CD86 and MyD88-downstream chemokines, CCL2 and CXCL10 following SNC compared to WT microglia. Preliminary flow cytometry data with WT mice showed increased total microglial and CD86+ microglial numbers within lumbar spinal cord post-SNC.
Conclusion: Our data so far appear to support the prediction that microglia from CD137L KO mice exhibit an increased anti-inflammatory profile compared to WT mice following SNC injury. CD137L and its downstream pathways could serve as potential drug targets.
Notalgia Paresthetica Successfully Treated with Cervical Epidural Injection and Occipital Nerve Block: A Case Report
1Department of Physical Medicine and Rehabilitation, NYU School of Medicine, Manhattan NY, USA; 2Spine Care, Active Orthopedics & Sports Medicine, LLC, Hackensack, NJ, USA
Correspondence: Fabienne Saint-Preux Department of Physical Medicine and Rehabilitation, NYU School of Medicine, 240 East 38th Street, 15th floor, Manhattan, NY 10016, USA Tel +1 212 263 6110 Fax +1 212 263 6251 Email [email protected]
Background: Notalgia paresthetica (NP) is a benign relapsing and remitting sensory neuropathic syndrome characterized by localized pruritus usually of the unilateral infrascapular region. Multiple treatment modalities focused on symptomatic relief exist, however, all with varying efficacy. The etiology of NP is not fully elucidated; however, it seems to be associated with underlying cervicothoracic spinal disease. Treatment of this associated comorbidity may confer relief and thus increase quality of life. Therefore, we present a treatment approach with epidural steroid injection (ESI) and adjunctive occipital nerve block.
Case Report: This case involves a 46-year-old woman with no significant history. She developed NP, with an atypical distribution, spontaneously without inciting event but was found to have positive radicular symptoms on exam and MRI confirming cervical disc disease. She failed conservative treatment and was eventually referred to pain management for assessment. She was treated with a C7-T1 interlaminar ESI and subsequent occipital nerve blocks which resulted in significant relief of her symptoms.
Conclusion: Treatment of underlying cervical spinal disease may be the key to successful, longer term treatment of NP. This can be achieved with minimally invasive interventions such as ESIs at the appropriate level.
Keywords: cutaneous neuropathy, pruritis, neuropathic itch, hyperpigmented patch, nerve block, steroid injection
Introduction
Notalgia paresthetica (NP) is a common cutaneous dysesthesia involving the dorsal spinal nerves that is largely under-recognized and underdiagnosed. It is characterized by a unilateral (usually) neuropathic pruritus normally occurring in the interscapular and paravertebral region resulting in pain, discomfort, scarring from incessant itching and decreased quality of life. NP is usually localized to the T2–T6 dermatomal distribution and accompanied by localized burning, tenderness, hyperalgesia and dysesthesia resulting in chronic itching and a post-inflammatory hyperpigmented patch that is esthetically distressing.1 The etiology of NP is not fully understood; however, it is widely accepted that the sensory neuropathy is caused by musculoskeletal compression of the cutaneous branches of the dorsal primary rami of thoracic spinal nerves T2–T6.1,2 While NP is associated with symptoms in the T2–T6 dermatomes, it has been associated with cervical spinal disease usually at the C4–C6 level even though the spinal pathology seen on imaging does not correlate with the dermatomal distribution of symptoms.2–6 Current first-line therapies include topical treatments such as lidocaine and capsaicin, conservative management with physical therapy, manual manipulation and/or transcutaneous electrical nerve stimulations (TENS) and medications such as gabapentin with varying and/or temporary success. Surgical and minimally invasive interventions are reserved for refractory cases and are rarely pursued. In this article, we present a case of NP that was successfully treated with a cervical interlaminar epidural steroid injection (ESI) and bilateral occipital nerve block, modalities typically used for cervical spinal pathology and cervical neuralgias, respectively, but not previously described for NP.
