Back to Journals » Therapeutics and Clinical Risk Management » Volume 11
Early myocardial damage assessment in dystrophinopathies using 99Tcm-MIBI gated myocardial perfusion imaging
Authors Zhang L, Liu Z, Hu K, Tian Q, Wei L, Zhao Z, Shen H, Hu J
Received 5 June 2015
Accepted for publication 31 August 2015
Published 10 December 2015 Volume 2015:11 Pages 1819—1827
DOI https://doi.org/10.2147/TCRM.S89962
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Deyun Wang
Li Zhang,1,* Zhe Liu,2,* Ke-You Hu,3 Qing-Bao Tian,3 Ling-Ge Wei,4 Zhe Zhao,5 Hong-Rui Shen,5 Jing Hu5
1Department of Cardiovascular Disorders, 2Department of Geriatrics, The Third Hospital of Hebei Medical University, 3The Public Health Department, Hebei Medical University, 4Department of Nuclear Medicine, 5Department of Neuromuscular Disorders, The Third Hospital of Hebei Medical University, Shijiazhuang, People’s Republic of China
*Li Zhang and Zhe Liu are first coauthors of this paper
Background: Early detection of muscular dystrophy (MD)-associated cardiomyopathy is important because early medical treatment may slow cardiac remodeling and attenuate symptoms of cardiac dysfunction; however, no sensitive and standard diagnostic method for MD at an earlier stage has been well-recognized. Thus, the aim of this study was to test the early diagnostic value of technetium 99m-methoxyisobutylisonitrile (99Tcm-MIBI) gated myocardial perfusion imaging (G-MPI) for MD.
Methods and results: Ninety-one patients underwent 99Tcm-MIBI G-MPI examinations when they were diagnosed with Duchenne muscular dystrophy (DMD) (n=77) or Becker muscular dystrophy (BMD; n=14). 99Tcm-MIBI G-MPI examinations were repeated in 43 DMD patients who received steroid treatments for 2 years as a follow-up examination. Myocardial defects were observed in nearly every segment of the left ventricular wall in both DMD and BMD patients compared with controls, especially in the inferior walls and the apices by using 99Tcm-MIBI G-MPI. Cardiac wall movement impairment significantly correlated with age in the DMD and BMD groups (rs=0.534 [P<0.05] and rs=0.784 [P<0.05], respectively). Intermittent intravenous doses of glucocorticoids and continuation with oral steroid treatments significantly improved myocardial function in DMD patients (P<0.05), but not in BMD patients.
Conclusion: 99Tcm-MIBI G-MPI is a sensitive and safe approach for early evaluation of cardiomyopathy in patients with DMD or BMD, and can serve as a candidate method for the evaluation of progression, prognosis, and assessment of the effect of glucocorticoid treatment in these patients.
Keywords: Duchenne muscular dystrophy, Becker muscular dystrophy, dystrophinopathy, 99Tcm-MIBI, cardiomyopathy
Introduction
Muscular dystrophy (MD) is a spectrum of muscle diseases caused by a mutation in the gene coding the protein, dystrophin,1 among which Duchenne muscular dystrophy (DMD) and Becker muscular dystrophy (BMD) are the most common types. DMD patients have a completely inactive dystrophin protein and BMD patients have reduced expression of dystrophin, which accounts for the differences between the two types of MD.2 Patients with DMD and BMD have progressive muscle weakness and muscle atrophy, which usually appears first in calf skeletal muscles and gradually extends to the shoulder girdle muscles, smooth muscles, and cardiac muscles.3 Respiratory failure and/or heart failure are major causes of death for these patients.4 Although skin biopsies, DNA testing, and electromyography are well-recognized tests for diagnosing MD, a standard method in the early detection of the pathophysiologic changes in cardiac muscle does not exist.5,6
Several cardiac imaging techniques have been used in clinics for the assessment of myocardial damage in MD patients, including echocardiography, cardiac magnetic resonance imaging (MRI), and nuclear medicine methods. Echocardiography is the most commonly used method, but has poor imaging windows due to scoliosis, lung hyperinflation, and chest wall deformities, which are commonly present in MD patients. In contrast, cardiac MRI has high resolution and sensitivity; however, such examinations take a relatively longer time than echocardiography, which is less tolerated by children, who are the major segment of the population afflicted by MD. Nuclear medicine, the third option in MD diagnosis, has become the major method in evaluating cardiac function by researchers. Thallium-201 chloride-single-photon emission tomography perfusion imaging and technetium 99m-methoxyisobutylisonitrile (99Tcm-MIBI) gated myocardial perfusion imaging (G-MPI) have been reported as valuable methods for evaluating myocardial abnormalities in patients with DMD7–9 because the combination of perfusion and functional information from these methods can greatly improve the accuracy for detecting myocardial dysfunction.9,10 The value of nuclear medicine in detecting early cardiac damage in MD patients and the value in assessment of prognosis has yet to be studied. Thus, this study was designed to evaluate the value of 99Tcm-MIBI G-MPI in the early diagnosis of MD and use in treatment follow-up and prognosis.
