Back to Journals » Clinical Ophthalmology » Volume 16
Early Ophthalmological Manifestations of Acute Myeloid Leukemia: Current Perspectives
Authors El Salloukh NA, Hage DG , Bashshur AZ, Kheir WJ
Received 5 April 2022
Accepted for publication 17 June 2022
Published 1 July 2022 Volume 2022:16 Pages 2119—2127
DOI https://doi.org/10.2147/OPTH.S342720
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Nasrine Anais El Salloukh,1 Dany G Hage,2 Anna Z Bashshur,1 Wajiha Jurdi Kheir1
1Ophthalmology Department, American University of Beirut Medical Center, Beirut, Lebanon; 2Tulane University School of Medicine, New Orleans, LA, USA
Correspondence: Wajiha Jurdi Kheir, Department of Ophthalmology, American University of Beirut Medical Center, Cairo Street, Beirut, Lebanon, Tel +961-1-350000, Fax +961-1-370837, Email [email protected]
Abstract: Acute myeloid leukemia (AML) is a hematological malignancy affecting different organ systems including the eye. The purpose of this review is to present and evaluate the medical literature regarding the early ophthalmological manifestations of acute myeloid leukemia. AML affects the ocular system through direct infiltration of tissues, secondary to hematological abnormalities, or in the form of chloroma or myeloid sarcoma in the brain or orbit consequently leading to a variety of manifestations depending on the ocular tissue involved. It is imperative for ophthalmologists to be aware of the early ophthalmological manifestations of AML which will allow for earlier diagnosis and treatment of this life-threatening disease.
Keywords: ocular involvement, myeloblastoma, chloroma, myeloid sarcoma, ocular granulocytic sarcoma, leukemic infiltration
Introduction
Acute myeloid leukemia (AML) is a malignant disorder of the hematopoietic stem cells characterized by abnormal proliferation of myeloid blast cells in the bone marrow and blood, preventing them from further differentiating into the specialized cells of the bone marrow and thus causing pancytopenia.1 Consequently, AML can affect various tissues and organs (liver, skin, central nervous system), including the eye and orbit.
Ophthalmic manifestations of leukemia are more frequent with acute than chronic leukemia and can affect all intraocular structures.2,3 The reported prevalence of ocular involvement with acute leukemia ranges from 32% to 35.5%.4–6 They are caused by direct neoplastic cell infiltration or indirect complications secondary to hematologic abnormalities (thrombocytopenia, anemia and hyperviscosity state).
Ophthalmic involvement can be the initial manifestation of the systemic disease or the first sign of relapse. Some studies associated ocular involvement with poorer prognosis7–10 while others found no difference in fatality.11 The purpose of this review is to present and evaluate the medical literature on the early ophthalmological manifestations of acute myeloid leukemia, which physicians should be aware of for an earlier and more efficient diagnosis and treatment.
Methodology
A literature review accessing PubMed, Embase, Science Direct, and Medline databases was performed between November and December 2021. PubMed, Embase, and Science Direct databases were searched with the keywords [(Acute Myeloid Leukemia) OR (AML)] AND (Ophthalmic Manifestations). Medline database was searched with the MeSH terms [(ocular.mp.) OR (exp Eye/ or exp Eye Abnormalities/) OR (ophthalmic.mp.) OR (opthamology.mp. or exp Ophthalmology/)] AND (acute myeloid leukemia.mp. exp *Leukemia, Myeloid, Acute/). Two hundred and ninety-seven studies were found after an initial search conducted by 2 independent reviewers. All peer-reviewed case reports published in the literature were included. Duplicates, non-English, and unavailable studies were removed. Titles and abstracts were screened for relevancy and full texts were obtained and evaluated for extractable data, resulting in 95 remaining studies.
Ocular Adnexa
Orbit
Myeloid sarcoma, also known as chloroma, granulocytic sarcoma or myeloblastoma is a type of extramedullary myeloid tumor. It is a rare manifestation of AML, accounting for 2.5% to 9.1% of AML cases.12 It most commonly affects children13 and is a highly unusual presentation in adults.14,15
In the pediatric population, the orbit is one of the most common sites of occurrence of myeloid sarcoma.12 The incidence of pediatric orbital chloroma varies by region16,17 but has been reported to be higher in Africa, Asia, Latin America and the Middle East.18 It can precede,17–21 appear synchronous18,21–23 or occur after a systemic leukemia diagnosis.17,18,21,24 It can also be a manifestation of leukemia relapse,25 even after bone marrow transplant.26,27 When granulocytic sarcoma precedes AML, the diagnosis can be difficult, especially as it has no clear characteristic radiologic features.18,23 Histopathological confirmation can also be challenging, seeing as the tumor is often not well differentiated.13,18 In this category of patients, systemic features usually develop within a year.12
The clinical features of orbital chloroma are variable. Proptosis is the most common presentation.20,21,23 Other symptoms include ptosis,28 eyelid swelling, decreased vision and diplopia. While the clinical presentations described above are not specific for granulocytic sarcoma, one constant feature of this type of tumor observed is its superior or supero-temporal location.13,23 Chloromas are also most often unilateral, but bilateral orbital involvement is considered a strong predictor of a diagnosis of myeloid sarcoma.29
As previously established, orbital involvement by AML is most frequently in the form of chloroma but can, less frequently, be secondary to infiltration of the lacrimal gland or extraocular muscles by leukemic cells.30–32 There is also a solitary report of a diagnosis of AML made in a patient presenting with dacryocystitis responding partially to antibiotics and fully to chemotherapy.33
Many authors have suggested that orbital involvement is a poor prognostic sign for AML patients,17,34 while others observed no change in prognosis.21,24 The latter group attributes this improvement in prognosis to earlier diagnosis of the disease, relating to advances in orbital imaging and better treatment modalities.