Case Report
A 46-year-old female presented to clinic with severe pruritus in her bilateral periscapular and occipital region for 1 year (Figure 1 ). The pruritus started in the neck and progressed cephalad and caudad. She had accompanying neck pain with occasional radiation down the left arm at the time of symptom onset and was presumed to have cervical radiculopathy. Her pain mostly resolved with a Medrol dose pack and a course of physical therapy, but pruritus persisted. On initial visit, physical exam demonstrated mild pain with cervical rotation at end range of motion, negative Spurling’s, but with scattered scarring across the medial scapular region bilaterally. She was prescribed topical anesthetic gel/ointment and gabapentin with no relief; her pruritus remained persistent and constant. The patient was referred to dermatology where she was diagnosed with suspected allergic reaction with residual irritation and prescribed 4 weeks of Allegra which conferred minimal relief. Imaging was obtained with cervical X-ray showing evidence of disc space narrowing at C5–6. Follow-up cervical MRI showed disc bulging at C4–5, C5–6. The patient subsequently underwent an interlaminar C7-T1 ESI using 1 mL of Depo-Medrol with near-complete resolution of pruritus in the scapular region but ongoing in the occipital region (Figure 2 ). She later underwent two occipital nerve blocks using 3 mL of 1% lidocaine and 0.25 mL celestone per side with 70% improvement in symptoms.
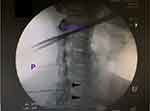 |
Figure 2 Fluoroscopic Image of C7-T1 Cervical Epidural Steroid Injectio.Notes: Contralateral oblique view of the cervical spine depicting epidural contrast spread (black arrows). |
Discussion
There are currently numerous treatment alternatives for NP; however, the majority only confer temporary symptomatic relief either losing efficacy over time or with cessation of treatment. Topical treatments include capsaicin7,8 tacrolimus9 and anesthetic creams.10 Intralesional treatments include botulinum toxin A,11–13 corticosteroids (triamcinolone)14 and cryolipolysis.15 Oral medications include oxcarbazepine,16 gabapentin17,18 and amitriptyline.19 Other therapies documented include TENS,20 EMS,21 Narrow band UV-B,22 osteopathic manipulation,23 acupuncture,24 exercise25 and physiotherapy.3 Paravertebral nerve blocks,26 intravenous lidocaine infusions27 and surgical decompression28 have also been described. ESI and occipital nerve block have not yet been described as viable treatments for NP. In order to understand why these interventions succeeded in conferring relief, we must acknowledge the following precepts: 1) pruritus can be a symptom of nerve damage with neuropathology as opposed to dermatopathology and 2) NP results from damage to the cutaneous branches of the dorsal primary rami of thoracic spinal nerves T2–T6 by compression or impingement from degenerative changes in the spine or entrapment from paraspinal muscle spasms.29 In 1979, Massey and Pleet suggested that, specifically, the posterior rami of spinal nerves T2–T6 are more susceptible to nerve entrapment and chronic trauma because they pass through the multifidus spinae muscle at a 90-degree angle.21,30 This theory of muscle spasms/entrapments coincides with the success of treatments such as physiotherapy which takes into account the anatomy of paraspinal muscles in relation to the spinal nerves. The upper six thoracic spinal nerves first pierce the rhomboid and trapezius prior to becoming cutaneous nerves. Fleischer et al used this knowledge to develop an exercise which involved strengthening the rhomboids and latissimus dorsi muscles and stretching the pectoralis muscles to reduce the angle of the nerve as it passes through the muscles. Extension of the spine through particular exercises may also reduce the angle of the nerve at the spinal level and thus the pressure it experiences.25
The second school of thought posits that nerve impingement and compression of the upper thoracic spinal nerves is caused by cervicothoracic muskuloskeletal degenerative processes; the nerves are entrapped as they exit the spine through the vertebral foramen. One could argue that the characteristic pruritus experienced is located along the T2–T6 dermatomes and does not correspond with a pathological cervical dermatomal distribution. Facet arthropathy, however, can present as referred pain in the upper thoracic and infrascapular region, with symptoms from the cervical area referred directly to the infrascapular back which is the characteristic site of pruritus in NP (Figure 3 ). While degenerative vertebral disk disease corresponding to the affected dermatome may also be observed, degenerative joint disease in the cervical region resulting in pain in a thoracic dermatome should not be discounted; through clinical observations, there exists a clear relationship between the upper thoracic region and the cervical spine.31