Methods
Patients and diagnosis
DMD and BMD patients and control subjects were recruited from the Neuromuscular Disorders Department of the Third Hospital of Hebei Medical University (Hebei Province, People’s Republic of China). Control subjects were patients with other neuromuscular disorders that received medical service from the Third Hospital of Hebei Medical University, which were age- and sex-matched with DMD and BMD patients. DMD and BMD diagnoses were excluded in these subjects based on histology reports from tissue biopsies, medical history, and clinical diagnosis according to the diagnostic criteria of DMD and BMD stated in this study. Confirmative diagnoses of DMD and BMD in this study included a combination of evidence of clinical characteristics, creatine kinase level, and skin biopsies. The antibodies used in immunofluorescence staining were anti-dystrophin (-R, -C, and -N) monoclonal antibodies (dilution ratio =1:50; Novocastra, Newcastle upon Tyne, UK) and anti-sarcoglycan (−α, −β, −γ, and −δ) monoclonal antibodies (dilution ratio =1:50; Novocastra). Patients with complete inactivated dystrophin on sarcolemma were diagnosed as DMD and patients with partial deficiency in dystrophin protein expression were diagnosed as BMD. The exclusion criteria included the presence of valvular heart disease, left ventricular hypertrophy, or other systemic diseases, in addition to MD. This study was approved by the Third Hospital of Hebei Medical University Ethics Committee, and all DMD and BMD patients and controls signed informed consent forms. The experiments conform to the principles outlined in the Declaration of Helsinki.
99Tcm-MIBI G-MPI and echocardiography data of 77 DMD children (aged 2–13 years) and 14 BMD patients (aged 5–25 years) were obtained. Twenty-eight and 12 age-matched patients served as controls for DMD (control group 1) and BMD (control group 2), respectively. All the subjects recruited in this study were from the neuromuscular database of the Third Hospital of Hebei Medical University between May 2008 and October 2012. Seventy DMD patients received glucocorticoid treatment after the diagnosis for 2 years and 99Tcm-MIBI G-MPI was performed again as a follow-up. The follow-up study was not done in BMD patients because glucocorticoid treatment is not the standard for this population.
99Tcm-MIBI imaging was acquired 1.5 hours after 99Tcm-MIBI injection. The dose was between 148 and 740 MBq, based on the following calculation: children 99Tcm-MIBI dose = weight (kg)/70× dose for adult.11 The imaging examination was performed using a dual-detector SPECT imaging system (Infinia Hawkeye 4, GE Healthcare, Piscataway, NJ, USA) with low-energy high-resolution collimators. The left ventricular ejection fraction (LVEF), end-diastolic volume (EDV), and end-systolic volume (ESV) were calculated using commercial software packages (Quantitative Gated SPECT, GE Healthcare). Briefly, EDV and ESV were standardized using the patient’s own body weight.12 The SPECT and polar maps were blindly analyzed by two experienced nuclear medicine physicians.
