Back to Journals » Patient Preference and Adherence » Volume 13
Ease of Use, Preference, and Safety of the Recombinant Human Growth Hormone Disposable Pen Compared with the Reusable Device: A Multicenter, Single-Arm, Open-Label, Switch-Over, Prospective, Phase IV Trial
Authors Lee JE , Kim SY, Yoo JH, Hwang IT, Lim JS, Yi KH , Rhie YJ , Lee GM, Nam HK , Chae HW, Kim EY , Cheon CK, Lee J , Shim YS , Lee Y, Kim EY, Hwang JS
Received 5 September 2019
Accepted for publication 5 December 2019
Published 20 December 2019 Volume 2019:13 Pages 2195—2205
DOI https://doi.org/10.2147/PPA.S229536
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Ji-Eun Lee,1 Se Young Kim,2 Jae-Ho Yoo,3 Il Tae Hwang,4 Jung Sub Lim,5 Kyung Hee Yi,6 Young-Jun Rhie,7 Gyung Min Lee,8 Hyo-Kyoung Nam,9 Hyun Wook Chae,10 Eun Young Kim,11 Chong Kun Cheon,12 Jieun Lee,13 Young Suk Shim,14 YuJin Lee,15 Eun Young Kim,15 Jin Soon Hwang16
1Department of Pediatrics, Inha University Hospital, Inha University Graduate School of Medicine, Incheon, Republic of Korea; 2Department of Pediatrics & Adolescent Medicine, Bundang Jeseang General Hospital, Daejin Medical Center, Seongnam, Republic of Korea; 3Department of Pediatrics, Dong-a University Hospital, Dong-a University College of Medicine, Busan, Republic of Korea; 4Department of Pediatrics, Kangdong Sacred Heart Hospital, Hallym University, College of Medicine, Seoul, Republic of Korea; 5Department of Pediatrics, Korea Cancer Center Hospital, Seoul, Republic of Korea; 6Department of Pediatrics, Wonkwang University Sanbon Medical Center, Gunpo, Republic of Korea; 7Department of Pediatrics, Korea University Ansan Hospital, Korea University College of Medicine, Ansan, Republic of Korea; 8Department of Pediatrics, Konyang University Hospital, Daejeon, Republic of Korea; 9Department of Pediatrics, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Republic of Korea; 10Department of Pediatrics, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Republic of Korea; 11Department of Pediatrics, Chosun University Hospital, Chosun University School of Medicine, Gwangju, Republic of Korea; 12Department of Pediatrics, Pusan National University Children’s Hospital, Pusan National University School of Medicine, Yangsan, Republic of Korea; 13Department of Pediatrics, Inje University Ilsan Paik Hospital, Inje University College of Medicine, Goyang, Republic of Korea; 14Department of Pediatrics & Adolescent Medicine, Hallym University Dongtan Sacred Heart Hospital, Hwaseong, Republic of Korea; 15Life Sciences, LG Chem, Ltd., Seoul, Republic of Korea; 16Department of Pediatrics & Adolescent Medicine, Ajou University Hospital, Ajou University School of Medicine, Suwon, Republic of Korea
Correspondence: Jin Soon Hwang
Department of Pediatrics & Adolescent Medicine, Ajou University Hospital, Ajou University School of Medicine, 164, World cup-ro, Yeongtong-gu, Suwon-si, Gyeonggi-do 16499, Republic of Korea
Tel +82 31 219 5166
Fax +82 31 219 5169
Email [email protected]
Purpose: To assess the usability and safety of the disposable pen compared to those of reusable devices in patients receiving recombinant human growth hormone (rhGH) treatment.
Patients and methods: This study was a multicenter, single-arm, open-label, switch-over, prospective, Phase IV trial. After screening, eligible patients who were previously treated with rhGH using a reusable device were enrolled to receive treatment with the disposable pen for 8 weeks. The ease of use, preference, and tolerability of the disposable pen compared to those of the reusable device were assessed by the subjects and/or their caregivers using a questionnaire. Adverse events were evaluated by the investigators.
