Back to Journals » Vascular Health and Risk Management » Volume 16
Early Detection of Undiagnosed Abdominal Aortic Aneurysm and Sub-Aneurysmal Aortic Dilatations in Patients with High-Risk Coronary Artery Disease: The Value of Targetted Screening Programme
Authors Saw ST, Leong BDK, Abdul Aziz DA
Received 24 February 2020
Accepted for publication 26 May 2020
Published 9 June 2020 Volume 2020:16 Pages 215—229
DOI https://doi.org/10.2147/VHRM.S250735
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Pietro Scicchitano
Siong Teng Saw, 1 Benjamin Dak Keung Leong, 2 Dayang Anita Abdul Aziz 3
1Faculty of Medicine, Universiti Kebangsaan Malaysia; Hospital Queen Elizabeth II, Kota Kinabalu, Sabah 88300, Malaysia; 2Hospital Queen Elizabeth II, Kota Kinabalu, Sabah 88300, Malaysia; 3Faculty of Medicine, Universiti Kebangsaan Malaysia, UKM Medical Center, Kuala Lumpur 56000, Malaysia
Correspondence: Dayang Anita Abdul Aziz Fax +603 9145 6684
Email [email protected]
Introduction: Abdominal aortic aneurysm (AAA) and coronary artery disease (CAD) share common risk factors. The objective of this study was to determine the prevalence of undiagnosed AAA in patients with angiographically diagnosed significant CAD.
Patients and Methods: Male patients aged 50 years and above (including indigenous people) with angiographically diagnosed significant CAD in the recent one year were screened for AAA. Standard definition of abdominal aortic aneurysm and CAD was used. All new patients were followed up for six months for AAA events (ruptured AAA and AAA-related mortality).
Results: A total of 277 male patients were recruited into this study. The total prevalence of undiagnosed AAA in this study population was 1.1% (95% CI 0.2– 3.1). In patients with high-risk CAD, the prevalence of undiagnosed AAA was 1.7% (95% CI 0.3– 4.8). The detected aneurysms ranged in size from 35.0mm to 63.8mm. Obesity was a common factor in these patients. There were no AAA-related mortality or morbidity during the follow-up. Although the total prevalence of undiagnosed AAA is low in the studied population, the prevalence of sub-aneurysmal aortic dilatation in patients with significant CAD was high at 6.6% (95% CI 3.9– 10.2), in which majority were within the younger age group than 65 years old.
Conclusion: This was the first study on the prevalence of undiagnosed AAA in a significant CAD population involving indigenous people in the island of Borneo. Targeted screening of patients with high-risk CAD even though they are younger than 65 years old effectively discover potentially harmful asymptomatic AAA and sub-aneurysmal aortic dilatations.
Keywords: abdominal aortic aneurysm, sub-aneurysmal aortic dilatation, coronary artery disease
Introduction
Abdominal aortic aneurysm (AAA) is a pathological condition in which weakening of the abdominal aortic wall causes it to bulge or balloon, resulting in a permanent focal dilatation of the abdominal aorta. AAA is defined as an abdominal aortic diameter of 3.0cm or more.1
AAA prevalence rates have decreased over the last 20 years, with the reported global pooled prevalence of AAA was 4.8%, being highest in male (6.0%) and aged 65–74 years (2.8%).1,2 Stratified analyses showed the pooled prevalence of AAA in America, Europe, Australia and Asia to be 2.2%, 2.5 %, 6.7% and 0.5% respectively.2 Abdominal aortic aneurysm continues to be a major threat to global health as its mortality reaches as high as 80% in the event of rupture.3–5 However, it remains a preventable cause of death in the elderly as the mortality rate for elective AAA repair ranges from 0.6 to 5.3%.6,7
As most AAAs are silent with high mortality rates if permitted to expand and rupture, it is crucial to identify screening strategies that could reduce AAA-related mortality and other adverse outcomes. Guidelines have recommended ultrasound as the primary screening tool due to the advantages of being non-invasive, user-friendly, and having both sensitivity and specificity approaching 100%.8 Population-based studies have demonstrated that ultrasound screening is cost effective and highly reproducible.9,10
An updated meta-analysis of the longest (≥ 13 years) follow-up results from four randomized controlled trials of AAA screening reported that invitation to AAA screening in ≥ 64-year-old men reduced both all-cause and AAA-related mortalities significantly.11–13 Based on a systematic review of evidence, several countries have provided their recommendations and guidelines on screening for AAA including Canada, USA and Europe. All these guidelines have strongly suggested not to screen women for AAA.1,14,15
Significant coronary artery disease (CAD) is defined as 50% stenosis in one or more involved vessels. High-risk CAD is defined as (i) three vessels with ≥ 70% stenosis, (ii) two vessels with ≥ 70% stenosis including the proximal left anterior descending artery, or (iii) stenosis of ≥ 50% in the left main artery.16–18 Revascularisation is required if the patient has high-risk CAD, which can be done by percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG).19 Recent epidemiological studies have suggested an association between AAA and atherosclerosis, CAD and peripheral vascular disease (PVD). Both AAA and CAD share common risk factors, such as male gender, age, and smoking.20,21 In fact, CAD has emerged as a significant independent risk factor for AAA, as a large proportion of patients whose AAA ruptured have previously diagnosed CAD.22
Overall, the prevalence of AAA in patients with CAD was 8.4%, which was significantly higher than those without CAD.23–25 A meta-analysis conducted by Hernesniemi et al showed that CAD is a strong predictor of future AAA events (fatal and non-fatal; meta-analysed hazard ratio of 3.49 with 95% CI of 2.56–4.76) and concluded that screening for AAA among CAD patients would be of possible benefit to survival and risk evaluations.26
Most AAA patients are asymptomatic, with aneurysms discovered incidentally during the diagnosis of other conditions. Even though evidence shows that ultrasound screening for AAA in the male population reduces the risk of AAA rupture and aneurysm-related death significantly by up to 50%, no population screening programme has been established in Southeast Asian countries, probably due to the lack of resources.11,12 Moreover, the low prevalence of AAA in Southeast Asia may render population screening programmes less cost effective. With no systematic population screening available, targeted screening of patients with CAD would effectively provide the same opportunity to discover potentially harmful asymptomatic AAA, and subsequently allow the provision of earlier surveillance or operative management if indicated.