In most cases, chemotherapy was used as the standard of treatment while radiation therapy has no well-defined role.35,36 While suggested by Puri et al,19 there is no evidence that chemotherapy for the treatment of isolated orbital chloroma can improve the prognosis if implemented prior to the development of systemic disease.
Conjunctiva
Myeloid sarcoma affecting the conjunctiva is rare. In postmortem studies, it was shown that ocular granulocytic sarcoma affects the conjunctiva in 2–4% of cases.37 It can present as an initial manifestation of primary disease37–41 or relapse,40,42–48 and can occur even in the absence of documented systemic relapse.43 Studies have found that extramedullary involvement in general, and conjunctival leukemic infiltrates in particular, were more common in myelomonocytic and monocytic leukemia than other forms of AML.49 Many authors suggested chemotherapy as the treatment of choice50 while others advocate local radiotherapy.37 Even with appropriate treatment, conjunctival granulocytic sarcoma has been associated with a poor prognosis.49 One case of bilateral subconjunctival hemorrhage in an infant as an initial AML manifestation has also been reported.51
Neuro-Ophthalmology and Strabismus
Neuro-ophthalmologic manifestations of AML are diverse and depend on the affected region of the nervous system. Gaze palsy has been reported as an initial presentation in patients with AML and was related to multiple different causes, namely, chloroma,52,53 brain infarction,54 leptomeningeal spread,55 hypercoagulable state56 or cranial nerve palsy.53,55,57 Chloroma of the central nervous system is rare and comprises only 1–6% of all chloromas.58,59 It was described causing gaze palsy in a patient with dorsal pons involvement52 and another with petrous apex involvement.53
An interesting presentation of a patient diagnosed with one and a half syndrome was reported by Hsu et al, and it was caused by brainstem infarction from AML.54 Cranial nerve palsy secondary to leukemia and involving the oculomotor nerve53,57 and the abducens nerve55 has also been documented. Pupillary abnormalities such as tonic pupil were reported in relapsed AML with choroidal thickening postulated to be due to damage to the ciliary nerve at the suprachoroidal level.60
Another rare but detrimental neuro-ophthalmic manifestation of AML is optic nerve infiltration. In a histopathological study by Allen and Straatsma, the optic nerve was affected in 34% of ocular leukemic cases, mostly in acute leukemia.61 While the optic nerve is a known site of disease relapse in patients with systemic or meningeal leukemia, it is rarely reported as an initial isolated presentation of disease without systemic relapse.62 It usually presents as a swollen optic nerve, often pale gray in color and with associated hemorrhages. It is considered a medical emergency, as severe, irreversible vision loss can occur.63 The recommended treatment is radiation therapy,64 as systemic chemotherapy is not shown to be beneficial and intrathecal chemotherapy is not enough to eradicate leukemic cells in the paraneural space of the optic nerve.62
Anterior Segment
Rare cases of hypopyon as an initial manifestation of AML have been documented,65–67 but most reported cases are in the context of relapsing disease. While acute lymphocytic leukemia (ALL) relapse presents as a pseudo-hypopyon in 2.5% of the cases in children, it remains very rare for acute myeloid leukemia relapse to present as hypopyon.68–73 In a prospective 2-year study of 53 patients undergoing treatment for AML, no patient presented with hypopyon uveitis.74 A review of 14 AML cases with leukemic hypopyon by Matano et al in 2000 concluded a more frequent occurrence of hypopyon in AML with monocytic blasts than other forms of AML.71 While leukemic hypopyon is strongly associated with the presence of extramedullary infiltration, especially CNS leukemia, some rare cases of isolated ocular relapse have been reported.70,72,75,76 Authors have suggested that leukemic hypopyon is associated with systemic relapse even when systemic evaluation does not detect leukemia and concluded that systemic chemotherapy combined with local therapy is advised in these patients.71
A few case reports have been published documenting other anterior segment presentations of AML such as corneal pseudomembrane in a patient with MDS,77 bilateral marginal corneal ring ulcers78,79 and iris leukemic infiltration.21,80,81 It is thus prudent to consider any atypical anterior segment sign as a masquerader for malignancy.