Data collection and analysis
The range of the myocardial abnormalities was analyzed by seven different regions of the cardiac walls, including the apex, inferior wall, anterior wall, anterolateral wall, inferior-lateral wall, anterior-septal wall, and inferior-septal wall. The degree of the myocardium abnormalities was analyzed by 17 segments based on the polar maps.13 Basal, mid-cavity, and apical segments, as part of the actual definition of the location of the abnormality along the long axis of the ventricle, were analyzed from the apex to base. The circumferential locations in the basal and mid-cavity were anterior, anterior-septal, inferior-septal, inferior, inferior-lateral, and anterior-lateral. The remaining segments include apical anterior, apical septal, apical inferior, and apical lateral.
The summed rest score (SRS) was used for the perfusion scores and the analysis of a 17-segment model of the left ventricle with a 5-point scoring system for perfusion defect severity as follows: normal uptake of 99Tcm-MIBI was scored as 0; slightly and moderately decreased uptake was scored as 1 and 2, respectively; severely decreased uptake was scored as 3; and absence of uptake was scored as 4.14 If there was a disagreement regarding the score of each patient between the two physicians, a final consensus was reached after discussion.
Statistical analysis
SPSS Version 13.0 statistical software (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses in this study. Data that are normally distributed were presented as the mean ± standard deviation, while data without a normal distribution were shown as the median and quartile range. Spearman’s rank correlation coefficient or Pearson’s correlation coefficient was used for correlation analysis. The Kruskal–Wallis test, Friedman test, independent samples t-test, and nonparametric test were used to compare the mean or median between groups. A P<0.05 was defined as significantly different.
Results
Pathologic diagnosis and cardiac function between patients with DMD and BMD and control groups
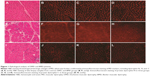
To diagnose DMD and BMD, all patients received skeletal muscle biopsies and representative images from the DMD, BMD, and control groups as presented in Figure 1. The pathologic changes suggested a phenotypic change in MD. H&E staining revealed normal morphology in controls (Figure 1A). Hematoxylin and eosin (H&E) staining from DMD patients showed muscle fiber diameters of varying sizes with a large number of muscle fibers undergoing degeneration, necrosis, and regeneration (Figure 1E). In addition, there was marked hyperplasia of connective tissues. H&E staining from BMD patients showed scattered muscle fiber degeneration, necrosis, regeneration, and connective tissue proliferation (Figure 1I).
Immunofluorescence staining against different terminal domains of dystrophin, including dystrophin-N, dystrophin-C, and dystrophin-R, showed negative muscle fiber membrane staining in DMD and decreased density of staining in muscle fiber membranes in BMD (Figure 1F–H and J–L). The dystrophin staining was normal in controls (Figure 1B–D).
There was no difference in age between DMD or BMD patients and controls after the age-matched strategy in control recruitment (P=0.81 for DMD vs control and P=0.962 for BMD vs control; Table 1). There was a significantly decreased cardiac perfusion score, as reflected by SRS between DMD or BMD patients and control groups (P=0.016 for DMD patients vs control group 1 and P=0.015 for BMD vs control group 2, respectively), indicating a cardiac perfusion deficiency in patients with MD. Decreased SRS was also independently associated with BMD and DMD after adjusting for factors, including age and LVEF from echocardiography (P<0.05); however, there was no difference in parameters for cardiac function, including LVEF, EDV index (EDVI), and ESV index (ESVI), using the Quantitative Gated SPECT method or echocardiography (all P>0.05).