Methodology
Study Design
This was a prospective cross-sectional study carried out over a period of one year. It was performed to determine the prevalence of undiagnosed abdominal aortic aneurysm (AAA) in patients with significant coronary artery disease (CAD) undergoing coronary angiography. The study was conducted at a tertiary hospital in major city in the state of Sabah, Malaysia (island in Borneo).
Study Population
All patients who underwent coronary angiography for a period of 12 months were screened for eligibility to participate in the study. Patients were included if they were male, aged 50 years and above, and angiographically diagnosed with significant CAD (one or more vessels with stenosis of ≥ 50%). Criteria for exclusion included previous diagnosis of treated AAA, previous diagnosis of CAD treated by medical therapy or revascularisation therapy including percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG), failure of abdominal ultrasound to delineate the size of the abdominal aorta due to obesity or bowel gas, presence of major life-threatening illnesses such as cardiogenic shock or acute pulmonary oedema, and unwillingness or inability to provide informed consent.
During the study period, a total of 704 male patients aged 50 years and above underwent coronary angiography. A group of 108 patients had normal coronary arteries and were not included into the study. Amongst 596 patients with angiographically-diagnosed significant CAD, three patients had previous diagnosis of treated AAA, 160 patients had previous diagnosis of treated CAD, and three patients passed away prior to recruitment. In addition, 61 patients were not interested in participating in the study, with 45 of them stating logistic issues as the reason for not taking part. From the remaining 369 patients, 277 were recruited into the study. Figure 1 shows the study flow diagram.
 |
Figure 1 Study flow diagram. |
Patients and Methods
Patients who fulfilled the inclusion criteria were invited for an abdominal ultrasound. Each patient received a thorough explanation and a patient information sheet regarding the study. Written informed consent was required before proceeding further. The relevant data were collected according to a standard questionnaire.
AAA was defined as an aortic diameter measuring ≥ 3.0cm.1 Sub-aneurysmal aortic dilatation was defined as a maximum aortic diameter between 25mm and 29mm.1 Significant CAD was defined as one or more coronary vessels with stenosis of ≥ 50%. High-risk CAD was defined as having (i) three vessels with ≥ 70% stenosis, or (ii) two vessels with ≥ 70% stenosis including the proximal left anterior descending artery, or (iii) left main artery with ≥ 50% stenosis.16–18 Information gathered and analysed included the patient’s age, body mass index (BMI), ethnicity, family history of AAA, smoking status, risk factors for AAA and abdominal aortic size.
Abdominal ultrasounds were performed with the General Electric Vingmed Ultrasound Vivid i ultrasonography machine. All examinations were done by single-trained and certified medical assistant to reduce inter-observer biasness. Ultrasonographic evaluation of the aorta was performed with the patient in supine position. Using a curvilinear probe, the abdominal aorta from the diaphragm to the aortic bifurcation was assessed in the longitudinal and transverse planes. An image of aorta in the transverse plane, as circular as possible, was obtained to ensure that the image chosen was perpendicular to the sagittal/longitudinal axis. The largest antero-posterior diameter was measured from the outer edge to the outer edge (OTO) and this was considered to represent the maximum infrarenal aortic diameter. AAA was defined as an aortic diameter of 3.0cm or larger. All results were verified by a vascular surgeon. The aortic diameter was recorded into the data collection form solely by the primary investigator. This was to ensure that both medical assistant and surgeon were blinded to the participant’s coronary angiogram finding. All ultrasound images were captured and stored in a computer.
Patients diagnosed with AAA in this study were referred to the vascular clinic to receive existing standard of care and medical consultation by vascular surgeons. The standard of care provided depended on the symptoms and size of AAA, in which appropriate primary treatment such as ultrasound surveillance, open AAA repair or Endovascular aneurysm repair (EVAR) was offered. The authors were not involved and did not interfere with the standard of care provided to patients. The role of the principal investigator after diagnosis of AAA was to follow-up and document the outcome (AAA events) after the patients received standard of care. All patients with newly diagnosed AAA were followed up from the day of diagnosis until six months later. The outcomes measured were AAA events including ruptured AAA and AAA-related mortality.
Measurement and Outcomes
The primary outcome of this study was to measure the prevalence of undiagnosed AAA in patients with angiographically diagnosed significant CAD. The secondary outcomes of this study were to compare the prevalence of undiagnosed AAA between patients with angiographically diagnosed high-risk CAD and non-high-risk CAD, to determine the association of risk factors and AAA in angiographically diagnosed high-risk CAD patients, to compare the abdominal aortic diameter between angiographically diagnosed high-risk CAD patients with and without risk factors, and to compare the prevalence of sub-aneurysmal aortic dilatation between patients with angiographically diagnosed high-risk CAD and non-high-risk CAD.
Statistical Analysis
Data analysis was performed with the SPSS (Statistical Package of Social Sciences) for Window version 25.0. Pearson’s chi-squared test and Fisher’s exact test were used for associations between categorical variables. Analysis of the difference between group medians was performed with Mann–Whitney U-test and Kruskal–Wallis test. The level of statistical significance was set at p < 0.05.
