Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 15
Early Clinically Important Improvement (ECII) and Exacerbation Outcomes in COPD Patients
Authors Kostikas K , Mackay AJ, Vogelmeier CF, Frent SM , Gupta P, Banerji D, Patalano F , Pfister PJ , Wedzicha JA
Received 1 February 2020
Accepted for publication 2 July 2020
Published 28 July 2020 Volume 2020:15 Pages 1831—1838
DOI https://doi.org/10.2147/COPD.S247966
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Konstantinos Kostikas,1 Alexander J Mackay,2 Claus F Vogelmeier,3 Stefan-Marian Frent,4 Pritam Gupta,5 Donald Banerji,6 Francesco Patalano,7 Pascal J Pfister,7 Jadwiga A Wedzicha8
1Respiratory Medicine Department, University of Ioannina Medical School, Ioannina, Greece; 2National Heart and Lung Institute, Imperial College London, London, UK; 3Department of Medicine, Pulmonary and Critical Care Medicine, Philipps-Universität Marburg, Member of the German Center for Lung Research (DZL), Marburg, Germany; 4Department of Pulmonology, University of Medicine and Pharmacy Timisoara, Timisoara, Romania; 5Novartis Healthcare Pvt. Ltd., Hyderabad, India; 6Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA; 7Novartis Pharma AG, Basel, Switzerland; 8Respiratory Clinical Science Section, National Heart and Lung Institute, Imperial College London, London, UK
Correspondence: Konstantinos Kostikas
Respiratory Medicine Department, University of Ioannina Medical School, Ioannina 45110, Greece
Tel +30-6944780616
Email [email protected]
Background: Chronic obstructive pulmonary disease (COPD) exacerbations are difficult outcomes to measure in clinical trials. It would be valuable to be able to predict which patients are likely to benefit in terms of exacerbation prevention based on their early response in lung function and symptoms.
Methods: This was a post-hoc analysis from the 52-week, randomized, double-blind, double-dummy, non-inferiority FLAME trial. Early clinically important improvement (ECII) was defined as achievement of minimal clinically important difference in trough forced expiratory volume in 1 second (FEV1; ≥ 100 mL increase) and one patient-reported outcome (PRO): either St. George’s Respiratory Questionnaire for COPD (≥ 4-unit reduction; D1), or COPD assessment test (≥ 2-point reduction; D2) at Week 4 or 12.
Results: Approximately 18– 20% of patients achieved ECII at Week 4 or 12 post-randomization according to any of the two definitions. The rate of subsequent exacerbations was lower in patients who achieved ECII at Week 4 (D1: ratio of rates [95% CI], 0.85 [0.74 to 0.98]; D2, 0.88 [0.77 to 1.00]) or at Week 12 (D1, 0.85 [0.74 to 0.98]; D2, 0.86 [0.75 to 1.00]) versus patients not achieving ECII. Patients who achieved ECII experienced longer time-to-first exacerbation between Week 4 or 12 to end of study. More patients achieved ECII with indacaterol/glycopyrronium versus salmeterol/fluticasone according to both definitions at Week 4 (D1, odds ratio [95% CI], 1.69 [1.40 to 2.04]; D2, 1.61 [1.34 to 1.93]), and 12 (D1, 2.01 [1.66 to 2.44]; D2, 1.80 [1.48 to 2.18]).
Conclusion: ECII is a novel composite endpoint, based on clinically relevant improvement in lung function and PROs in the early phase of treatment intervention that may predict subsequent exacerbation risk and may be used in clinical trials.
Keywords: exacerbations, ECII, indacaterol/glycopyrronium, lung function, PROs
Introduction
Chronic obstructive pulmonary disease (COPD) exacerbations contribute to a decline in lung function and impaired quality of life in patients with COPD; the frequency and severity of exacerbations are positively correlated with disease progression.1–5 Limited data are available on factors that could be predictive of exacerbations;6,7 however, a history of exacerbations in the previous year is considered to be a good predictor of the occurrence of subsequent exacerbations.8
Unlike outcomes such as lung function, breathlessness and exercise capacity that respond quickly to treatment,9,10 COPD exacerbations are difficult outcomes to measure in clinical trials. This is mainly because exacerbations may occur at variable time points during follow-up, and the frequency of exacerbations is relatively low in trials comparing effective treatments, even in those including exacerbating COPD patients.11–13 Hence, trials with a large patient population and long follow-up duration are required to evaluate the effect of a treatment on exacerbations.13,14 Therefore, it would be of value to identify soon after the commencement of a treatment regimen, whether that specific treatment may prevent exacerbations later, during the course of the disease. There is limited evidence on whether early clinical outcomes predict a reduction of exacerbations in the long term.