Posterior Segment
The retina and choroid are the most common ocular tissue affected by leukemia.82 Duke-Elder estimated that up to 90% of patients with leukemia will show fundus changes at some point in their disease course.83 Overall, ocular manifestations are more common in acute versus chronic and in myelogenous versus lymphocytic leukemia,84 which suggests that posterior segment changes may be a presenting sign of AML. The prevalence of ocular changes at the time of AML diagnosis was reported to be as high as 35%.5
Posterior segment involvement with leukemia may occur from direct invasion of tissue, known as “leukemic infiltrate”, or secondary to leukemic blood dyscrasia (anemia and thrombocytopenia), known as “leukemic retinopathy”.82,85 While leukemic retinopathy is the most commonly reported clinical ophthalmic manifestation, autopsy studies suggest that subclinical choroidal infiltration is the most common ophthalmic involvement by leukemia.84 Ocular involvement in general and posterior segment infiltrate (not retinopathy) in particular, entails a poorer prognosis, and is associated with a higher rate of bone marrow relapse and CNS involvement.82,86 Studies have shown that more than 50% of cases with intraocular leukemia have CNS involvement and this incidence is even higher in those with posterior segment infiltrates.87
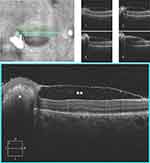
Leukemic retinopathy is present in around 31.6% of all leukemia5 and is the most commonly reported ophthalmic manifestation.84 It is frequently asymptomatic,88 and presents as white centered hemorrhages known as Roth spots,89 cotton wool spots,90 vascular tortuosity and dilatation,91 microaneurysms and neovascularizations.88,92,93 Hemorrhages are predominantly intraretinal but may occasionally be subretinal, sub-internal limiting membrane or subhyaloid (Figure 1).88,90 In AML, intraretinal hemorrhages are statistically correlated with low platelet count.94 No direct treatment for leukemic retinopathy is needed and the focus is on the treatment of the primary hematological disease.83
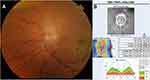
Central retinal vein occlusion (CRVO) is a very rare manifestation of leukemia, most likely caused by hyperviscosity associated with leukocytosis.88 However, it is important not to overlook retinal vein or artery occlusions simply as a manifestation of hypercoagulability, as they can be caused by leukemic infiltration (Figure 2). In a study of 288 patients known to have leukemia with ocular involvement, 3 developed CRVO.5 Infiltration causing vein/artery occlusion often presents with optic disc edema and/or subretinal infiltrates. Khair et al reported a case of bilateral retinal artery occlusions as a first manifestation of CNS involvement in relapsed AML, but the patient did not have disc edema or retinal infiltrates. This suggests that vein/artery occlusions can result from microscopic infiltration.95 Therefore, in cases of vein/artery occlusions, work-up of CNS involvement (imaging, lumbar puncture, etc.) is critical for treatment strategy and patient prognosis.
While clinically apparent leukemic choroidal infiltrates are infrequently reported, histologically detected choroidal leukemic infiltration has been found in up to 65% of patients with leukemia87 and in 31% of post-mortem eyes of fatal leukemia.96 This makes it the most common ophthalmic involvement by leukemia.84 It is more common in relapsing leukemia than in primary disease87 and is most commonly associated with CNS and systemic relapse.
Choroidal involvement usually presents clinically as serous retinal detachment,91 which has been documented as a presenting symptom of AML in adults and children.97 The suggested theory is that leukemic choroidal infiltration causes decreased blood flow to the choriocapillaris, inducing ischemia of the retinal pigment epithelium (RPE). This causes disruption of the inter-cellular tight junctions and thus of the ability of the RPE to effectively pump fluid,98 consequently inducing exudative retinal detachment.99 Choroidal infiltration has been also reported to cause angle closure glaucoma.98,100 To our knowledge, only two cases of choroidal infiltration as first manifestation of leukemic relapse in the absence of CNS involvement or systemic relapse have been reported.87,101 Biopsy of the involved choroid is the most appropriate diagnostic tool in this category of patients.