Sample Size Calculation
To estimate the prevalence of undiagnosed AAA in patients undergoing coronary angiography, we needed 241 patients to achieve a 3.0% precision in estimating prevalence, which was 6.0% in Miura et al.27 The sample size was calculated using the Sample Size Calculator for Prevalence Studies.28 With an estimated 15% drop out rate, we included an additional 36 subjects in our sample size. Therefore, the total sample size required was 277 patients.
Ethical Consideration
This study was a project for thesis submission of the first author; the corresponding author was the main supervisor. This study was approved by the Research Ethics Committee of the National University of Malaysia (Project Code: UKM PPI/11/8/JEP-2018-148), Faculty of Medicine (Project Code: FF-2018-095) as well as the Medical Research Ethics Committee (MREC) of the Ministry of Health Malaysia (Project Code: NMRR-17-3031-39208). This study was conducted in accordance with the Declaration of Helsinki.
Results
Patient Characteristics
A total of 277 male patients aged 50 years and above who had angiographically diagnosed significant coronary artery disease (CAD) were recruited into this study. They had a mean age of 60 years (Mean = 60.25, Standard Deviation = 6.86) with most patients falling within the age group of 50 to 59 years old (51.6%). The Chinese was the commonest race affected at 25.3%, followed by the indigenous: Kadazan-Dusun (23.1%) and Bajau (14.1%). The remaining patients were made up of other races and indigenous groups in Sabah. Majority of patients were obese (57.8%) with a mean body mass index (BMI) of 25.9. Amongst 277 patients, 65.0% had high-risk CAD whereby 6.1% received medical therapy, 65.6% received PCI, and 28.3% were scheduled for CABG. Table 1 shows the demographic profile of patients with significant CAD.
 |
Table 1 Demographic Profile of Patients with Significant CAD |
Prevalence of Undiagnosed Abdominal Aortic Aneurysm in Patients with High-Risk Coronary Artery Disease
Three new AAAs were detected in 277 patients who had angiographically diagnosed significant CAD, and all three fulfilled the criteria of high-risk CAD. (Figure 2) The total prevalence of undiagnosed AAA in this study population was 1.1% (95% CI 0.2–3.1). In patients with high-risk CAD undergoing coronary angiography, the prevalence of undiagnosed AAA was 1.7% (95% CI 0.3–4.8). There was no significant difference in the prevalence of undiagnosed AAA between patient with high-risk and non-high-risk CAD undergoing coronary angiography (p = 0.554). Table 2 shows the association of risk factors and AAA in patients with significant CAD.
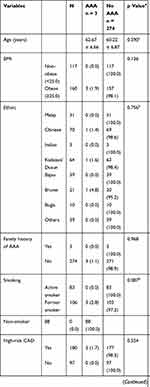 | 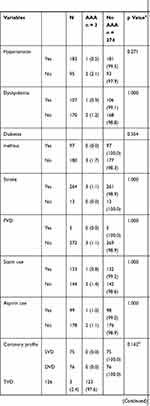 |  |
Table 2 Abdominal Aortic Aneurysm in Patients with Significant Coronary Artery Disease |
 |
Figure 2 Abdominal aortic aneurysm in patients with significant coronary artery disease. Abbreviations: AAA, abdominal aortic aneurysm; CAD, coronary artery disease. |
Table 3 shows the individual profile of these three patients. The size of AAA detected in this study ranged from 35.0mm to 63.8mm. All three patients were obese, two of them were former smokers, with only one patient having hypertension and dyslipidemia as additional risk factors. None of them had diabetes mellitus, stroke, PVD or a family history of AAA. All of them had triple vessel disease whereby one patient underwent PCI and two were scheduled for CABG.
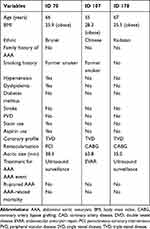 |
Table 3 Individual Profile of Abdominal Aortic Aneurysm in Patients with High-Risk Coronary Artery Disease |
All three patients received existing standard of care based on the size of AAA, in which two patients with smaller AAA received ultrasound surveillance whereas the patient with the largest detected AAA was planned for EVAR after his CABG. No AAA event (ruptured AAA or AAA-related mortality) was detected during the follow-up period of six months.
Sub-Aneurysmal Aortic Dilatation and Coronary Artery Disease
A total of 18 sub-aneurysmal aortic dilatations were detected in 274 patients who had angiographically diagnosed significant CAD, which confirmed a prevalence of 6.6% (95% CI 3.9–10.2) in this study population. The prevalence of sub-aneurysmal aortic dilatation in patients with high-risk CAD was 8.5% (95% CI 4.8–13.6). In contrast, a prevalence of 3.1% (95% CI 0.6–8.8) was demonstrated in patients with non-high-risk CAD. (Figure 3) There was no significant difference in the prevalence of sub-aneurysmal aortic dilatation between patient with high-risk and non-high-risk CAD (p = 0.086). In addition, there was no significant association between age, BMI, ethnicity, family history of AAA, smoking history, hypertension, dyslipidaemia, stroke, PVD, aspirin use, statin use, coronary profile, revascularisation and sub-aneurysmal aortic dilatation. Interestingly, majority of our patients with sub-aneurysmal aortic dilatation were within the age group of 50 to 64 years old, the population which is not included in Western screening programmes. (Figure 4).
 |
Figure 3 Sub-aneurysmal aortic dilatation in patients with significant coronary artery disease. |
 |
Figure 4 Age distribution in patients with sub-aneurysmal aortic dilatation. |
Abdominal Aortic Diameter and Coronary Artery Disease
The median abdominal aortic diameter in this study was 19.2mm, with a minimum diameter of 10.0mm and maximum diameter of 63.8mm. (Figure 5) The median abdominal aortic diameter was 19.3mm in high-risk CAD and 18.9mm in non-high-risk CAD. (Figure 6) There was no significant difference between the median of abdominal aortic diameter in both groups (p = 0.317).