Early Clinical Important Improvement (ECII) is a composite endpoint, defined as the clinically relevant improvement in both lung function (forced expiratory volume in 1 second, FEV1) and a patient-reported outcome (PRO) at 4 or 12 weeks, which may be useful in predicting responders early in terms of exacerbation prevention in the longer term. In the present analysis, we evaluated this novel composite endpoint as a predictor of exacerbation risk during the subsequent follow-up, using data from the FLAME study.13
Methods
Study Details
This was a post-hoc analysis from the 52-week, multicenter, randomized, double-blind, double-dummy, non-inferiority FLAME trial (ClinicalTrials.gov number, NCT01782326). Details of the study design have been published previously.13 Briefly, the study enrolled symptomatic patients with moderate-to-very severe COPD (FEV1 ≥25% and <60% predicted), and a history of ≥1 moderate or severe COPD exacerbation(s) in the previous year. These patients received either indacaterol/glycopyrronium (IND/GLY) 110/50 μg once-daily (q.d.) or salmeterol/fluticasone (SFC) 50/500 μg twice-daily (b.i.d.). All patients provided written informed content for participation in the FLAME trial.
Early Clinically Important Improvement
ECII is defined as an improvement measured by reaching minimal clinically important difference (MCID) in (i) lung function (≥100 mL in trough FEV1) and (ii) a PRO (COPD assessment test [CAT] or St. George’s Respiratory Questionnaire for COPD [SGRQ-C]) at Week 4 or Week 12 (Table 1). The SGRQ-C and CAT are well recognized, validated and easy-to-use health questionnaires developed for patients with COPD; a reduction of ≥4 units in the SGRQ-C total score indicates MCID,15,16 and a reduction of ≥2 points is considered as the MCID for the CAT score.17
 |
Table 1 Definitions of ECII |
Two time points were selected for the analysis of ECII, with Week 4 selected to discern early improvements in aforementioned outcomes and predicting early responders in terms of exacerbation prevention in the longer term, and Week 12 for further validation of these outcomes. While CAT and SGRQ both evaluate impairment in health status, evidence suggests that CAT is a more sensitive tool to detect symptoms (cough and sputum) and SGRQ is more reflective of pathophysiology.18,19 Hence, both PROs were included in the ECII definition.
Assessments
Exacerbations were defined as a worsening of two or more major symptoms (dyspnea, sputum volume and sputum purulence) for at least two consecutive days, or worsening of any one major symptom together with an increase in any one of the minor symptoms (sore throat, colds, fever without other cause, cough and wheeze) for at least two consecutive days, occurring after randomization, compared with the baseline levels. These symptom-defined exacerbations were captured using an electronic diary, as worsening of symptoms. During the course of the study, a moderate exacerbation was defined as a worsening of COPD symptoms requiring treatment with systemic corticosteroids (SCS) or antibiotics or both; an exacerbation was defined as severe if hospitalization was required in addition to treatment with SCS and/or antibiotics. These investigator-assessed exacerbations requiring healthcare use were recorded on an electronic case report form.20
The proportion of patients achieving ECII at Week 4 and/or Week 12 was calculated based on the number of patients who had the relevant data available both on Day 1 and at Week 4 or Week 12, as applicable. The rates of moderate/severe exacerbations, and the time-to-first subsequent moderate or severe exacerbation and corresponding hazard ratio (HR) were compared between patients achieving ECII and those who did not achieve ECII at Week 4 or 12. The rates and time-to-first subsequent moderate or severe exacerbations were assessed between Weeks 4 and 52 for the evaluation of ECII at Week 4, and between Weeks 12 and 52, for the evaluation of ECII at Week 12. The effect of treatment on ECII was assessed by comparing the proportion of patients achieving ECII with IND/GLY versus those receiving SFC, using both definitions of ECII.