Conclusion
Ophthalmological manifestations of acute myeloid leukemia can be an initial presentation of the primary disease or a sign of relapse and can affect different parts of the eye and orbit. The retina and choroid are the most commonly affected ocular tissues in the form of direct leukemic infiltration or secondary to blood dyscrasia, mostly manifesting as intraretinal hemorrhages. In the orbit, AML mainly presents as an extramedullary myeloid tumor or chloroma, most commonly inducing proptosis. Rarely, AML can affect the anterior segment in the form of pseudo-hypopyon, usually in the context of relapsing disease. AML can also present as gaze palsy related to infarctions, brain chloroma or cranial nerve palsies. It can infiltrate the optic nerve, resulting in severe decrease in vision, and this constitutes a medical emergency requiring urgent radiation therapy. Intraocular leukemic involvement has been associated with CNS disease in 50% of cases, and the incidence is higher with posterior segment involvement. Recognizing ocular signs of AML is therefore crucial in expediting diagnosis and subsequent treatment, which may aid in saving patients from this life-threatening disease.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lowenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999;341(14):1051–1062. doi:10.1056/NEJM199909303411407
2. Skarsgård LS, Andersson MK, Persson M, et al. Clinical and genomic features of adult and paediatric acute leukaemias with ophthalmic manifestations. BMJ Open Ophthalmol. 2019;4(1):e000362. doi:10.1136/bmjophth-2019-000362
3. Soman S, Kasturi N, Srinivasan R, Vinod KV. Ocular manifestations in leukemias and their correlation with hematologic parameters at a tertiary care setting in South India. Ophthalmol Retin. 2018;2(1):17–23. doi:10.1016/j.oret.2017.05.009
4. Dhasmana R, Prakash A, Gupta N, Verma SK. Ocular manifestations in leukemia and myeloproliferative disorders and their association with hematological parameters. Ann Afr Med. 2016;15(3):97–103. doi:10.4103/1596-3519.188887
5. Reddy SC, Jackson N, Menon BS. Ocular involvement in leukemia–a study of 288 cases. Ophthalmologica. 2003;217(6):441–445. doi:10.1159/000073077
6. Eze BI, Ibegbulam GO, Ocheni S. Ophthalmic manifestations of leukemia in a tertiary hospital population of adult Nigerian Africans. Middle East Afr J Ophthalmol. 2010;17(4):325–329. doi:10.4103/0974-9233.71599
7. Ohkoshi K, Tsiaras WG. Prognostic importance of ophthalmic manifestations in childhood leukaemia. Br J Ophthalmol. 1992;76(11):651–655. doi:10.1136/bjo.76.11.651
8. Ridgway EW, Jaffe N, Walton DS. Leukemic ophthalmopathy in children. Cancer. 1976;38(4):1744–1749. doi:10.1002/1097-0142(197610)38:4<1744::aid-cncr2820380449>3.0.co;2-3
9. Cavdar AO, Babacan E, Gözdaşoğlu S, et al. High risk subgroup of acute myelomonocytic leukemia (AMML) with orbito-ocular granulocytic sarcoma (OOGS) in Turkish children. Retrospective analysis of clinical, hematological, ultrastructural and therapeutical findings of thirty-three OOGS. Acta Haematol. 1989;81(2):80–85. doi:10.1159/000205531
10. Curto ML, Zingone A, Acquaviva A, et al. Leukemic infiltration of the eye: results of therapy in a retrospective multicentric study. Med Pediatr Oncol. 1989;17(2):134–139. doi:10.1002/mpo.2950170212
11. Bitirgen G, Belviranli S, Caliskan U, Tokgoz H, Ozkagnici A, Zengin N. Ophthalmic manifestations in recently diagnosed childhood leukemia. Eur J Ophthalmol. 2016;26(1):88–91. doi:10.5301/ejo.5000647
12. AlSemari MA, Perrotta M, Russo C, et al. Orbital myeloid sarcoma (chloroma): report of 2 cases and literature review. Am J Ophthalmol Case Rep. 2020;19:100806. doi:10.1016/j.ajoc.2020.100806
13. Qian X, Gigantelli JW, Abromowitch M, Morgan LA, Suh DW. Myeloid sarcoma in the orbit. J Pediatr Ophthalmol Strabismus. 2016;53:e64–e68. doi:10.3928/01913913-20161102-01
14. Maka E, Lukáts O, Tóth J, Fekete S. Orbital tumour as initial manifestation of acute myeloid leukemia: granulocytic sarcoma: case report. Pathol Oncol Res. 2008;14(2):209–211. doi:10.1007/s12253-008-9028-x
15. Watkins LM, Remulla HD, Rubin PA. Orbital granulocytic sarcoma in an elderly patient. Am J Ophthalmol. 1997;123(6):854–856. doi:10.1016/s0002-9394(14)71146-8
16. Panda A, Sudan R, Nainiwal S. Childhood proptosis. The invaluable but overlooked peripheral blood smear. Indian J Ophthalmol. 2002;50(3):247.
17. Cavdar AO, Arcasoy A, Babacan E, Gözdaşoğlu S, Topuz U, Fraumeni JF. Ocular granulocytic sarcoma (chloroma) with acute myelomonocytic leukemia in Turkish children. Cancer. 1978;41(4):1606–1609. doi:10.1002/1097-0142(197804)41:4<1606::aid-cncr2820410451>3.0.co;2-y
18. Stockl FA, Dolmetsch AM, Saornil MA, Font RL, Burnier MN. Orbital granulocytic sarcoma. Br J Ophthalmol. 1997;81(12):1084–1088. doi:10.1136/bjo.81.12.1084
19. Puri G. Granulocytic sarcoma of orbit preceding acute myeloid leukaemia: a case report. Eur J Cancer Care (Engl). 1999;8(2):113–115. doi:10.1046/j.1365-2354.1999.00140.x
20. Swami G, Arya A, Chowdhry B. Acute myeloid leukemia presenting with sudden bilateral proptosis as a sole manifestation. Indian Pediatr. 2001;38(10):1199–1200.