In 2000, Savk and Savk demonstrated that of 10 patients with NP, 7 showed evidence of degenerative changes in the vertebrae. While most changes were most prominent in the vertebrae that corresponded with the dermatomal cutaneous lesion, the study did demonstrate cervicothoracic disease corresponding with T4–T8 NP localization.5 In 2005, they evaluated 43 patients with NP and found that 79.1% of the cohort had positive radiographic findings consistent with degenerative changes or herniated nucleus pulposus in the cervicothoracic region.32 Other studies have also shown a correlation between spinal pathology and NP.3,20 As described earlier, the patient continued to have occipital and upper cervical pain even after the cervical ESI. This is likely because the upper cervical spinal pathology noted on imaging was not adequately penetrated with the C7-T1 ESI. In addition, repetitive upper body and neck twisting associated with incessantly scratching ones back likely contributed to a concomitant cervicalgia. It is likely that the occipital nerve block conferred near-complete resolution of neck pain and irritation by accessing the upper cervical region as well as providing anesthetic relief to cervical muscle strain. It is also possible that the neck and occipital pain experienced was a superimposed atypical occipital neuralgia.
Conclusion
Notalgia Paresthetica, while defined as sensory neuropathy, may also be a cutaneous sign of underlying degenerative cervical or cervicothoracic disk disease. This is supported, in this case, by the significant improvement of scapular pruritis, in a patient with underlying cervical disk disease, who received a cervical ESI. In patients who present with NP, cervical spinal imaging is appropriate, and first-line treatment should include addressing the underlying spinal pathology. Topical and/or conservative measures will likely only confer temporary relief.
Evaluation of Umbilical Cord derived Wharton’s Jelly for Regenerative Medicine Applications
1BioIntegrate LLC, New York, NY, USA; 2South Texas Orthopaedic Research Institute, Laredo, TX, USA; 3Department of Psychology, Illinois Wesleyan University, Bloomington, IL, USA; 4Department of Biomedical Engineering, Columbia University, New York, NY, USA; 5Morris Brown College, Atlanta, GA, USA; 6Orthopaedic Sports Medicine, Gwinnett Medical Hospital, Duluth, GA, USA
Correspondence: Saadiq F. El-Amin III BioIntegrate LLC, 423 West 127th Street, New York, NY 10027, USA Email: [email protected]
Introduction: Musculoskeletal injuries have traditionally been treated with activity-modification, physical therapy, pharmacological agents and surgical procedures. These modalities have limitations and potential side-effects. Over the last decade, there has been an increased interest in the use of biologics for regenerative medicine applications (RMA), including umbilical cord (UC) derived Wharton’s Jelly (WJ). Despite this increase, there is insufficient literature assessing the amount of growth factors, cytokines, hyaluronic acid (HA) and extracellular vesicles (EV) including exosomes in these products. The purpose of this study was to develop a novel WJ formulation and evaluate the presence of growth factors, cytokines, HA and EV including exosomes.
Materials and Methods: Human-UC was obtained from consenting C-section donors. The WJ was then isolated from the procured UC and formulated into an injectable form. Randomly selected samples from different batches were analyzed for sterility testing and presence of growth factors, cytokines, HA, and particles in the EV size range.
Results: The results revealed that all samples passed the sterility test. Growth factors including IGFBP 1, 2, 3, 4 and 6, TGF-α, PDGF-AA were detected. Expression of several immunomodulatory cytokines, such as RANTES, IL-6R, IL-16, were also detected. Expression of pro-inflammatory cytokines MCSFR, MIP-1a; anti-inflammatory cytokines TNF-RI, TNF-RII, IL-1RA; and homeostatic cytokines TIMP-1 and TIMP-2 were observed. Cytokines associated with wound-healing, ICAM-1, GDF-15, and regenerative properties, growth hormone, were also expressed. High concentrations of HA were observed. Particles in the EV size range (30–150 nm) were detected and were enclosed by the membrane, indicative of true EV.