 |
Figure 5 Distribution of abdominal aortic diameter in patients with significant coronary artery disease. |
 |
Figure 6 Distribution of abdominal aortic diameter in patients with high-risk and non-high-risk coronary artery disease. |
Further analysis was performed to determine the relationship between abdominal aortic diameter and cardiovascular risk factors in the high-risk CAD group. Mann–Whitney U-test indicated that the abdominal aortic diameter was smaller for diabetic patients (Median = 18.20) than for non-diabetic patients (Median = 19.70), p = 0.014. Similarly, the abdominal aortic diameter was smaller for patients who consumed statins (Median = 18.50) than for patients who did not (Median = 19.65), p = 0.033. There was no significant association between median abdominal aortic diameter and age, BMI, ethnicity, family history of AAA, smoking history, hypertension, dyslipidaemia, stroke, PVD, aspirin use, revascularisation and significant CAD. Table 4 shows the median abdominal aortic diameter of high-risk CAD patients with diabetes mellitus and those who consumed statin.
 |
Table 4 Comparing Median Abdominal Aortic Diameter Among Variables in Patients with High-Risk Coronary Artery Disease |
Discussion
Abdominal Aortic Aneurysm in High-Risk Coronary Artery Disease Population
In this study, the prevalence of undiagnosed AAA in angiographically diagnosed high-risk CAD patients was found to be 1.7% (95% CI 0.3–4.8). Compared to epidemiological studies conducted in Western countries, our study showed a relatively low prevalence of AAA in the local population. Nevertheless, this low prevalence is consistent with the results of other studies performed within the Asian region.2 A possible explanation for the observed low prevalence rate in Asian countries might be the lack of routine screening programs which allow asymptomatic silent AAA to remain undetected. Consequently, AAAs are only either discovered when symptomatic or incidentally during the work-up of other disease conditions. Another reason for the low prevalence rate of AAA in our study might be the smaller abdominal aortic size found in the local people compared to Caucasians, considering the obvious body size differences between these two populations. Our study reported that the median abdominal aortic diameter was 19.2mm (range 10.0–63.8mm), which was much smaller than that found in Caucasians.29 Several authors have suggested that a lower threshold might thus be more appropriate in some Asian populations.1 Therefore, the use of 30mm as the definition for AAA may not be appropriate in our local setting. An alternative definition of a 50% increase from the normal abdominal aortic diameter has been proposed, however, the demarcation between aneurysmal and disease-free would be highly variable due to the wide dissimilarity of abdominal aortic diameter in different populations. Ethnicity-related or unmeasured genetic factors may also play an independent role in the prevalence of AAA in different ethnic groups in Sabah, despite the presence of cardiovascular risk factors.
The reported prevalence of 1.7% in our study population is higher than the pooled prevalence of AAA in Asia which was 0.5%, given that our study population (men with mean age of 62.7 ± 6.7 years) has younger age distribution compared with reported individual Asian prevalence studies in meta-analysis.2 Similarly, our reported prevalence was found to exceed that of China and Taiwan.30,31 Meta-analysis has shown that the overall prevalence of AAA is significantly higher in patients with CAD.23,26 In our study, the prevalence was comparable to the only local study available on the prevalence of AAA in patients with acute coronary syndrome (ACS), which was found to be 2.0%.32 To the best of our knowledge, our study is the first in literature which specifically investigates AAA in high-risk CAD population. Given that the prevalence of AAA is higher in patients with high-risk CAD, a concurrent diagnosis of AAA should be considered in this population as AAA screening is almost unique and proven in reducing both cause-specific and all-cause mortality.11
A total of three AAAs was detected in our study population, all of whom had angiographically diagnosed high-risk CAD. The youngest patient was 55 years old with the largest aortic diameter of 63.8mm. We might have overlooked this patient if the study had been performed only in patients aged ≥ 65 years. Most of the recent guidelines recommend one-time AAA screening for all men at the age of 65 years and above, but these recommendations are based on population studies performed in Western countries.1,14,15,33,34 The Japan Circulation Society guideline (JCS 2011) is the only AAA guideline available in Asian countries.35 However, no specific recommendation is made regarding AAA screening.35,36 According to the Malaysia Annual Report of the Percutaneous Coronary Intervention (PCI) Registry from year 2015–2016, the mean age of patients presenting with ACS was 58.5 years and 64.3% of male patients who underwent PCI were aged 50 years and above.37 Hence, AAA screening based on the age criteria proposed by Western guidelines might not be suitable for local adoption considering the age distribution of patients with significant CAD in our local population. The presence of risk factors, especially CAD, should be factored into the framework of a local targeted screening for a population with such a low prevalence of AAA, rather than following screening criteria from Western guidelines.
In our present study, all three patients with AAA were obese, with two of them being former smokers. One of them had both hypertension and dyslipidemia. None of them had a family history of AAA or diabetes mellitus. Surprisingly, the patient with the largest aortic diameter did not have any medical illness besides CAD. No significant relationship was found between AAA and cardiovascular risk factors due to the low prevalence of AAA in our study. Smoking is the strongest risk factor for AAA, as there is a 5-fold and 2-fold increase in the risk of AAA among current and former smokers compared to never smokers, respectively.34,38-40 Hypertension has been reported to have a strong association with AAA.41–43 There is inconsistency in the literature regarding the association of obesity with the presence of AAA, with some studies showing that the relationship between obesity and AAA may be more significant in conjunction with atherosclerotic disease.44–48
Even though our study reported a AAA prevalence rate of 1.7%, we were able to detect a AAA with a size of 63.8mm in which surgical intervention was indicated. Population-based AAA screening programs in men have demonstrated a significant cost-effective reduction in all-cause mortality and AAA-related mortality even with an AAA prevalence rate of as low as 0.5%.49,50 Therefore, a targeted AAA screening programme tailored to specific risk factors would be more effective in detecting AAA in local populations.