21. Zimmerman LE, Font RL. Ophthalmologic manifestations of granulocytic sarcoma (myeloid sarcoma or chloroma). The third Pan American Association of Ophthalmology and American Journal of Ophthalmology Lecture. Am J Ophthalmol. 1975;80(6):975–990. doi:10.1016/0002-9394(75)90326-8
22. Olson JL, May MJ, Stork L, Kadan N, Bateman JB. Acute megakaryoblastic leukemia in Down syndrome: orbital infiltration. Am J Ophthalmol. 2000;130(1):128–130. doi:10.1016/S0002-9394(00)00459-1
23. Murthy R, Vemuganti GK, Honavar SG, Naik M, Reddy V. Extramedullary leukemia in children presenting with proptosis. J Hematol Oncol. 2009;2:4. doi:10.1186/1756-8722-2-4
24. Bidar M, Wilson MW, Laquis SJ, et al. Clinical and imaging characteristics of orbital leukemic tumors. Ophthal Plast Reconstr Surg. 2007;23(2):87–93. doi:10.1097/IOP.0b013e3180333a85
25. Sahu KK, Malhotra P. Re: “Granulocytic sarcoma of the orbit presenting as a fulminant orbitopathy in an adult with acute myeloid leukemia”. Ophthal Plast Reconstr Surg. 2015;31(5):421. doi:10.1097/IOP.0000000000000523
26. Sun X, Rong X, Nie H, Yan X. Isolated retro-orbital granulocytic sarcoma relapse of Acute Myeloid Leukemia after allogeneic hematopoietic stem cell transplantation: a case report. Eur J Ophthalmol. 2020;1120672120976551. doi:10.1177/1120672120976551
27. Tan L, Hwang W, Looi A. Proptosis in a patient with known graft versus host disease. Ophthal Plast Reconstr Surg. 2019;35(6):e142–e145. doi:10.1097/IOP.0000000000001469
28. Angsubhakorn N, Suvannasankha A. Multifocal myeloid sarcomas: a rare presentation of AML. BMJ Case Rep. 2017;2017. doi:10.1136/bcr-2017-222659
29. Shields JA, Stopyra GA, Marr BP, et al. Bilateral orbital myeloid sarcoma as initial sign of acute myeloid leukemia: case report and review of the literature. Arch Ophthalmol. 2003;121(1):138–142. doi:10.1001/archopht.121.1.138
30. Chen E, Morrison DG, Donahue SP. Acute myeloid leukemia presenting as bilateral proptosis from diffuse extraocular muscle infiltration. Am J Ophthalmol. 2004;137(5):948–950. doi:10.1016/j.ajo.2003.10.050
31. Goldberg L, Tao A, Romano P. Severe exophthalmos secondary to orbital myopathy not due to Graves’s disease. Br J Ophthalmol. 1982;66(6):392–395. doi:10.1136/bjo.66.6.392
32. Consul BN, Kulshrestha OP, Mehrotra AS. Bilateral proptosis in acute myeloid leukaemia. Br J Ophthalmol. 1967;51(1):65–67. doi:10.1136/bjo.51.1.65
33. Wirostko WJ, Garcia GH, Cory S, Harris GJ. Acute dacryocystitis as a presenting sign of pediatric leukemia. Am J Ophthalmol. 1999;127(6):734–736. doi:10.1016/S0002-9394(99)00023-9
34. Yaghouti F, Nouri M, Mannor GE. Ocular adnexal granulocytic sarcoma as the first sign of acute myelogenous leukemia relapse. Am J Ophthalmol. 1999;127(3):361–363. doi:10.1016/s0002-9394(98)00363-8
35. Baldwin S, Mian A. More than meets the eye: a presentation of extramedullary infiltration (AML-M7). Clin Pediatr (Phila). 2010;49(10):986–988. doi:10.1177/0009922810364661
36. Avni B, Koren-Michowitz M. Myeloid sarcoma: current approach and therapeutic options. Ther Adv Hematol. 2011;2(5):309–316. doi:10.1177/2040620711410774
37. Fleckenstein K, Geinitz H, Grosu A, Goetze K, Werner M, Molls M. Irradiation for conjunctival granulocytic sarcoma. Strahlentherapie und Onkol. 2003;179(3):187–190. doi:10.1007/s00066-003-1002-7
38. Douglas RS, Goldstein SM, Nichols C. Acute myelogenous leukaemia presenting as a conjunctival lesion and red eye. Acta Ophthalmol Scand. 2002;80(6):671–672. doi:10.1034/j.1600-0420.2002.800623_1.x