Conclusion: Our results confirmed there are numerous growth factors, cytokines, HA and EV present in the WJ formulation we analyzed. We believe the presence of multiple factors within one WJ formulation may play a role in reducing inflammation, pain and augment healing of musculoskeletal injuries. This offers a potential expanded use for RMA.
Keywords: regenerative medicine, umbilical cord, Wharton’s jelly, growth factors, cytokines, exosomes
References
1. Massey EW, Pleet AB. Notalgia paresthetica. JAMA. 1979;241(14):1464. doi:10.1001/jama.1979.03290400024012
2. Situm M, Kolic M, Franceschi N, Pećina M. Notalgia paresthetica. Acta Clin Croat. 2018;57(4):721–725. doi:10.20471/acc.2018.57.04.14
3. Raison-peyron N, Meunier L, Acevedo M, Meynadier J. Notalgia paresthetica: clinical, physiopathological and therapeutic aspects. A study of 12 cases. J Eur Acad Dermatol Venereol. 1999;12(3):215–221. doi:10.1111/j.1468-3083.1999.tb01031.x
4. Shumway NK, Cole E, Fernandez KH. Neurocutaneous disease: neurocutaneous dysesthesias. J Am Acad Dermatol. 2016;74(2):215–228. doi:10.1016/j.jaad.2015.04.059
5. Savk E, Savk O, Bolukbasi O, et al. Notalgia paresthetica: a study on pathogenesis. Int J Dermatol. 2000;39(10):754–759. doi:10.1046/j.1365-4362.2000.00080.x
6. Huesmann T, Cunha PR, Osada N, et al. Notalgia paraesthetica: a descriptive two-cohort study of 65 patients from Brazil and Germany. Acta Derm Venereol. 2012;92(5):535–540. doi:10.2340/00015555-1344
7. Wallengren J, Klinker M. Successful treatment of notalgia paresthetica with topical capsaicin: vehicle-controlled, double-blind, crossover study. J Am Acad Dermatol. 1995;32(2 Pt 1):287–289. doi:10.1016/0190-9622(95)90152-3
8. Andersen HH, Sand C, Elberling J. Considerable variability in the efficacy of 8% capsaicin topical patches in the treatment of chronic pruritus in 3 patients with notalgia paresthetica. Ann Dermatol. 2016;28(1):86–89. doi:10.5021/ad.2016.28.1.86
9. Ochi H, Tan LX, Tey HL. Notalgia paresthetica: treatment with topical tacrolimus. J Eur Acad Dermatol Venereol. 2016;30(3):452–454. doi:10.1111/jdv.12830
10. Layton AM, Cotterill JA. Notalgia paraesthetica – report of three cases and their treatment.Clin. Exp Dermatol. 1991;16(3):197–198. doi:10.1111/ced.1991.16.issue-3
11. Pérez-Pérez L, García-gavín J, Allegue F, Caeiro JL, Fabeiro JM, Zulaica A. Notalgia paresthetica: treatment using intradermal botulinum toxin A. Actas Dermosifiliogr. 2014;105(1):74–77. doi:10.1016/j.ad.2013.09.003
12. Weinfeld PK. Successful treatment of notalgia paresthetica with botulinum toxin type. A Arch Dermatol. 2007;143(8):980–982. doi:10.1001/archderm.143.8.980
13. Maari C, Marchessault P, Bissonnette R. Treatment of notalgia paresthetica with botulinum toxin A: a double-blind randomized controlled trial. J Am Acad Dermatol. 2014;70(6):1139–1141. doi:10.1016/j.jaad.2013.12.006
14. Weber PJ, Poulos EG. Notalgia paresthetica. Case reports and histologic appraisal. J Am Acad Dermatol. 1988;18(1 Pt 1):25–30. doi:10.1016/S0190-9622(88)70003-1