Sub-Aneurysmal Aortic Dilatation
Sub-aneurysmal aortic dilatation can be defined as a maximum aortic diameter between 25mm and 29mm. It is a topic of current interest as more than half of these aortas will exceed 30mm within 5 years and one quarter will reach 55mm within 10 to 15 years.51 A multicentre observational study reported that 3.1% of patients with sub-aneurysmal aortic dilatation progressed to ruptured AAA at 10 years follow up, with the mortality rate as high as 71.4%.52 Guidelines have suggested that men with an aortic size of 2.5mm to 2.9mm at initial screening may be considered for rescreening after 5 to 10 years, even with the current limited evidence regarding clinical relevance and cost effectiveness of surveillance.1,53 Our subgroup analysis showed that 18 patients had sub-aneurysmal aortic dilatation with a prevalence of 6.6% (95% CI 3.9–10.2). Surprisingly, 14 patients (77.8%) with sub-aneurysmal aortic dilatation were within the age group of 50 to 64 years old. Again, we would like to emphasize that targeted screening of AAA should be tailored to a younger demographic in our local setting compared to the Western population, given that a high prevalence of CAD is found in younger patients. We suggest surveillance of sub-aneurysmal aortic dilatation as a future study to investigate the impact of the initial targeted screening.
The guidelines from European Society for Vascular Surgery (ESVS) defines sub-aneurysmal aortic dilatation as a maximum aortic diameter between 25mm and 29mm. The population study was in men aged beyond 65 years old.1 In our cohort of 177 patients with high-risk CAD (majority were 50–59 years old), 15 sub-aneurysmal aortic dilatations were detected, which in turn confirmed a prevalence of 8.5% (95% CI 4.8–13.6). This finding is comparable to the prevalence of sub-aneurysmal aortic dilatation reported in the Italian general population of men between the age of 50–64 years old.54 Our finding was in keeping with the prevalence results of a systematic review which reported a prevalence of sub-aneurysmal aortas ranging from 1.1% to 8.5%. However their population study was in older population aged above 65 years old.53 Hence, it was rather an unexpected finding from our study was that 12 high-risk CAD patients (80.0%) with sub-aneurysmal aortic dilatation were within the age group of 50 to 64 years old - an age group which is not included in major Western screening programmes (USA, UK and Canada). Our finding highlighted that sub-aneurysmal aortas can be found in patients with cardiovascular risk factors in younger age group. The identification of AAA or potential AAA based on cardiovascular risk factors would prove to be more beneficial rather than a strict age criterion.
Our study has detected an unexpected number of sub-aneurysmal aortic dilatations despite the low prevalence of AAA in the study population. Therefore, this specific population deserves special attention as they could develop AAA in the future. It has been reported that more than half of these aortas will exceed 30mm within 5 years and one quarter will reach 55mm within 10 to 15 years.51 A multicentre observational study reported that 3.1% of patients with sub-aneurysmal aortic dilatation progressed to ruptured AAA at 10 years of follow up, with a mortality rate as high as 71.4%.52 Guidelines have suggested that men with an aortic size of 25mm to 29mm at initial screening may be considered for rescreening after 5 to 10 years, even with the current limited evidence regarding clinical relevance and cost effectiveness of surveillance.1,53 Hence, we strongly believe that surveillance of sub-aneurysmal aortic dilatation in our local population as a future study would be of value to investigate the impact of initial targeted screening. Certainly, the cohort of our patients with sub-aneurysmal aortic dilatation will be monitored closely.
Protective Factors for Abdominal Aortic Aneurysm
Diabetes mellitus (DM), one of the strongest coronary atherosclerotic factors, appears to be independently and negatively associated with AAA formation.55 In our study, we were unable to prove any significant relationship between AAA and diabetes mellitus due to the limitation of a small sample size. However, our analysis of the high-risk CAD group has shown that the abdominal aortic diameter was smaller in diabetic patients compared to non-diabetic patients (p = 0.014). Meta-analyses have demonstrated that DM is negatively associated with the presence, expansion, and rupture of AAA, and suggested that individuals with DM have a reduction in risk of AAA by 42%.56–58 It has been explained that hyperglycemia in DM is associated with glycation of protein precursors of the extracellular matrix, thus resulting in increased synthesis of advanced glycation end products (AGEs). Formation of AGEs has been linked to increased smooth muscle cell proliferation which in turn increases the matrix protein in the aortic wall. Diabetes mellitus also suppresses matrix metalloproteinase (MMPs) and elastase which are responsible for degradation of the medial elastic lamellae in the aortic wall.59
In our study, the abdominal aortic diameter was smaller for patients with high-risk CAD who consumed statins than for patients who did not (p = 0.033). To date, no recognised pharmacological drug has been shown to be effective in reducing the rate of small aneurysm growth, although statins have been suggested to be beneficial. A highly significant reduction in AAA prevalence was observed in a population with statins.60 Moreover, a recently published meta-analysis has concluded that statin therapy may be associated with reduction in AAA progression and rupture.61 A higher dose of statin has been shown to exhibit an inhibitory effect on endoplasmic reticulum stress-associated apoptosis signaling pathways and inflammatory response in clinically relevant AAA mice model, which in turn suppresses the development of AAA effectively.62
Limitations
There was limitation in this study whereby we were mainly focused on a high-risk CAD population; only three AAAs were found. Thus, with the low prevalence of AAA detected in this study, the analysis for interesting variables such as smoking and hypertension could not be performed. Even though the high-risk CAD population has been identified as being suitable for AAA screening, more randomised control trials are required to evaluate the long-term benefits and cost-effectiveness of targeted screening in this population. In comparison to all other screening models or programme, our suggestion of targeted screening in a small population may look pale in comparison but certainly one life was saved and more lives could be saved and that made a huge difference to patients and their families.