Statistical Analysis
The analysis was performed using the full analysis set (FAS) from the FLAME study. FAS included all randomized patients who received at least one dose of the study drug. The number of moderate or severe exacerbations that occurred during the follow-up treatment periods (from Week 4 or 12 to the end of treatment for the ECII evaluation at Weeks 4 and 12, respectively) was analyzed using a generalised linear model assuming a negative binomial distribution for the rates of moderate or severe exacerbations experienced during the follow-up treatment period. The model included ECII response status at either Week 4 or 12, baseline smoking status, inhaled corticosteroid (ICS) use at screening, baseline severity of airflow limitation, baseline total symptom score, history of COPD exacerbations in the 1 year prior to screening and region as terms of fixed effects for comparing the rate of moderate or severe exacerbations experienced by the two treatment groups. Time-to-first COPD exacerbation was analyzed using a Cox regression model that included the same terms as the generalized linear model. In the FLAME study, exacerbations were reported in <50% of patients treated with IND/GLY; hence, the time-to-first-exacerbation was evaluated in terms of the time at which at least 25% of patients had a first moderate or severe exacerbation instead of the median time.13 A logistic regression model was used to analyze the proportion of patients achieving ECII at either Week 4 or Week 12. The model included terms of treatment (IND/GLY vs SFC), baseline FEV1, baseline CAT/SGRQ-C score (as appropriate), ICS use at screening, baseline smoking status, baseline severity of airflow limitation and region as fixed effects.
Patients
A total of 3362 patients were randomized (1:1) to IND/GLY 110/50 μg q.d. (N = 1680) and SFC 50/500 μg b.i.d. (N = 1682) in the FLAME study. Of these, 3354 patients (IND/GLY, 1675; SFC, 1679) were included in this analysis. Baseline demographics and disease characteristics were well balanced between the treatment arms (Table 2). Detailed demographics are provided in the original FLAME study publication.13
 |
Table 2 Baseline Demographics and Clinical Characteristics (Full Analysis Set) |
Results
ECII Analysis
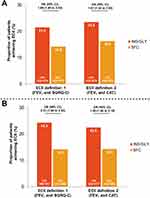
Of the patients who had Day 1 and post-baseline values (either at Week 4 or 12), approximately 18–20% of patients achieved ECII at Week 4 or 12 post-randomization according to any of the two definitions (Figure 1).
Annualized Rate of Moderate or Severe COPD Exacerbations by ECII Definition
The ratios of annualized exacerbations rates were 12–15% lower in patients achieving ECII at Weeks 4 or 12, irrespective of the definition of ECII used (Figure 2).
Time-to-First Moderate or Severe COPD Exacerbation by ECII Definition
Patients achieving ECII by either definition had a longer time-to-first subsequent COPD exacerbation compared with those who did not achieve ECII at Week 4 (25th percentile of the time-to-first exacerbation – ECII definition 1: 173 versus 116 days; HR, 0.81; ECII definition 2: 151 versus 118 days; HR, 0.88; Figure 3A) or Week 12 (25th percentile of the time-to-first exacerbation – ECII definition 1: 163 versus 132 days; HR, 0.82; ECII definition 2: 160 versus 133 days; HR, 0.86; Figure 3B).
Proportion of Patients Achieving ECII with IND/GLY and SFC
The proportions of patients achieving ECII with IND/GLY vs SFC are presented in Figure 4. Overall, more patients achieved ECII with IND/GLY vs SFC both at Week 4 (odds ratio [OR] 95% CI, 1.69 (1.40 to 2.04) and 1.61 (1.34 to 1.93) for ECII definitions 1 and 2, respectively; Figure 4A) and at Week 12 (OR (95% CI), 2.01 (1.66 to 2.44) and 1.80 (1.48 to 2.18) for ECII definitions 1 and 2, respectively; Figure 4B).
Discussion
In this analysis, we evaluated the novel composite endpoint of ECII for the first time using data from the FLAME study.13 We have shown that patients who achieve ECII at Week 4 or 12 are at a lower risk of subsequent COPD exacerbations. Our data suggest that ECII endpoint may be used as an early predictor of exacerbation prevention in clinical trials of COPD. More patients treated with IND/GLY achieved ECII compared with those treated with SFC based on all definitions, both at Week 4 and at Week 12, confirming the potential role of this composite endpoint as a measure of early prediction of exacerbation prevention in the FLAME trial.
An important observation is that a minority of patients (18–20% across definitions) achieved ECII after 4 and 12 weeks, most likely due to the strict criterion of achieving MCID in two variables (lung function and a PRO). Despite this low proportion, the composite endpoint indicated treatment differences during the remainder of the follow-up period. Importantly, the differences between the groups and between treatments were evident from Week 4, suggesting that this composite endpoint may provide clinically relevant information on the subsequent exacerbation risk and prevention by treatments early in the course of a study. Results from this analysis suggest that ECII might be a useful assessment tool to predict subsequent exacerbation risk.