Cardiac function and cardiac perfusion analysis of DMD and BMD patients by 99Tcm-MIBI G-MPI
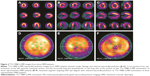
We analyzed the cardiac damage and cardiac functional changes in DMD and BMD patients using 99Tcm-MIBI G-MPI. Seven different regions of the cardiac walls, including the apex, inferior wall, anterior wall, anterolateral wall, inferior-lateral wall, anterior-septal wall, and inferior-septal wall, were analyzed. 99Tcm-MIBI G-MPI showed that there were 74 of 77 DMD patients (96.1%) who had cardiac damage with detailed regions involved in the cardiac wall, as summarized in Table 2. Twelve of 14 BMD patients (85.7%) had cardiac damage based on 99Tcm-MIBI G-MPI (Table 2). No cardiac wall abnormality on 99Tcm-MIBI G-MPI was observed in control groups (data not shown). The seven regional walls of 99Tcm-MIBI G-MPI revealed cardiac abnormalities in every wall of the left ventricle in DMD, and was particularly common in the apex and inferior wall of the left ventricle (Figure 2A–C). Myocardial perfusion of the overall left ventricle, left ventricular inferior wall, and apex was greatly reduced in DMD patients (Figure 2A–E for the inferior wall and Figure 2C and F for the apex). A semiquantitative method of 17 segments of G-MPI revealed the presence of cardiac involvement in every segment, and we observed a significant difference among segments in DMD (P<0.01). The most severe cardiac impairment was observed at the apical, apical inferior, and apical anterior areas in DMD patients (significance score: α’ = α/55=0.05/55=0.0009; Figure 2D–F).
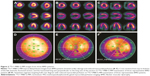
Although the overall left ventricle perfusion was normal in patients with BMD, similar cardiac damage patterns were also observed in the left ventricular inferior wall and apex in patients with BMD (Figure 3A–C). The most severe damage in BMD was located in the mid-inferior and apical inferior areas in these patients (significance score: α’ = α/55=0.05/55=0.0009; Figure 3D–F).
Effect of hormone therapy on DMD patients evaluated using 99Tcm-MIBI G-MPI
Seventy DMD patients received glucocorticoid treatment for 2 years after the initial diagnosis by skin biopsy in this study. A follow-up 99Tcm-MIBI G-MPI imaging examination for cardiac damage was performed and compared between DMD patients with or without hormone therapy. Due to the limited number for each age group, we selected the 7- to 10-year-old group (n=10–12) for this analysis, which is summarized in Table 3. Cardiac function was greatly protected in each age group after 2 years of hormone treatment compared with the baseline level without hormone therapy (all P<0.05; Table 3); however, there was no significant difference in LVEF, EDVI, and ESVI in these DMD patients with and without hormone therapy (all P>0.05; Table 3).
Correlation between SRS and age in DMD and BMD patients
SRS was positively correlated with age in DMD and BMD patients (rs=0.534 [P<0.05] for DMD and rs=0.784 [P<0.05] for BMD; Figure 4A and B). In contrast, the LVEF of DMD and BMD patients was negatively associated with age (rs=−0.627 [P<0.05] for DMD and rs=−0.831 [P<0.05] for BMD; Figure 4C and D). EDVI or ESVI was positively correlated with age (rs=0.396, P<0.05 for EDVI and rs=0.479, P<0.05 for ESVI, respectively) in DMD patients (Figure 4E and F). There was no correlation between EDVI/ESVI and age (data not shown).
Discussion
DMD and BMD are MD disorders due to a mutation of the DMD gene, leading to either inactivation or partial dysfunction of dystrophin protein. Such a defect of dystrophin results in skeletal and cardiac muscle degeneration and necrosis. Patients usually show impaired mobility during the early stage of the disease. As the disease progresses, exercise capacity gradually diminishes, muscles atrophy becomes severe, and patients develop heart and respiratory failure, which are the leading causes of death for MD patients.1,3 Because patients do not develop significant cardiac dysfunction-related symptoms during the early stage, dilated cardiomyopathy, life-threatening arrhythmias, and sudden cardiac death occur frequently due to the delayed detection and earlier intervention in DMD and BMD patients.1,5,6 Therefore, early evaluation and treatment of cardiomyopathy in MD patients are important in an effort to improve the overall survival rate in these patients. In the current study, we evaluated the value of using 99Tcm-MIBI G-MPI for cardiac perfusion and cardiac damage assessment in patients with DMD or BMD as an earlier diagnostic method.