Results: Of 116 subjects enrolled in this study, 115 received treatment with the disposable pen and 109 completed the study. The mean age of the subjects was 9.4 years. Compared to the previous reusable device, the disposable pen was assessed as significantly easier to use (mean value 7.88, 95% confidence interval (CI) [7.45–8.30] on a numerical scale ranging from 0 (far less easy) to 10 (far easier)). Furthermore, the percentage of subjects who preferred the disposable pen to the previously used reusable device was 75.7% (95% CI [67.6%–83.8%]). The percentages of subjects who rated pain and discomfort at the injection site as “not at all” were higher after using the disposable pen compared to the reusable device. No specific safety concerns were identified.
Conclusion: The disposable pen is easier to use than the reusable devices and is preferred by approximately 75% of patients receiving rhGH treatment. Moreover, the disposable pen is safe and acceptable. Therefore, it could be a good alternative to reusable devices. The disposable pen is expected to provide benefits to patients receiving rhGH treatment.
Clinicaltrials.gov identifier: NCT03015909.
Keywords: growth hormone, disposable equipment, patient preference, usability, safety
Introduction
Recombinant human growth hormone (rhGH) is used to treat various growth disorders. The general therapeutic goals of rhGH treatment in pediatric growth disorders are for the patients to reach a normal adult height or approach their target height.1–3 To achieve these goals, continuous and long-term treatment is required. However, rhGH products which are usually administered subcutaneously on a daily basis, are likely to result in poor treatment adherence,4,5 which can make it difficult to reach therapeutic goals in pediatric growth disorders.
Growth rates are significantly lower in patients with poor adherence to rhGH treatment than in those with better adherence.6 Some studies have shown that poor adherence to long-term rhGH therapy is associated with needle injection therapy, but injection devices that are easier to use have been shown to improve adherence, and this ultimately enhances clinical outcomes.6–11 Therefore, it is essential that administration devices are convenient, easy, safe to use, and acceptable to patients.5
There have been several advances addressing usability and tolerability in rhGH injection devices over the years. Reusable devices were introduced in the 1990s and are currently often used. However, several steps are required for the patient and/or caregiver to prepare reusable devices for injection, including the exchange of a cartridge. In particular, in case of some devices, the reconstitution procedure is required prior to the injection. Therefore, disposable pens for the administration of rhGH were developed to avoid the disadvantages of reusable devices. Eutropin Pen (LG Chem, Ltd., Seoul, Republic of Korea) is a disposable pen prefilled with cartridge containing a liquid formulation of rhGH and does not require replacement of the cartridge. The objective of the study was to assess the usability and safety of the disposable pen (Eutropin Pen) compared to those of reusable devices.
Subjects and Methods
Study Subjects
Patients between 4 to 15 years of age were included in the study if they met following criteria: being currently treated with rhGH using the same reusable device for ≥3 months before screening; the person who mainly injected rhGH using the previous reusable device (for at least 6 weeks in the last 3 months) can continuously inject rhGH to the patient during the study period; and eligibility for the study treatment, such as growth failure due to growth hormone deficiency (GHD), Turner syndrome (TS), chronic renal failure (CRF), being born small for gestational age (SGA), or idiopathic short stature (ISS).
Exclusion criteria included patients with any contraindication to use of rhGH. Patients who were scheduled to receive injections on a regular basis other than the investigational product during the study period were also excluded.
Study Design
This study was a multicenter, single-arm, open-label, switch-over, prospective, Phase IV study conducted at 15 centers in the Republic of Korea from August 2016 to December 2017. The study was conducted in compliance with the ethical guidelines of the Declaration of Helsinki and Good Clinical Practices and was approved by the institutional review board of each study site. Written informed consent was obtained from all subjects and their legally authorized representatives, and the study was registered at ClinicalTrials.gov (NCT03015909).