Previous studies have shown that both FEV1 and PROs, especially health status, are associated with exacerbations. A pooled analysis of 23,213 patients from 23 clinical trials showed that greater improvements in trough FEV1 were associated with significantly fewer exacerbations, and better PROs. In this analysis, the improvement in lung function was evaluated at a similar time frame to the occurrence of exacerbations.21 A post-hoc analysis of three 12-month randomized trials in patients with moderate-to-very severe COPD showed that FEV1 response at 2 months predicts the risk of a future exacerbation at 12 months, with the rate of exacerbations during 3–12 months being significantly lower in FEV1 responders at 2 months (patients with improvement in FEV1 ≥100 mL) compared with non-responders.22 ECII not only supports the results from these analyses but also provides evidence on short-term outcomes as early as 4 weeks, which are further validated by comparable outcomes at Week 12.
ECII definitions evaluated here use trough FEV1, SGRQ-C and CAT scores, all of which are frequently used clinical trial endpoints that show responses to treatment within days or weeks.13,23,24 The MCIDs of these endpoints indicate a clinically significant change in response to pharmacological treatment. Our analysis showed that using these endpoints to assess clinically important improvement at Week 4 and/or Week 12 can predict the risk of future exacerbation.
Other tools have been developed or markers identified that can predict the risk of exacerbations in both the short and longer term. Clinically important deterioration (CID) is a composite endpoint that measures disease worsening in terms of rate of decline in lung function, exacerbation rate and health status. CID has been used as a composite endpoint in studies of long-acting bronchodilators, mostly of ≤26 weeks duration to enrich for deterioration events in the short term.25–28 One post-hoc analysis of two 3-year studies (TORCH and ECLIPSE) has been reported to date, where CID was used to predict long-term worsening of COPD; a CID occurring within 6–12 months of follow-up was found to be associated with sustained loss of lung function and health-status and increased exacerbation and all-cause mortality risk.29 Make et al proposed SCOPEX, a score that can predict the short-term risk of exacerbations over the next 6 months.30 Unlike ECII, which predicts the risk of subsequent exacerbation early after an intervention in a clinical trial, based on improvements in clinical outcomes, SCOPEX score is an indicator of the risk of exacerbation in general, based on clinical characteristics and disease history.
Exacerbations occur at relatively low frequency in trials, and often not necessarily as early as 4 or 12 weeks after baseline. The advantage of ECII is that it predicts the reduction in the rate of exacerbation, without measuring actual exacerbations, based on improvements in lung function and a patient-reported outcome, as early as Week 4. Predicting exacerbation risk as early as 4 weeks may allow for more adaptive and novel designs of clinical trials evaluating treatments aiming at exacerbation reduction. The finding that the results at 4 and 12 weeks were very similar supports the use of ECII at 4 weeks.
This analysis has certain limitations. The composite endpoint, ECII, is defined based on MCIDs of the component endpoints included in the analysis. These thresholds need to be validated in large prospective trials. In the present analysis, ECII was defined using FEV1, SGRQ-C and CAT score as endpoints. We recognize that there is no universal definition of ECII and selection of endpoints may be a potential limitation. While SGRQ and CAT both assess impairment in health status, the extent of information captured is different. In addition, the preference for the use of a specific PRO may differ between clinical practice and clinical trials. Hence, it might be preferable to have ECII assessed based on either of these PROs. Future studies may help provide more information on which PRO is a more sensitive predictor, and could help in further enhancements to the definition. Factors such as change in lifestyle, comorbidities, multi-morbidities or cardiovascular disease were not adjusted for this analysis. Also, the ECII outcomes presented here are based on results from the FLAME study. Similar analyses from other studies and prospective trials using ECII are required to further validate the application of this composite endpoint, but the initial results from this analysis are promising. Besides its potential usefulness in clinical trials design and evaluation, ECII may also be useful in clinical practice as an objective measure of how patients will respond to specific treatments in terms of exacerbation prevention, providing clinicians with a tool to predict future risk.
Conclusion
ECII is a novel composite endpoint, based on clinically relevant improvement in lung function and PROs in the early phase of a treatment intervention that may predict subsequent exacerbation risk. This composite endpoint may be useful in the design of future clinical trials on pharmacotherapy for COPD exacerbation prevention.