39. Nau JA, Shields CL, Shields JA, Eagle RC, Rice E. Clinicopathologic reports, case reports, and small case series: acute myeloid leukemia manifesting initially as a conjunctival mass in a patient with acquired immunodeficiency syndrome. Arch Ophthalmol. 2002;120(12):1741–1742. doi:10.1001/archopht.120.12.1741
40. Lee DA, Su WP. Acute myelomonocytic leukemia cutis presenting as a conjunctival lesion. Int J Dermatol. 1985;24(6):369–370. doi:10.1111/j.1365-4362.1985.tb05803.x
41. Meel R, Desai A, Gaur N, Bakhshi S. Myeloid sarcoma presenting as an unusual limbal mass. BMJ Case Rep. 2019;12(1):
42. Hong ES, Longmuir SQ, Goins KM. Ocular myeloid sarcoma in a 10-year-old child. J Am Assoc Pediatr Ophthalmol Strabismus. 2011;15(5):504–505. doi:10.1016/j.jaapos.2011.06.003
43. Mozaheb Z, Khooei A. Bilateral conjunctival infiltration as an extramedullary relapse of AML. Case Rep Hematol. 2018;2018:1–3. doi:10.1155/2018/9590469
44. Hon C, Shek TW, Liang R. Conjunctival chloroma (granulocytic sarcoma). Lancet. 2002;359(9325):2247. doi:10.1016/S0140-6736(02)09294-2
45. Font RL, Mackay B, Tang R. Acute monocytic leukemia recurring as bilateral perilimbal infiltrates. Ophthalmology. 1985;92(12):1681–1685. doi:10.1016/S0161-6420(85)34091-5
46. Mansour AM, Traboulsi EI, Frangieh GT, Jarudi N. Caruncular involvement in myelomonocytic leukemia: a case report. Med Pediatr Oncol. 1985;13(1):46–47. doi:10.1002/mpo.2950130111
47. Tsumura T, Sakaguchi M, Shiotani N, Sugita A, Nakahara T. A case of acute myelomonocytic leukemia with subconjunctival tumor. Jpn J Ophthalmol. 1991;35(2):226–231.
48. Park JH, Son Y, Hyon JY, Lee JY, Jeon HS. Relapsed acute myeloid leukemia presenting as conjunctival myeloid sarcoma: a case report. BMC Ophthalmol. 2022;22(1):65. doi:10.1186/s12886-022-02286-1
49. Rosenberg C, Finger PT, Furlan L, Iacob CE. Bilateral epibulbar granulocytic sarcomas: a case of an 8-year-old girl with acute myeloid leukaemia. Graefe’s Arch Clin Exp Ophthalmol. 2006;245(1):170–172. doi:10.1007/s00417-006-0341-3
50. Ohanian M, Borthakur G, Quintas-Cardama A, et al. Ocular granulocytic sarcoma: a case report and literature review of ocular extramedullary acute myeloid leukemia. Clin Lymphoma Myeloma Leuk. 2013;13(1):93–96. doi:10.1016/j.clml.2012.07.008
51. Kougou Ntoutoume AR, Madbouhi K, Mekyna S, Matsanga RO, Tachfouti S, Cherkaoui O. Ecchymoses en lunettes, hémorragies sous-conjonctivales et rétiniennes bilatérales consécutives à une manœuvre de Valsalva et révélant une leucémie myéloïde aiguë. J Fr Ophtalmol. 2020;43(10):1117–1119. doi:10.1016/j.jfo.2019.12.028
52. Sykakis E, Patwary SN. Acute myeloid leukaemia presenting as gaze palsy. Case Rep Ophthalmol. 2011;2(3):343–346. doi:10.1159/000334272
53. Gong J, Li J, Liang H. Extramedullary relapse presenting as trigeminal neuralgia and diplopia after allogeneic hematopoietic stem cell transplantation. Intern Med. 2011;50(10):1117–1119. doi:10.2169/internalmedicine.50.4909
54. Hsu W-H, Chu S-J, Tsai W-C, Tsao Y-T. Acute myeloid leukemia presenting as one-and-a-half syndrome. Am J Emerg Med. 2008;26(4):
55. Fozza C, Dore F, Isoni MA, et al. Strabismus and diplopia in a patient with acute myeloid leukemia. Am J Case Rep. 2014;15:288–290. doi:10.12659/AJCR.890526
56. Lucas J, Bathini A, Greenberg K. Acute myeloid leukemia presenting as horizontal diplopia. Am J Emerg Med. 2021;49:
57. Al-Mujaini AS, Al-Dhuhli HH, Dennison DJ. Acute unilateral third nerve palsy as an early manifestation of central nervous system relapse in a patient with acute myeloid leukemia. Saudi Med J. 2009;30(7):961–963.