The study was conducted over 8 weeks. All eligible subjects were enrolled at Visit 1 (Day 1), and ease of use and tolerability of the previous reusable device were assessed by the subjects and/or their caregivers through the questionnaire. The reusable device being used was replaced with the disposable pen at this time. There was no washout period. The regimen of rhGH treatment was determined at the discretion of the investigator according to the approved dosage for each indication. All subjects and their caregivers were trained by the investigator or nurse on how to use the disposable pen. Subjects were to receive rhGH treatment for 8 weeks. The subjects visited the study site at Visit 2 (Day 57) for evaluation of ease of use, preference, and safety assessments of the disposable pen compared to the previous reusable device.
Assessment Methods
Ease of use, fear of the needle of both the disposable pen and the reusable device, and preference for the disposable pen against the previous reusable device were assessed using the questionnaire (supplementary material). The subjects and/or their caregivers completed a questionnaire about the previously used reusable device at Visit 1 and a questionnaire about the disposable pen at Visit 2. The person who completed the questionnaire at Visit 1 was to have filled out the questionnaire at Visit 2.
The primary endpoints were ease of use and preference for the disposable pen against the previous reusable device, assessed after 8 weeks of using the disposable pen. Ease of use was measured using a quantitative scale ranging from 0 (far less easy) to 10 (far easier), and preference was chosen from three choices (prefer the disposable pen, prefer the reusable device, or no preference). The secondary endpoints included ease of use based on each injection step, preparation time for injection, and fear of the needle of both the disposable pen and the reusable device. Ease of use based on each injection step and fear of the needle were measured using a 5-point scale. The benefits of the disposable pen and treatment adherence to the disposable pen were also assessed as secondary endpoints.
Safety assessments included the monitoring of adverse events and an evaluation of tolerability. Pain and discomfort at the injection site were measured using a 5-point scale through the questionnaire for the tolerability evaluation.
Statistical Analysis
The sample size to assess the ease of use and preference for the disposable pen against the previous reusable device was determined using a significance level of 2.5% and a one-sided test. The planned enrollment was 116 subjects, which would provide 90% power to detect that the lower limit of a 95% confidence interval (CI) of the mean value of the numerical scale in ease of use of the disposable pen against the reusable device was greater than the neutral value of 5. This was assuming a mean value of 6.89 and a standard deviation (SD) of 2.52. The lower limit of the 95% CI also would be used to detect that the percentage of subjects who prefer the disposable pen to the previous reusable device or do not prefer either was greater than 50%, assuming a percentage of 65.2%. This calculation was based on the assumption of a 10% drop-out rate.
Usability analyses were based on the per protocol set consisting of all subjects with treatment adherence greater than 80% during the 8-week treatment period and who completed the study without protocol deviations that had a significant impact on the evaluation. Safety analyses were performed on the safety set, which included all enrolled subjects who received at least one dose of the investigational product.
For continuous usability variables, the descriptive statistics and the 95% CI were summarized. For categorical data, frequency and percentage were presented, and the differences between pre- and post-treatment of the investigational product were analyzed using Bhapkar’s test. For adverse events, the number and percentage of the subjects who had experienced at least one adverse event and the total number of events were summarized. For tolerability data, the number and percentage of the subjects were presented, and the differences between pre- and post-treatment of the investigational product were analyzed using Bhapkar’s test. Statistical data analyses were performed using SAS® version 9.4 (SAS Institute, Inc., Cary, NC, USA).
Results
Subject Disposition and Baseline Characteristics
A total of 116 subjects were enrolled, and 115 were treated with the investigational product at least once and included in the safety analyses. A total of 109 subjects completed the study, and 107 were included in the usability analyses (Figure 1). A total of 9 subjects were excluded from the usability analyses, for the following reasons: 5 subjects deviated from the inclusion criteria, 3 did not complete the study (2 “withdrawal of consent” and 1 “other reason”), and 1 had less than 80% adherence to the study treatment.
 |
Figure 1 Subject disposition. |
The mean (SD) age of the subjects was 9.4 (2.8) years, with a range of 4–15 years of age (Table 1). The most common indication for rhGH treatment was ISS (57.9%), followed by GHD (28.0%). The mean duration since diagnosis was 26.3 months. For the 3 months prior to enrollment, 43.0% and 42.1% of subjects had been using an electronic device with a liquid cartridge and a pen with a dual-chamber cartridge, respectively, and the remaining subjects (15.0%) had been using a pen with a liquid cartridge.