Abbreviations
b.i.d., twice-daily; CAT, COPD assessment test; CID, clinically important deterioration; COPD, chronic obstructive pulmonary disease; ECII, Early Clinically Important Improvement; FAS, full analysis set; FEV1, forced expiratory volume in 1 second; HR, hazard ratio; ICS, inhaled corticosteroid; IND/GLY, indacaterol/glycopyrronium; MCID, minimal clinically important difference; OR, odds ratio; PRO, patient-reported outcome; q.d., once-daily; SCS, systemic corticosteroids; SGRQ-C, St. George’s Respiratory Questionnaire for COPD.
Data Sharing Statement
Novartis is committed to sharing access to patient-level data and supporting documents from eligible studies with qualified external researchers. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations.
Ethics Approval and Written Informed Consent
The FLAME study protocol and all amendments were reviewed by an Independent Ethics Committee or Institutional Review Board for each center in each country (Japan, Korea, Latvia, Lithuania, Mexico, Netherlands, Norway, Philippines, Poland, Portugal, Romania, Russia, Slovakia, South Africa, Spain, Sweden, Taiwan, Turkey, United Kingdom, Thailand, Croatia, Serbia, Argentina, Austria, Belgium, Bulgaria, Canada, Chile, China, Colombia, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Guatemala, Hong Kong, Hungary, Iceland, India, Italy). The study was conducted as per the guidelines in the Declaration of Helsinki and Good Clinical Practice guidelines. All patients provided written informed content for participation in the FLAME trial.
Acknowledgments
Under the direction of authors, Vatsal Vithlani, Archana Jayaraman and Cathy McDonnell (professional medical writers; Novartis) assisted in the preparation of this article in accordance with the third edition of Good Publication Practice guidelines. Medical writing support was funded by the study sponsor. A part of this paper was presented in the European Respiratory Society International Congress in 2018 (Paris, 15–19 September 2018) as an oral presentation. The oral presentation’s abstract was published in “Oral Abstracts” in the European Respiratory Journal 2018;52:OA1657; doi: 10.1183/13993003.congress-2018.OA1657; available from: https://erj.ersjournals.com/content/52/suppl_62/OA1657.
Author Contributions
All authors contributed towards data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Disclosure
KK was an employee of Novartis Pharma AG at the time of the conduct of this analysis and has received honoraria for presentations and/or consulting services and reports grants and/or personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, ELPEN, GlaxoSmithKline, Menarini, Novartis, Nuvoair, and Sanofi, not related to this work. AJM was a European Respiratory Society Fellow in Industry at Novartis Pharma AG at the time of conduct of this study and is currently employed and hold shares of AstraZeneca. CV reports grants and personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Grifols, Mundipharma, Novartis, personal fees from Cipla, Berlin Chemie/Menarini, CSL Behring, Teva, German Federal Ministry of Education and Research (BMBF) Competence Network Asthma and COPD (ASCONET), and grants from Bayer Schering Pharma AG, MSD, Pfizer, outside the submitted work. SF was an ERS Fellow at Novartis Pharma AG during this project and reports personal fees from Astra Zeneca and Novartis, outside the submitted work. PG is an employee of Novartis. DB, FP and PP are employees and shareholders of Novartis. JAW reports grants from GlaxoSmithKline, Johnson and Johnson, AstraZeneca, Boehringer Ingelheim, Chiesi, and Novartis, outside the submitted work.
References
1. Guimaraes M, Bugalho A, Oliveira AS, Moita J, Marques A. COPD control: can a consensus be found? Rev Port Pneumol. 2016;22(3):167–176. doi:10.1016/j.rppnen.2016.01.004
2. Seemungal TA, Donaldson GC, Bhowmik A, Jeffries DJ, Wedzicha JA. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161(5):1608–1613. doi:10.1164/ajrccm.161.5.9908022
3. Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847–852. doi:10.1136/thorax.57.10.847
4. Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1418–1422. doi:10.1164/ajrccm.157.5.9709032
5. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease 2018. 2018. Available from: http://goldcopd.org/.