As for the strength of this study, targeted group was most suited for our population study as trying to get them to come for separate screening programme would have been logistically impossible. The geographical and cultural factors related to the state of Sabah (in the island of Borneo) and most developing south-east Asian countries (ASEAN) required us to screen patients as and when they presented to the hospital rather than the usual public screening programme. This is the first study evaluating the prevalence of AAA in those aged 50 years and above in a high-risk CAD population in Malaysia with involvement of indigenous people. There was no inter-observer biasness as the abdominal ultrasound was performed by single-qualified and trained medical assistant. Both the medical assistant and vascular surgeon were blinded to the patient’s coronary angiogram findings in order to reduce interviewer and performance biasness.
Future Research
The early detection of AAA and sub-aneurysmal aortic dilatation in this study serves as a pilot study to explore the potential selection criteria in establishing a targeted screening programme in multicultural population of Malaysia. Given the limitations of this study, a high power, larger population study which its sample size calculation based on proposed multivariate regression analysis will be efficient to generate a generalized equation; which in turn helps to establish an appropriate screening programme suitable for local high-risk CAD population. Based on this, we can foresee that age, obesity, and other specific risk factors for AAA will be included. We hope to also evaluate long-term outcome and study the cost-benefit analysis for future screening programmes.
Conclusion
To the best of our knowledge, our study is the first in literature which specifically investigated AAA in high-risk CAD population. This is also the first prevalence study of undiagnosed AAA in the population of the state of Sabah, Malaysia (in the island of Borneo). Although the prevalence of AAA in the high-risk CAD population appeared to be low, it was present in younger age group than other studies. The prevalence of sub-aneurysmal aortic dilatation in this younger group was also significant and made it worth screening. The proposed screening criteria in Western countries may not be suitable for our local population and other resource-limited countries. Therefore, targeted screening of patients based on risk factors and screening at the first opportunity of meeting them would effectively discover potentially harmful asymptomatic AAA which may require surgical intervention or follow-up.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wanhainen A, Verzini F, Van Herzeele I, et al. Editor’s choice – european society for vascular surgery (ESVS) 2019 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2019;57(1):8–93. doi:10.1016/j.ejvs.2018.09.020
2. Li X, Zhao G, Zhang J, Duan Z, Xin S. Prevalence and trends of the abdominal aortic aneurysms epidemic in general population-a meta-analysis. PLoS One. 2013;8(12):1–11. doi:10.1371/journal.pone.0081260
3. Sidloff D, Stather P, Dattani N, et al. Aneurysm Global Epidemiology Study. Circulation. 2013;129(7):747–753. doi:10.1161/circulationaha.113.005457
4. Guo W, Zhang T. Abdominal aortic aneurysm prevalence in China. Endovasc Today. 2014;76–82.
5. Mani K, Lees T, Beiles B, et al. Treatment of abdominal aortic aneurysm in nine countries 2005–2009: a vascunet report. Eur J Vasc Endovasc Surg. 2011;42(5):598–607. doi:10.1016/j.ejvs.2011.06.043
6. Naghavi M, Abajobir AA, Abbafati C, et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2017;390(10100):1151–1210. doi:10.1016/S0140-6736(17)32152-9
7. Becquemin JP, Pillet JC, Lescalie F, et al. A randomized controlled trial of endovascular aneurysm repair versus open surgery for abdominal aortic aneurysms in low- to moderate-risk patients. J Vasc Surg. 2011;53(5):1167–1173.e1. doi:10.1016/j.jvs.2010.10.124
8. Janelle M, Guirguis-Blake, Beil TL, Senger CA, Whitlock EP. Review systematic evidence review for the u. S. Preventive services task force. Ann Intern Med. 2014;160(5):321–329. doi:10.7326/M13-1844
9. Glover MJ, Kim LG, Sweeting MJ, Thompson SG, Buxton MJ. Cost-effectiveness of the National Health Service abdominal aortic aneurysm screening programme in England. Br J Surg. 2014;101(8):976–982. doi:10.1002/bjs.9528
10. Stather PW, Dattani N, Bown MJ, Earnshaw JJ, Lees TA. International variations in AAA screening. Eur J Vasc Endovasc Surg. 2013;45(3):231–234. doi:10.1016/j.ejvs.2012.12.013
11. Takagi H, Ando T, Umemoto T. Abdominal aortic aneurysm screening reduces all-cause mortality: make screening great again. Angiology. 2018;69(3):205–211. doi:10.1177/0003319717693107
12. Thompson SG, Ashton HA, Gao L, Buxton MJ, Scott RAP. Final follow-up of the multicentre aneurysm screening study (MASS) randomized trial of abdominal aortic aneurysm screening. Br J Surg. 2012;99(12):1649–1656. doi:10.1002/bjs.8897