15. Cohen PR. Notalgia paresthetica: a novel approach to treatment with cryolipolysis. Cureus. 2017;9(9):e1719.
16. Savk E, Bolukbasi O, Akyol A, Karaman G. Open pilot study on oxcarbazepine for the treatment of notalgia paresthetica. J Am Acad Dermatol. 2001;45(4):630–632. doi:10.1067/mjd.2001.116228
17. Maciel AA, Cunha PR, Laraia IO, Trevisan F. Efficacy of gabapentin in the improvement of pruritus and quality of life of patients with notalgia paresthetica. An Bras Dermatol. 2014;89(4):570–575. doi:10.1590/abd1806-4841.20142777
18. Loosemore MP, Bordeaux JS, Bernhard JD. Gabapentin treatment for notalgia paresthetica, a common isolated peripheral sensory neuropathy. J Eur Acad Dermatol Venereol. 2007;21(10):1440–1441. doi:10.1111/jdv.2007.21.issue-10
19. Yeo B, Tey HL. Effective treatment of notalgia paresthetica with amitriptyline. J Dermatol. 2013;40(6):505–506. doi:10.1111/jde.2013.40.issue-6
20. Savk E, Savk O, Sendur F. Transcutaneous electrical nerve stimulation offers partial relief in notalgia paresthetica patients with a relevant spinal pathology. J Dermatol. 2007;34(5):315–319. doi:10.1111/j.1346-8138.2007.00279.x
21. Wang CK, Gowda A, Barad M, Mackey SC, Carroll IR. Serratus muscle stimulation effectively treats notalgia paresthetica caused by long thoracic nerve dysfunction: a case series. J Brachial Plex Peripher Nerve Inj. 2009;4:17.
22. Pérez-pérez L, Allegue F, Fabeiro JM, Caeiro JL, Zulaica A. Notalgia paresthetica successfully treated with narrow-band UVB: report of five cases. J Eur Acad Dermatol Venereol. 2010;24(6):730–732. doi:10.1111/j.1468-3083.2009.03479.x
23. Richardson BS, Way BV, Speece AJ. 3rd osteopathic manipulative treatment in the management of notalgia paresthetica. J Am Osteopath Assoc. 2009;109(11):605–608.
24. Stellon A. Neurogenic pruritus: an unrecognised problem? A retrospective case series of treatment by acupuncture. Acupunct Med. 2002;20(4):186–190. doi:10.1136/aim.20.4.186
25. Fleischer AB, Meade TJ, Fleischer AB. Notalgia paresthetica: successful treatment with exercises. Acta Derm Venereol. 2011;91(3):356–357. doi:10.2340/00015555-1039
26. Goulden V, Toomey PJ, Highet AS. Successful treatment of notalgia paresthetica with a paravertebral local anesthetic block. J Am Acad Dermatol. 1998;38(1):114–116. doi:10.1016/S0190-9622(98)70552-3
27. Chtompel Y, Eghtesadi M, Vargas-schaffer G. A case report of refractory notalgia paresthetica treated with lidocaine infusions. Am J Case Rep. 2017;18:1225–1228. doi:10.12659/AJCR.905676
28. Williams EH, Rosson GD, Elsamanoudi I, Dellon AL. Surgical decompression for notalgia paresthetica: a case report. Microsurgery. 2010;30(1):70–72. doi:10.1002/micr.20702
29. Ellis C. Notalgia paresthetica: the unreachable itch. Dermatol pract concept. Dermatol Pract Conceptual 2013;3(1):3–6. doi:10.5826/dpc.0301a02
30. Pećina MM, Krmpotić-nemanić J, Markiewitz AD. Tunnel Syndromes: Peripheral Nerve Compression Syndromes.
31. Alai AN, Skinner HB, Nabili ST, et al. Notalgia paresthetica associated with cervical spinal stenosis and cervicothoracic disk disease at C4 through C7. Cutis. 2010;85:77–81.
32. Savk O, Savk E. Investigation of spinal pathology in notalgia paresthetica. J Am Acad Dermatol. 2005;52:1085–1087. doi:10.1016/j.jaad.2005.01.138
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