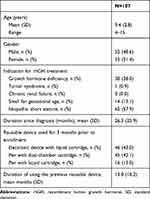 |
Table 1 Baseline Characteristics (Per Protocol Set) |
Usability results
The mean value (SD) of ease of use of the disposable pen against the previous reusable device was 7.88 (2.22), with a 95% CI of 7.45–8.30. Since the lower limit of the 95% CI of the mean value of ease of use was greater than 5, it was confirmed that the disposable pen was easier to use than the previous reusable device. Furthermore, the percentage of subjects who preferred the disposable pen to the previous reusable device (n = 81) or who did not prefer either (n = 0) was 75.7% (81/107 subjects), with a 95% CI of 67.6%–83.8%. Since the lower limit of the 95% CI of the percentage of subjects was greater than 50%, it was confirmed that the disposable pen was preferred to the previous reusable device.
For the primary endpoints, subgroup analyses were performed (Table 2). Compared to the subgroups of those who had used an electronic device with a liquid cartridge or those who had used a pen with a liquid cartridge, the subgroup that had been using a pen with a dual-chamber cartridge had the highest ease of use score (mean value = 8.40) and the highest percentage of subjects who preferred the disposable pen to the previous reusable device or who did not prefer either (80.0%).
 |
Table 2 Ease of Use and Preference for the Disposable Pen by the Previous Reusable Device (Per Protocol Set) |
Overall ease of use of the disposable pen based on each injection step was considered “very easy” by 71 subjects (66.4%) and the percentage of subjects was higher compared to that of the previous reusable device (27.1%, p<0.0001). In addition, the injection step of replacing the cartridge when the remaining doses were exhausted was rated as “very easy” by 91 subjects (85.0%). This was the biggest change compared to the previous reusable device, which was evaluated as “very easy” by 33 subjects (30.8%, p<0.0001). A greater percentage of subjects, based on each injection step, considered the disposable pen to be “very easy” or “a little easy” to use compared with the previous reusable device (Figure 2). Although the step of checking for completion of drug injection showed no statistically significant difference of ease of use between the previous reusable device and the disposable pen (p=0.3884), the percentage of subjects who rated this injection step of the disposable pen as “very easy” was numerically higher by 10% compared to the previous reusable device.
 |
Figure 2 Ease of use based on each injection step (Per protocol set). *p<0.05, p-value was obtained from Bhapkar’s test. |
For 73 subjects (68.2%), the injection preparation time of the disposable pen took <2 mins. Of the remaining subjects, 28 (26.2%) and 4 (3.7%) were able to prepare the disposable pen for injection in 2–5 mins and 5–10 mins, respectively, and 2 subjects (1.9%) needed more than 10 mins (Figure 3). In general, the injection preparation time shortened when using the disposable pen compared to the previous reusable device (p=0.0023).
The benefit of the disposable pen, that was most frequently rated high by subjects, was simple operation (no need of cartridge replacement), followed by the large capacity with a long replacement cycle (Figure 4). Fear of the needle tended to be reduced when using the disposable pen compared to the previous reusable device; 15 (14.0%) responded that they were not afraid of the needle at all when injecting using the reusable device, while 26 subjects (24.3%) responded that they were not afraid of the needle at all when injecting using the disposable pen (p=0.0025, Figure 5).
 |
Figure 4 Benefits of the disposable pen (Per protocol set). Notes: Multiple choices were allowed for each subject. |
Safety Results
Mean treatment adherence to the disposable pen, assessed through subjects’ diaries, was 94.9%. Mean (SD) dose of rhGH injected during the 8-week treatment period was 0.84 (0.14) IU/kg/week.