We found that earlier changes in myocardial perfusion deficiency could be detected in DMD and BMD patients using 99Tcm-MIBI G-MPI when echocardiography parameters, including LVEF, EDVI, and ESVI, were not sensitive to detect myocardial dysfunction, clearly suggesting an earlier detection advantage of the 99Tcm-MIBI G-MPI examination compared with traditional imaging methods. Our results are not only consistent with another report involving DMD patients by Fu et al,9 but has also provided evidence of applying 99Tcm-MIBI G-MPI in BMD patients for early cardiac perfusion deficiency for the first time.
Skeletal MD increases with age,15,16 and cardiac decompensation may ultimately lead to dilated cardiomyopathy during the late stage of DMD and BMD. Nigro et al17 showed that <10% of patients with cardiac dysfunction were younger than 14 years of age, while 80% of these patients developed cardiac decompensation after 4 years of age. Using 99Tcm-MIBI G-MPI, we found that children with DMD <13 years of age had a defect in cardiac function judged by EDVI and ESVI. The positive correlation between SRS and aging and the negative correlation between LVEF and aging in our BMD and DMD patients served as additional supporting evidence that was consistent with the finding using 99Tcm-labeled human serum albumin radionuclide ventriculography and cardiac MRI studies, which showed a positive correlation;18,19 however, we did not find a correlation between EDVI/ESVI and age in BMD patients. We considered this negative result to be due to an insufficient sample size in our BMD population (n=14).
Dystrophin-deficient myocardium is more vulnerable to pressure overload in vivo than normal myocardium.20–22 The inferior wall of the left ventricle should be the most vulnerable site for myocardial perfusion deficiency and cardiac damage because wall stress in this location is greater than other parts of the left ventricle.23 In addition, the apex of the left ventricle is the second vulnerable site for cardiac damage because it contains the most abundant amount of myocardial cells, which is rich in dystrophin protein.24,25 Although 99Tcm-MIBI G-MPI images revealed the presence of cardiac damage in almost every left ventricular wall and segment of the left ventricle in this study, the most severe damage was present in the interior wall and apex. Less severe, but notable cardiac damage also existed in apical, apical inferior, and apical anterior areas in DMD patients and in the mid-inferior and apical inferior areas in BMD patients. In the current study, we found a higher detection rate of heart failure in BMD patients (21.4%) than DMD patients (1.3%), suggesting a potential value of 99Tcm-MIBI G-MPI in the early detection of cardiac dysfunction in BMDs, which had rarely been reported previously.17,26 The 99Tcm-MIBI examination has been widely used in other cardiovascular diseases, including coronary artery disease.27 Ciccone et al27 used 99Tcm-MIBI in myocardial stress-rest testing in parallel with echocardiography and confirmed the value of the former in diagnosing myocardial function. Therefore, with the maturation of 99Tcm-MIBI technology, the application of such examinations in less common diseases (DMD and BMD) is practical and promising based on its application in other common diseases. Several studies have shown that glucocorticoid therapy can alleviate DMD progression.28,29 Therefore, we also did a follow-up study using 99Tcm-MIBI G-MPI to evaluate the effect of early intervention with glucocorticoid therapy in preventing cardiac damage. We found a significant improvement in myocardial perfusion in the hormone therapy group compared with the untreated group or compared with the baseline level without hormone therapy. It has been reported that low-dose hormone treatment could increase muscle cell regeneration, induce muscle formation, and improve motor function.30,31 Thus, early intervention with hormone therapy could have a beneficial effect for patients with DMD. In addition, intravenous glucocorticoid treatment (0.75 mg/kg/d) can reduce ventricular load, slow progression of cardiomyopathy, and postpone ventricular dysfunction.31 We also found that 99Tcm-MIBI G-MPI is a stable technique with highly reproducible features, easier postprocedure evaluation, and can provide dynamic observation of the cardiac pathogenesis progression;28,29,32 however, the long-term effect of glucocorticoids on cardiac structure and function needs further investigation.