58. Simpson TA, Anderson ML, Garcia JH, Barton JC. Myeloblastoma of the brain. Acta Neuropathol. 1989;78(4):444–447. doi:10.1007/BF00688182
59. Imrie KR, Kovacs MJ, Selby D, et al. Isolated chloroma: the effect of early antileukemic therapy. Ann Intern Med. 1995;123(5):351–353. doi:10.7326/0003-4819-123-5-199509010-00005
60. Vishwanath MR, Charles SJ. Tonic pupil as the presenting sign of relapsed acute myeloid leukemia. J Neuroophthalmol. 2007;27(4):310–311. doi:10.1097/WNO.0b013e31815b9a7f
61. Allen RA, Straatsma BR. Ocular involvement in leukemia and allied disorders. Arch Ophthalmol. 1961;66:490–508. doi:10.1001/archopht.1961.00960010492010
62. Lin Y-C, Wang A-G, Yen M-Y, Hsu W-M. Leukaemic infiltration of the optic nerve as the initial manifestation of leukaemic relapse. Eye. 2004;18(5):546–550. doi:10.1038/sj.eye.6700701
63. Patel SA. Acute myeloid leukemia relapse presenting as complete monocular vision loss due to optic nerve involvement. Case Rep Hematol. 2016;2016:3794284. doi:10.1155/2016/3794284
64. Patel N, RIch BJ, Patel S, et al. Emergent radiotherapy for leukemia-induced cranial neuropathies refractory to intrathecal therapy. Cureus. 2021;13(5):e15212. doi:10.7759/cureus.15212
65. Tabbara KF, Beckstead JH. Acute promonocytic leukemia with ocular involvement. Arch Ophthalmol. 1980;98(6):1055–1058. doi:10.1001/archopht.1980.01020031045006
66. Ells A, Clarke WN, Noel LP. Pseudohypopyon in acute myelogeneous leukemia. J Pediatr Ophthalmol Strabismus. 1995;32(2):123–124. doi:10.3928/0191-3913-19950301-15
67. Scoville NM, Feng S, Taylor MR, et al. Bilateral pseudo-hypopyon as presenting symptom of acute monocytic leukemia in an 8-month-old infant. J Pediatr Ophthalmol Strabismus. 2021;58(5):e30–e33. doi:10.3928/01913913-20210708-05
68. Ayliffe W, Foster CS, Marcoux P, et al. Relapsing acute myeloid leukemia manifesting as hypopyon uveitis. Am J Ophthalmol. 1995;119(3):361–364. doi:10.1016/S0002-9394(14)71181-X
69. Birnbaum AD, Tessler HH, Goldstein DA. A case of hypopyon uveitis nonresponsive to steroid therapy and a review of anterior segment masquerade syndromes in childhood. J Pediatr Ophthalmol Strabismus. 2005;42(6):372–377. doi:10.3928/01913913-20051101-08
70. Touitou V, Bodaghi B, Thepot S, et al. When the eye gives it all: diagnosis of relapsing acute myeloblastic leukemia with anterior chamber tap of a chronic hypopyon. Am J Hematol. 2014;89(8):858–859. doi:10.1002/ajh.23693
71. Matano S, Ohta T, Nakamura S, Kanno M, Sugimoto T. Leukemic hypopyon in acute myelogenous leukemia. Ann Hematol. 2000;79(8):455–458. doi:10.1007/s002770000164
72. Kulbacki E, Schneider E, Wang E. “Hypopyon” in the anterior chamber: unilateral ocular relapse of acute myeloid leukaemia in a 2-year-old girl. Br J Haematol. 2013;162(3):293. doi:10.1111/bjh.12418
73. Arbuthnot CD, Bradbury M, Darbyshire PJ. Relapsing acute myeloid leukaemia manifesting as uveitis with hypopyon. Br J Haematol. 2007;136(4):520. doi:10.1111/j.1365-2141.2006.06380.x
74. Karesh JW, Goldman EJ, Reck K, Kelman SE, Lee EJ, Schiffer CA. A prospective ophthalmic evaluation of patients with acute myeloid leukemia: correlation of ocular and hematologic findings. J Clin Oncol. 1989;7(10):1528–1532. doi:10.1200/JCO.1989.7.10.1528
75. Hegde SP, Ursekar AT, Chitale AA. Relapsing acute myeloid leukemia presenting as hypopyon uveitis. Indian J Ophthalmol. 2011;59(5):391–393. doi:10.4103/0301-4738.83621
76. Alten F, Ehlert K, Böhm MR, Grenzebach UH. Leukemic hypopyon in acute myeloid leukemia. Eur J Ophthalmol. 2013;23(2):252–254. doi:10.5301/ejo.5000177
77. Kurup SK, Coleman H, Chan -C-C. Corneal pseudomembrane from acute inflammatory response and fibrin formation to acute myeloid leukemic infiltrate. Am J Ophthalmol. 2005;139(5):921–923. doi:10.1016/j.ajo.2004.10.049
78. Wood WJ, Nicholson DH. Corneal ring ulcer as the presenting manifestation of acute monocytic leukemia. Am J Ophthalmol. 1973;76(1):69–72. doi:10.1016/0002-9394(73)90013-5
79. Bhadresa GN. Changes in the anterior segment as a presenting feature in leukaemia. Br J Ophthalmol. 1971;55(2):133–135. doi:10.1136/bjo.55.2.133
80. Perry HD, Mallen FJ. Iris involvement in granulocytic sarcoma. Am J Ophthalmol. 1979;87(4):530–532. doi:10.1016/0002-9394(79)90243-5
81. Sun N, Shao Y, Zheng Y, Zhang X. Uveal infiltration in an acute myeloid leukemia case. Ocul Immunol Inflamm. 2020;1–4. doi:10.1080/09273948.2020.1802487
82. Khaja WA, Pogrebniak AE, Bolling JP. Combined orbital proptosis and exudative retinal detachment as initial manifestations of acute myeloid leukemia. J AAPOS off Publ Am Assoc Pediatr Ophthalmol Strabismus. 2015;19(5):479–482. doi:10.1016/j.jaapos.2015.05.018