A total of 8 adverse events were reported by 5 subjects (4.3%). All adverse events were mild in severity, and no adverse drug reactions related to the investigational product or serious adverse events were reported. Pain and discomfort at the injection site of the reusable device, assessed through the questionnaire, were considered “not at all” by 7 (6.1%) and 18 subjects (15.7%), respectively. On the other hand, pain and discomfort at the injection site of the disposable pen were rated as “not at all” by 16 (14.0%) and 30 subjects (26.3%), respectively, and the percentages of subjects who considered pain and discomfort at the injection site as “not at all” were higher compared to those of the previous reusable device (p<0.0001, Figure 6).
Discussion
In this study, ease of use of the disposable pen was confirmed compared to the previous reusable device. In addition, a greater percentage of subjects considered the disposable pen to be “very easy” or “a little easy” to use based on each injection step compared to the reusable device, and the biggest change was observed in the injection step of replacing the cartridge when the remaining doses were exhausted.
There was no limit on the types of reusable devices that subjects used previously. As a result, subjects who had used an electronic device with a liquid cartridge, a pen with a dual-chamber cartridge, or a pen with a liquid cartridge were enrolled in this study, and the subgroup that had been using a pen with a dual-chamber cartridge showed a higher score in ease of use of the disposable pen than the subgroups previously using an electronic device with a liquid cartridge or a pen with a liquid cartridge. Since a pen with a dual-chamber cartridge needs replacement of the cartridge and reconstitution, subjects would have considered the disposable pen, which does not require these steps, easier to use. This finding is supported by the results that most subjects (92.5%) rated simple operation (no need for cartridge replacement) as a benefit of the disposable pen, 94.4% of subjects took less than 5 mins preparing the disposable pen for injection, and the preparation time significantly shortened compared with the reusable devices. In other studies,7,12–14 the ease of use of two different injection devices was compared because the comparator was fixed. In this study, ease of use of the disposable pen was compared with those of three reusable devices by not limiting the comparator when enrolling subjects. Although the results of the subgroup analyses of this study should be carefully interpreted because of the small number of subjects included in each subgroup, the results are expected to provide more information to patients who are receiving rhGH treatment and their caregivers, as well as healthcare providers when selecting injection devices.
Three-quarters (75.7%) of subjects preferred the disposable pen to the previous reusable device. This preference for the disposable pen was consistent with the results of other studies that compared a disposable pen and a reusable device.7,12 Based on the findings of this study that simple operation and less pain considered as a benefit of the disposable pen by 92.5% and 20.6% of subjects, respectively, these characteristics of the disposable pen, which provide ease of use and less pain, may have affected the preference of subjects and their caregivers. In addition, considering the highest percentage of subjects who preferred the disposable pen to the previous reusable device in the subgroup that had been using a pen with a dual-chamber cartridge which required multiple steps to prepare for injections, this supports the idea that ease of use of the disposable pen may have affected the preference.
Treatment adherence to the disposable pen, calculated by the number of days of administration, was 94.9%. Although the methods for measuring treatment adherence were slightly different in each study, the results of this study showed high levels of adherence. The features of the disposable pen, which is easy to use, easy to set an administration dose, and less painful and uncomfortable to use, may have contributed to the overall increase in treatment adherence. The results of this study support previous findings that easier injection devices resulted in high treatment adherence.6,7 In addition, considering better adherence to treatment leads to increased clinical outcomes,7,10 the disposable pen is expected to improve clinical outcomes of patients who receiving rhGH treatment. However, because treatment adherence to the reusable device was not measured in the study, a direct increase in adherence after using the disposable pen could not be assessed. Another limitation of the study is that the training method was not evaluated. Because only the treatment period of use of the disposable pen was included in the study, the training method of the reusable device could not be controlled and assessed. Therefore, the training methods of the reusable device and the disposable pen may have been different, which might have affected the results of usability and treatment adherence. Nevertheless, the subjects and their caregivers were trained on how to use the reusable device and the disposable pen according to common practice, and the results from the study reflect general medical care. In addition, the usability of the reusable device and the disposable pen were evaluated by the same person, and reliable results could be obtained by using the same scale through the questionnaire. Further studies designed as cross-over studies that include all treatment periods using comparable injection devices are needed to control the training methods and evaluate treatment adherence and ease of training between the injection devices.