There were several limitations in this study that should be mentioned. First, this was a single-center, cross-sectional, small-scale clinical study. Although the power calculation showed that a sufficient sample size was included in this study to obtain a statistically convincing result, a large-scale study further investigating the early diagnostic value of 99Tcm-MIBI G-MPI is needed to verify the findings of the current study. Second, although we have shown that cardiac perfusion deficiency change on 99Tcm-MIBI G-MPI manifested earlier than cardiac dysfunction, as shown by standard examination echocardiography, we were not able to distinguish how early in the development of DMD and BMD the 99Tcm-MIBI G-MPI would exhibit an advantage versus echocardiography. When to suggest a 99Tcm-MIBI G-MPI examination for DMD and BMD patients and what are the benefits from early diagnosis remain to be addressed in a further study.
Conclusion
99Tcm-MIBI G-MPI in the assessment of myocardial damage is a suitable method for early evaluation of cardiomyopathy in patients with DMD and BMD, which was first reported in the latter population. 99Tcm-MIBI G-MPI could also serve as a method for evaluating the progression and prognosis of dystrophinopathy.
Acknowledgments
The authors would like to thank Drs Qingbao Tian, Lingge We, and Zhe Zhao for critical comments and valuable suggestions. This work was supported by the grant from the Third Hospital of Hebei Medical University and the Public Health Department of Hebei Medical University.
Disclosure
The authors report no conflicts of interest in this work.
References
Emery AE. The muscular dystrophies. Lancet. 2002;359:687–695. | ||
Jennekens FG, ten Kate LP, de Visser M, Wintzen AR. Diagnostic criteria for Duchenne and Becker muscular dystrophy and myotonic dystrophy. Neuromuscul Disord. 1991;1:389–391. | ||
Zatz M, Rapaport D, Vainzof M, et al. Serum creatine-kinase (CK) and pyruvate-kinase (PK) activities in Duchenne (DMD) as compared with Becker (BMD) muscular dystrophy. J Neurol Sci. 1991;102:190–196. | ||
Verhaert D, Richards K, Rafael-Fortney JA, Raman SV. Cardiac involvement in patients with muscular dystrophies: magnetic resonance imaging phenotype and genotypic considerations. Circ Cardiovasc Imaging. 2011;4:67–76. | ||
Finsterer J, Stollberger C. The heart in human dystrophinopathies. Cardiology. 2003;99:1–19. | ||
Cox GF, Kunkel LM. Dystrophies and heart disease. Curr Opin Cardiol. 1997;12:329–343. | ||
Naruse H, Miyagi J, Arii T, Ohyanagi M, Iwasaki T, Jinnai K. The relationship between clinical stage, prognosis and myocardial damage in patients with Duchenne-type muscular dystrophy: five-year follow-up study. Ann Nucl Med. 2004;18:203–208. | ||
Kumita S, Cho K, Nakajo H, et al. Clinical applications of ECG-gated myocardial perfusion SPECT. J Nippon Med Sch. 2006;73:248–257. | ||
Fu P, Hu L, Sinzinger J, Huang J, Liu X. Assessment of cardiac abnormalities in Duchenne’s muscular dystrophy by (99m)Tc-MIBI gated myocardial perfusion imaging. Hell J Nucl Med. 2012;15:114–119. | ||
Sun Y, Ma P, Bax JJ, et al. 99mTc-MIBI myocardial perfusion imaging in myocarditis. Nucl Med Commun. 2003;24:779–783. | ||
Barker S, Lees DM, Wood EG, Corder R. Inhibitory effect of adrenomedullin on basal and tumour necrosis factor alpha-stimulated endothelin-1 synthesis in bovine aortic endothelial cells is independent of cyclic AMP. Biochem Pharmacol. 2002;63:149–156. | ||
Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317:1098. | ||
Cerqueira MD, Weissman NJ, Dilsizian V, et al. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. | ||