13. McCaul KA, Lawrence-Brown M, Dickinson JA, Norman PE. Long-term outcomes of the western australian trial of screening for abdominal aortic aneurysms: secondary analysis of a randomized clinical trial. JAMA Intern Med. 2016;176(12):1761–1767. doi:10.1001/jamainternmed.2016.6633
14. Singh H, Bell N, Dickinson JA, et al. Recommendations on screening for abdominal aortic aneurysm in primary care. Cmaj. 2017;189(36):E1137–E1145. doi:10.1503/cmaj.170118
15. LeFevre ML. Screening for abdominal aortic aneurysm: U.S. Preventive services task force recommendation statement. Ann Intern Med. 2014;161(4):281–290. doi:10.7326/M14-1204
16. Chang H, Cheng VY, Chinnaiyan K, et al. A clinical model to identify patients with high-risk coronary artery disease. JACC. 2015;8(4). doi:10.1016/j.jcmg.2014.11.015
17. Nakanishi R, Gransar H, Slomka P, et al. HHS Public Access. Anal Chem. 2019;23(3):530–541. doi:10.1007/s12350-015-0150-3.Predictors
18. Udelson JE, Jones WS, Sorrell VL. Predictive model for high-risk coronary. Circulation. 2019;12:1–10. doi:10.1161/CIRCIMAGING.118.007940
19. Fihn SD, Blankenship JC, Alexander KP, et al. ACC/AHA/AATS/PCNA/SCAI/STS focused update 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease a report of the american college of cardiology. JThoracic Cardiovasc Surg. 2014;149:1749–1767. doi:10.1161/CIR.00000000000
20. Toghill BJ, Saratzis A, Bown MJ. Abdominal aortic aneurysm—an independent disease to atherosclerosis? Cardiovasc Pathol. 2017;27:71–75. doi:10.1016/j.carpath.2017.01.008
21. Erbel R, Aboyans V, Boileau C, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases. Russ J Cardiol. 2015;123(7):7–72. doi:10.15829/1560-4071-2015-07-7-72
22. Chun KC, Teng KY, Chavez LA, et al. Risk factors associated with the diagnosis of abdominal aortic aneurysm in patients screened at a regional veterans affairs health care system. Ann Vasc Surg. 2014;28(1):87–92. doi:10.1016/j.avsg.2013.06.016
23. Elkalioubie A, Haulon S, Duhamel A, et al. Meta-analysis of abdominal aortic aneurysm in patients with coronary artery disease. Am J Cardiol. 2015;116(9):1451–1456. doi:10.1016/j.amjcard.2015.07.074
24. Vänni V, Hakala T, Mustonen J, et al. Ultrasound screening of men with coronary artery disease for abdominal aortic aneurysms: a prospective dual center study. Scand J Surg. 2016;105(4):235–240. doi:10.1177/1457496915626839
25. Durieux R, Van Damme H, Labropoulos N, et al. High Prevalence of abdominal aortic aneurysm in patients with three-vessel coronary artery disease. Eur J Vasc Endovasc Surg. 2014;47(3):273–278. doi:10.1016/j.ejvs.2013.12.011
26. Hernesniemi JA, Vänni V, Hakala T. The prevalence of abdominal aortic aneurysm is consistently high among patients with coronary artery disease. J Vasc Surg. 2015;62(1):232–240.e3. doi:10.1016/j.jvs.2015.02.037
27. Miura T, Soga Y, Aihara H, Yokoi H, Iwabuchi M. Prevalence and clinical outcome of polyvascular atherosclerotic disease in patients undergoing coronary intervention. Circ J. 2013;77(5):1349. doi:10.1253/circj.cj-13-0212
28. Naing L, Winn T, Rusli BN. Practical issues in calculating the sample size for prevalence studies. Arch Orofac Sci. 2006;1(Ci):9–14.
29. Li W. Predictors associated with increased prevalence of abdominal aortic aneurysm in chinese patients with atherosclerotic risk factors. Eur J Vasc Endovasc Surg. 2017;2:43–49. doi:10.1016/j.ejvs.2017.04.004
30. Li K, Zhang K, Li T, Zhai S. Primary results of abdominal aortic aneurysm screening in the at-risk residents in middle China. BMC cardiovasc disord. 2018;18:1–6.
31. Wang S, Huang Y, Huang J, Chiu C. Epidemiology, clinical features, and prescribing patterns of aortic aneurysm in Asian population from 2005 to 2011. Medicine. 2015;94(41):1–6. doi:10.1097/MD.0000000000001716
32. Dak Keung B, Leong, Zainal AA, Chuah JA, Voo SY. Prevalence of peripheral arterial disease and abdominal aortic aneurysm among patients with acute coronary syndrome. Med J Malaysia. 2013;68(1):2012–2014.
33. Kapila V, Jetty P, Wooster D. The canadian society for vascular surgery 2018 screening for abdominal aortic aneurysms in canada: review and position statement from the canadian society of vascular surgery. Can Soc Vasc Surg. 2018. Available from: https://vascular.ca/resources/Documents/Clinical-Guidelines/FINAL-2018-CSVS-Screening-Recommendations.pdf
34. Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67(1):2–77.e2. doi:10.1016/j.jvs.2017.10.044
35. Joint Working Group J; JCS Joint Working Group. Guidelines for diagnosis and treatment of aortic aneurysm and aortic dissection (JCS 2011). Circ J. 2013;77(3):789–828. doi:10.1253/circj.cj-66-0057
36. Rozado J, Martin M, Pascual I, Hernandez-Vaquero D, Moris C. comparing American, European and Asian practice guidelines for aortic diseases. J Thorac Dis. 2017;9(Suppl 6):S551–S560. doi:10.21037/jtd.2017.03.97