Conclusion
The disposable pen is easier to use than reusable devices and was preferred by approximately 75% of patients receiving rhGH treatment. Moreover, the disposable pen is safe and tolerable to patients and caregivers. Therefore, the disposable pen could be a good alternative to reusable devices. It is expected that the disposable pen would provide benefits to patients receiving rhGH treatment.
Abbreviations
CI, confidence interval; CRF, chronic renal failure; GHD, growth hormone deficiency; ISS, idiopathic short stature; rhGH, recombinant human growth hormone; SD, standard deviation; SGA, small for gestational age; TS, Turner syndrome.
Ethics Approval and Informed Consent
The study was approved by the institutional review board at 15 study sites. Written informed consent was obtained from all individual participants and their legally authorized representatives.
The full title of each institution and the corresponding ethical review board is as follows:
Inha University Hospital: Inha University Hospital Institutional Review Board
Bundang Jeseang General Hospital: Institutional Review Board of Bundang Jeseang General Hospital
Dong-A University Hospital: Institutional Review Board of Dong-A University Hospital
Kangdong Sacred Heart Hospital: Kangdong Sacred Heart Hospital Institutional Review Board
Korea Cancer Center Hospital: Korea Institute of Radiological & Medical Sciences Institutional Review Board
Wonkwang University Sanbon Medical Center: Institutional Review Board of Wonkwang University Sanbon Hospital
Korea University Ansan Hospital: Institutional Review Board of Korea University Ansan Hospital
Konyang University Hospital: Konyang University Hospital Institutional Review Board
Korea University Guro Hospital: Institutional Review Board of Korea University Guro Hospital
Gangnam Severance Hospital: Institutional Review Board of Gangnam Severance Hospital
Chosun University Hospital: Chosun University Hospital Institutional Review Board
Pusan National University Children’s Hospital: Institutional Review Board of Pusan National University Yangsan Hospital
Inje University Ilsan Paik Hospital: Inje University Ilsan Paik Hospital Institutional Review Board
Hallym University Dongtan Sacred Heart Hospital: Hallym University Dongtan Sacred Heart Hospital Institutional Review Board
Ajou University Hospital: Institutional Review Board of Ajou University Hospital
Data Sharing Statement
The data supporting the findings of the study are available from the corresponding author on reasonable request.
Acknowledgments
The study was funded by LG Chem, Ltd. The affiliation of Gyung Min Lee was changed after this work, and the current affiliation is Lee Gyung Min Pediatrics, Daejeon, Republic of Korea. The authors acknowledge the efforts of all the investigators, site staffs, subjects, and caregivers who devoted their time and energy to this trial. The authors thank Yunae Eom of LG Chem, Ltd., for writing assistance in preparing the manuscript.
Author Contributions
Ji-Eun Lee contributed to the data interpretation and wrote the manuscript. YuJin Lee supported conducting the study, and contributed to conception and design and project administration. Eun Young Kim of LG Chem, Ltd. contributed to the data analysis. All authors, except YuJin Lee and Eun Young Kim of LG Chem, Ltd., contributed to investigation and acquisition of data. All authors contributed to drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The study was funded by LG Chem, Ltd. The sponsor was involved in the design of the study, data analysis, and reviewing the manuscript, but played no role in the collection and interpretation of data. YuJin Lee and Eun Young Kim are employees of LG Chem, Ltd. Dr Ji-Eun Lee, Dr Se Young Kim, Dr Jae-Ho Yoo, Dr Il Tae Hwang, Dr Jung Sub Lim, Dr Kyung Hee Yi, Dr Young-Jun Rhie, Dr Gyung Min Lee, Dr Hyo-Kyoung Nam, Dr Hyun Wook Chae, Dr Eun Young Kim, Dr Chong Kun Cheon, Dr Jieun Lee, Dr Young Suk Shim, and Dr Jin Soon Hwang report grants and non-financial support from LG Chem, Ltd., during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Takeda A, Cooper K, Bird A, et al. Recombinant human growth hormone for the treatment of growth disorders in children: a systematic review and economic evaluation. Health Technol Assess. 2010;14(42):1–209. doi:10.3310/hta14420
2. Rose SR, Cook DM, Fine MJ. Growth hormone therapy guidelines: clinical and managed care perspectives. Am J Pharm Benefits. 2014;6(5):e134–e146.