Berman DS, Hachamovitch R, Kiat H, et al. Incremental value of prognostic testing in patients with known or suspected ischemic heart disease: a basis for optimal utilization of exercise technetium-99m sestamibi myocardial perfusion single-photon emission computed tomography. J Am Coll Cardiol. 1995;26:639–647. | ||
Romfh A, McNally EM. Cardiac assessment in duchenne and becker muscular dystrophies. Curr Heart Fail Rep. 2010;7:212–218. | ||
Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling – concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000;35:569–582. | ||
Nigro G, Comi LI, Politano L, Bain RJ. The incidence and evolution of cardiomyopathy in Duchenne muscular dystrophy. Int J Cardiol. 1990;26:271–277. | ||
Saito M, Kawai H, Akaike M, Adachi K, Nishida Y, Saito S. Cardiac dysfunction with Becker muscular dystrophy. Am Heart J. 1996;132:642–647. | ||
Nagamachi S, Inoue K, Jinnouchi S, et al. Cardiac involvement of progressive muscular dystrophy (Becker type, Limb-girdle type and Fukuyama type) evaluated by radionuclide method. Ann Nucl Med. 1994;8:71–74. | ||
Lapidos KA, Kakkar R, McNally EM. The dystrophin glycoprotein complex: signaling strength and integrity for the sarcolemma. Circ Res. 2004;94:1023–1031. | ||
Colan SD. Evolving therapeutic strategies for dystrophinopathies: potential for conflict between cardiac and skeletal needs. Circulation. 2005;112:2756–2758. | ||
Kamogawa Y, Biro S, Maeda M, et al. Dystrophin-deficient myocardium is vulnerable to pressure overload in vivo. Cardiovasc Res. 2001;50:509–515. | ||
Perloff JK, Henze E, Schelbert HR. Alterations in regional myocardial metabolism, perfusion, and wall motion in Duchenne muscular dystrophy studied by radionuclide imaging. Circulation. 1984;69:33–42. | ||
Ervasti JM, Sonnemann KJ. Biology of the striated muscle dystrophin-glycoprotein complex. Int Rev Cytol. 2008;265:191–225. | ||
Jung C, Martins AS, Niggli E, Shirokova N. Dystrophic cardiomyopathy: amplification of cellular damage by Ca2+ signalling and reactive oxygen species-generating pathways. Cardiovasc Res. 2008;77:766–773. | ||
Townsend D, Daly M, Chamberlain JS, Metzger JM. Age-dependent dystrophin loss and genetic reconstitution establish a molecular link between dystrophin and heart performance during aging. Mol Ther. 2011;19:1821–1825. | ||
Ciccone MM, Niccoli-Asabella A, Scicchitano P, et al. Cardiovascular risk evaluation and prevalence of silent myocardial ischemia in subjects with asymptomatic carotid artery disease. Vasc Health Risk Manag. 2011;7:129–134. | ||
Miura P, Andrews M, Holcik M, Jasmin BJ. IRES-mediated translation of utrophin A is enhanced by glucocorticoid treatment in skeletal muscle cells. PLoS One. 2008;3:e2309. | ||
St-Pierre SJ, Chakkalakal JV, Kolodziejczyk SM, Knudson JC, Jasmin BJ, Megeney LA. Glucocorticoid treatment alleviates dystrophic myofiber pathology by activation of the calcineurin/NF-AT pathway. FASEB J. 2004;18:1937–1939. | ||
Beenakker EA, Fock JM, Van Tol MJ, et al. Intermittent prednisone therapy in Duchenne muscular dystrophy: a randomized controlled trial. Arch Neurol. 2005;62:128–132. | ||
Parreira SL, Resende MB, Zanoteli E, Carvalho MS, Marie SK, Reed UC. Comparison of motor strength and function in patients with Duchenne muscular dystrophy with or without steroid therapy. Arq Neuropsiquiatr. 2010;68:683–688. | ||
Markham LW, Kinnett K, Wong BL, Woodrow Benson D, Cripe LH. Corticosteroid treatment retards development of ventricular dysfunction in Duchenne muscular dystrophy. Neuromuscul Disord. 2008;18:365–370. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.