37. Wan Ahmad WA ANNUAL REPORT OF THE NCVD-PCI REGISTRY YEAR 2015–2016. 2018;19. http://www.acrm.org.my/ncvd.
38. Aune D, Schlesinger S, Norat T, Riboli E, Schlesinger S. Tobacco smoking and the risk of abdominal aortic aneurysm: a systematic review and meta-analysis of prospective studies. J Diabetes Complications. 2018;8(1):1–9. doi:10.1016/j.jdiacomp.2018.09.009
39. Altobelli E, Rapacchietta L, Profeta VF, Fagnano R. Risk factors for abdominal aortic aneurysm in population-based studies: a systematic review and meta-analysis. Int J Environ Res Public Health. 2018;15(12):12. doi:10.3390/ijerph15122805
40. Han SA, Joh JH, Park HC. Risk factors for abdominal aortic aneurysm in the Korean population. Ann Vasc Surg. 2017;41:135–140. doi:10.1016/j.avsg.2016.08.044
41. Howard DPJ, Banerjee A, Fairhead JF, Handa A, Silver LE, Rothwell PM. Age-specific incidence, risk factors and outcome of acute abdominal aortic aneurysms in a defined population. Br J Surg. 2015;102(8):907–915. doi:10.1002/bjs.9838
42. Fukuda S, Watanabe H, Iwakura K, Daimon M, Ito H, Yoshikawa J. Multicenter investigations of the prevalence of abdominal aortic aneurysm in elderly Japanese patients with hypertension. Circ J. 2015;79(3):524–529. doi:10.1253/circj.CJ-14-0972
43. Kobeissi E, Hibino M, Pan H, Aune D. Blood pressure, hypertension and the risk of abdominal aortic aneurysms: a systematic review and meta-analysis of cohort studies. Eur J Epidemiol. 2019;34(6):547–555. doi:10.1007/s10654-019-00510-9
44. Cronin O, Liu D, Bradshaw B, et al. Visceral adiposity is not associated with abdominal aortic aneurysm presence and growth. Vasc Med. 2014;19(4):272–280. doi:10.1177/1358863X14537883
45. Stackelberg O, Wolk A, Eliasson K, et al. Lifestyle and risk of screening-detected abdominal aortic aneurysm in men. J Am Heart Assoc. 2017;6(5). doi:10.1161/JAHA.116.004725
46. Cronin O, Walker PJ, Golledge J. The association of obesity with abdominal aortic aneurysm presence and growth. Atherosclerosis. 2013;226(2):321–327. doi:10.1016/j.atherosclerosis.2012.10.041
47. Wang L, Djousse L, Song Y, et al. Associations of diabetes and obesity with risk of abdominal aortic aneurysm in men. J Obes. 2017;2017:1–11. doi:10.1155/2017/3521649
48. Larsson SC, Bäck M, Rees JMB, Mason AM, Burgess S. Body mass index and body composition in relation to 14 cardiovascular conditions in UK Biobank: a Mendelian randomization study. Eur Heart J. 2019;1–8. doi:10.1093/eurheartj/ehz388
49. Lindholt JS, Sørensen J, Søgaard R, Henneberg EW. Long-term benefit and cost-effectiveness analysis of screening for abdominal aortic aneurysms from a randomized controlled trial. Br J Surg. 2010;97(6):826–834. doi:10.1002/bjs.7001
50. Ying AJ, Affan ET. General reviews abdominal aortic aneurysm screening: a systematic review and meta-analysis of efficacy and cost. Ann Vasc Surg. 2019;54:298–303.e3. doi:10.1016/j.avsg.2018.05.044
51. Sweeting MJ, Turton G, Parkin D, et al. Lessons learned about prevalence and growth rates of abdominal aortic aneurysms from a 25-year ultrasound population screening programme. Br J Surg. 2018;105:68–74. doi:10.1002/bjs.10715
52. Wild JB, Stather PW, Biancari F, et al. A multicentre observational study of the outcomes of screening detected sub-aneurysmal aortic dilatation. Eur J Vasc Endovasc Surg. 2013;45(2):128–134. doi:10.1016/j.ejvs.2012.11.024
53. Hamel C, Ghannad M, McInnes MDF, et al. Potential benefits and harms of offering ultrasound surveillance to men aged 65 years and older with a subaneurysmal (2.5-2.9 cm) infrarenal aorta. J Vasc Surg. 2018;67(4):1298–1307. doi:10.1016/j.jvs.2017.11.074
54. Gianfagna F, Veronesi G, Tozzi M, Tarallo A, Borchini R, Ferrario MM. Prevalence of abdominal aortic aneurysms in the general population and in subgroups at high cardiovascular risk in Italy. Results RoCAV Popul Based Stud. 2018;633–639. doi:10.1016/j.ejvs.2018.01.008
55. Lederle FA, Noorbaloochi S, Nugent S, et al. Multicentre study of abdominal aortic aneurysm measurement and enlargement. Br J Surg. 2015;102(12):1480–1487. doi:10.1002/bjs.9895
56. Aune D, Schlesinger S, Norat T, Riboli E. Diabetes mellitus and the risk of abdominal aortic aneurysm: a systematic review and meta-analysis of prospective studies. J Diabetes Complications. 2018. doi:10.1016/j.jdiacomp.2018.09.009
57. Xiong J, Wu Z, Chen C, Wei Y, Guo W. Association between diabetes and prevalence and growth rate of abdominal aortic aneurysms: a meta-analysis. Int J Cardiol. 2016;221:484–495. doi:10.1016/j.ijcard.2016.07.016
58. Takagi H. Association of diabetes mellitus with presence, expansion, and rupture of abdominal aortic aneurysm: “Curiouser and curiouser!” cried ALICE. Semin Vasc Surg. 2016;29(1–2):18–26. doi:10.1053/j.semvascsurg.2016.06.003
59. Pafili K, Gouni-Berthold I, Papanas N, Mikhailidis DP. Abdominal aortic aneurysms and diabetes mellitus. J Diabetes Complications. 2015;29(8):1330–1336. doi:10.1016/j.jdiacomp.2015.08.011
60. Persson SE, Boman K, Wanhainen A, Carlberg B, Arnerlöv C. Decreasing prevalence of abdominal aortic aneurysm and changes in cardiovascular risk factors. J Vasc Surg. 2017;65(3):651–658. doi:10.1016/j.jvs.2016.08.091
61. Salata K, Hussain MA, Mestral C, et al. Statins reduce abdominal aortic aneurysm growth, rupture, and perioperative mortality: a systematic review and meta‐analysis. J Am Heart Assoc. 2018;7(19). doi:10.1161/JAHA.118.008657
62. Li Y, Lu G, Sun D, Zuo H, Wang DW, Yan J. Inhibition of endoplasmic reticulum stress signaling pathway: a new mechanism of statins to suppress the development of abdominal aortic aneurysm. PLoS One. 2017;12(4):1–19. doi:10.1371/journal.pone.0174821
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