3. Tauber M, Jaquet D, Jesuran-Perelroizen M, et al. User assessment of Norditropin NordiFlex®, a new prefilled growth hormone pen: a Phase IV multicenter prospective study. Patient Prefer Adherence. 2013;7:455–462. doi:10.2147/PPA.S43460
4. Smith SL, Hindmarsh PC, Brook CG. Compliance with growth hormone treatment–are they getting it? Arch Dis Child. 1993;68(1):91–93. doi:10.1136/adc.68.1.91
5. Dumas H, Panayiotopoulos P, Parker D, Pongpairochana V. Understanding and meeting the needs of those using growth hormone injection devices. BMC Endocr Disord. 2006;6(5):1–6. doi:10.1186/1472-6823-6-5
6. Desrosiers P, O’Brien F, Blethen S. Patient outcomes in the GHMonitor: the effect of delivery device on compliance and growth. Pediatr Endocrinol Rev. 2005;2(Suppl 3):S327–S331.
7. Hey-Hadavi J, Pleil A, Deeb LC, et al. Ease of use and preference for a new disposable self-injection pen compared with a reusable pen for administering recombinant human growth hormone: a multicenter, 2-month, single-arm, open-label clinical trial in patient-caregiver dyads. Clin Ther. 2010;32(12):2036–2047. doi:10.1016/j.clinthera.2010.11.007
8. Kappelgaard AM, Mikkelsen S, Knudsen TK, Fuchs GS. Patient preference for a new growth hormone injection device: results of an open-label study in Japanese pediatric patients. J Pediatr Endocrinol Metab. 2011;24(7–8):489–496. doi:10.1515/jpem.2011.252
9. Kapoor RR, Burke SA, Sparrow SE, et al. Monitoring of concordance in growth hormone therapy. Arch Dis Child. 2008;93(2):147–148. doi:10.1136/adc.2006.114249
10. Haverkamp F, Johansson L, Dumas H, et al. Observations of nonadherence to recombinant human growth hormone therapy in clinical practice. Clin Ther. 2008;30(2):307–316. doi:10.1016/j.clinthera.2008.02.017
11. Rosenfeld RG, Bakker B. Compliance and persistence in pediatric and adult patients receiving growth hormone therapy. Endocr Pract. 2008;14(2):143–154. doi:10.4158/EP.14.2.143
12. Pleil AM, Darendeliler F, Dörr HG, Hutchinson K, Wollmann HA. Results from an international multicenter trial evaluating the ease-of-use of and preference for a newly developed disposable injection pen for the treatment of growth hormone deficiency in treatment-naïve children and adults. Med Devices (Auckl). 2014;7:61–71. doi:10.2147/MDER.S59821
13. Adachi M. Assessment of user-friendliness of the Norditropin FlexPro for pediatric patients treated with recombinant human growth hormone: results of an open-label user survey. J Pediatr Endocrinol Metab. 2013;26(11–12):1105–1110. doi:10.1515/jpem-2013-0071
14. Rapaport R, Saenger P, Schmidt H, et al. Validation and ease of use of a new pen device for self-administration of recombinant human growth hormone: results from a two-center usability study. Med Devices (Auckl). 2013;6:141–146. doi:10.2147/MDER.S50088
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