83. Stewart Duke-Elder CC. New book. In: New Book. 1964:425.
84. Green W, Rao PK, Harocopos GJ. Extramedullary relapse of acute myelogenous leukemia presenting as a large serous retinal detachment. Ocul Oncol Pathol. 2017;3(2):95–100. doi:10.1159/000450858
85. Schachat AP, Markowitz JA, Guyer DR, Burke PJ, Karp JE, Graham ML. Ophthalmic manifestations of leukemia. Arch Ophthalmol. 1989;107(5):697–700. doi:10.1001/archopht.1989.01070010715033
86. Tseng M-Y, Chen Y-C, Lin -Y-Y, Chu S-J, Tsai S-H. Simultaneous bilateral central retinal vein occlusion as the initial presentation of acute myeloid leukemia. Am J Med Sci. 2010;339(4):387–389. doi:10.1097/MAJ.0b013e3181cf31ac
87. Kassam F, Gale JS, Sheidow TG. Intraocular leukemia as the primary manifestation of relapsing acute myelogenous leukemia. Can J Ophthalmol. 2003;38(7):613–616. doi:10.1016/s0008-4182(03)80120-5
88. Wechsler DZ, Tay TS, McKay DL. Life-threatening haematological disorders presenting with opthalmic manifestations. Clin Experiment Ophthalmol. 2004;32(5):547–550. doi:10.1111/j.1442-9071.2004.00878.x
89. Chandra A, Chakraborty U, Ganai S, Ray AK. Roth spots in acute myeloid leukaemia. BMJ Case Rep. 2020;13(9):e238133. doi:10.1136/bcr-2020-238133
90. Zhuang I, Gupta I, Weng CY. Retinal hemorrhages in a patient with petechiae. JAMA Ophthalmol. 2019;137(4):459–460. doi:10.1001/jamaophthalmol.2018.6220
91. Sharma H, Majumder PD, Rao C, Biswas J. A case of acute myeloid leukemia masquerading as unilateral exudative detachment. Am J Ophthalmol Case Rep. 2016;4:47–49. doi:10.1016/j.ajoc.2016.08.004
92. Balubaid MM, Alqahtani AS. A case of acute promyelocytic leukemia with retinal hemorrhages beneath internal limiting membrane during clinical remission. Cureus. 2021;13(2):e13387. doi:10.7759/cureus.13387
93. Yang X, Xu J, Yang J, et al. Unilateral macular choroidal neovascularization-a rare manifestation in acute myelocytic leukemia: case report. Medicine. 2018;97(16):e0344. doi:10.1097/MD.0000000000010344
94. Reddy SC, Jackson N. Retinopathy in acute leukaemia at initial diagnosis: correlation of fundus lesions and haematological parameters. Acta Ophthalmol Scand. 2004;82(1):81–85. doi:10.1046/j.1600-0420.2003.00197.x
95. Khair D, Mehanna C-J, Ghannam AB, Kheir WJ. Bilateral retinal artery occlusions as the first manifestation of extramedullary central nervous system involvement in relapsed acute myeloid leukaemia. BMJ Case Rep. 2021;14(4):e239795. doi:10.1136/bcr-2020-239795
96. Thill M, Schwartz R, Fiedler W, Linke S. Bilateral retinal pigment epithelial detachment as the presenting symptom of acute myeloid leukaemia. Eye. 2006;20(7):851–852. doi:10.1038/sj.eye.6702025
97. Naseripour M, Abdolalizadeh P, Abdi F, Mehrvar A, Tashvighi M. Serous retinal detachment as an initial presentation of childhood acute myeloid leukemia. Can J Ophthalmol. 2019;54(4):e170–e173. doi:10.1016/j.jcjo.2018.10.008
98. Patel AV, Miller JB, Nath R, et al. Unilateral eye findings: a rare herald of acute leukemia. Ocul Oncol Pathol. 2016;2(3):166–170. doi:10.1159/000442951
99. Wu L, Calderón M, Hernández G, Marbis J, Ramírez V. Bilateral exudative retinal detachment as the first sign of relapsing acute myelogenous leukaemia. Clin Experiment Ophthalmol. 2006;34(6):623–625. doi:10.1111/j.1442-9071.2006.01291.x
100. Tumuluri K, Woo T, Crowston J, Healey PR, Gottlieb D, Maloof AJ. Bilateral leukemic orbital infiltration presenting as proptosis and narrow-angle glaucoma. Ophthal Plast Reconstr Surg. 2004;20(3):248–250. doi:10.1097/01.iop.0000129018.17256.38
101. Uozumi K, Takatsuka Y, Ohno N, et al. Isolated choroidal leukemic infiltration during complete remission. Am J Hematol. 1997;55(3):164–165.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.