6. Bertens LC, Reitsma JB, Moons KG, et al. Development and validation of a model to predict the risk of exacerbations in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2013;8:493–499. doi:10.2147/COPD.S49609
7. Holgate ST. Priorities for respiratory research in the UK. Thorax. 2007;62(1):5–7. doi:10.1136/thx.2006.073882
8. Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi:10.1056/NEJMoa0909883
9. Aliverti A, Rodger K, Dellaca RL, et al. Effect of salbutamol on lung function and chest wall volumes at rest and during exercise in COPD. Thorax. 2005;60(11):916–924. doi:10.1136/thx.2004.037937
10. O’Donnell DE, Lam M, Webb KA. Spirometric correlates of improvement in exercise performance after anticholinergic therapy in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160(2):542–549. doi:10.1164/ajrccm.160.2.9901038
11. Papi A, Vestbo J, Fabbri L, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet. 2018;391(10125):1076–1084. doi:10.1016/S0140-6736(18)30206-X
12. Calverley PMA, Anzueto AR, Carter K, et al. Tiotropium and olodaterol in the prevention of chronic obstructive pulmonary disease exacerbations (DYNAGITO): a double-blind, randomised, parallel-group, active-controlled trial. Lancet Respir Med. 2018;6(5):337–344. doi:10.1016/S2213-2600(18)30102-4
13. Wedzicha JA, Banerji D, Chapman KR, et al. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med. 2016;374(23):2222–2234. doi:10.1056/NEJMoa1516385
14. Wedzicha JA, Decramer M, Ficker JH, et al. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group study. Lancet Respir Med. 2013;1(3):199–209. doi:10.1016/S2213-2600(13)70052-3
15. Jones PW. Interpreting thresholds for a clinically significant change in health status in asthma and COPD. Eur Respir J. 2002;19(3):398–404. doi:10.1183/09031936.02.00063702
16. Jones PW, Quirk FH, Baveystock CM. The St George’s respiratory questionnaire. Respir Med. 1991;85(Suppl B):
17. Kon SS, Canavan JL, Jones SE, et al. Minimum clinically important difference for the COPD assessment test: a prospective analysis. Lancet Respir Med. 2014;2(3):195–203. doi:10.1016/S2213-2600(14)70001-3
18. Ayora AF, Soler LM, Gasch AC. Analysis of two questionnaires on quality of life of chronic obstructive pulmonary disease patients. Rev Lat Am Enfermagem. 2019;27:e3148. doi:10.1590/1518-8345.2624.3148
19. Morishita-Katsu M, Nishimura K, Taniguchi H, et al. The COPD assessment test and St George’s respiratory questionnaire: are they equivalent in subjects with COPD? Int J Chron Obstruct Pulmon Dis. 2016;11:1543–1551. doi:10.2147/COPD.S104947
20. Frent SM, Chapman KR, Larbig M, et al. Capturing exacerbations of chronic obstructive pulmonary disease with EXACT. A subanalysis of FLAME. Am J Respir Crit Care Med. 2019;199(1):43–51. doi:10.1164/rccm.201801-0038OC
21. Donohue JF, Jones PW, Bartels C, et al. Correlations between FEV1 and patient-reported outcomes: a pooled analysis of 23 clinical trials in patients with chronic obstructive pulmonary disease. Pulm Pharmacol Ther. 2018;49:11–19. doi:10.1016/j.pupt.2017.12.005
22. Calverley PM, Postma DS, Anzueto AR, et al. Early response to inhaled bronchodilators and corticosteroids as a predictor of 12-month treatment responder status and COPD exacerbations. Int J Chron Obstruct Pulmon Dis. 2016;11:381–390. doi:10.2147/COPD.S93303
23. Bateman ED, Ferguson GT, Barnes N, et al. Dual bronchodilation with QVA149 versus single bronchodilator therapy: the SHINE study. Eur Respir J. 2013;42(6):1484–1494. doi:10.1183/09031936.00200212
24. Buhl R, Gessner C, Schuermann W, et al. Efficacy and safety of once-daily QVA149 compared with the free combination of once-daily tiotropium plus twice-daily formoterol in patients with moderate-to-severe COPD (QUANTIFY): a randomised, non-inferiority study. Thorax. 2015;70(4):311–319. doi:10.1136/thoraxjnl-2014-206345
25. Singh D, D’Urzo AD, Chuecos F, Munoz A, Garcia Gil E. Reduction in clinically important deterioration in chronic obstructive pulmonary disease with aclidinium/formoterol. Respir Res. 2017;18(1):106. doi:10.1186/s12931-017-0583-0
26. Singh D, Maleki-Yazdi MR, Tombs L, Iqbal A, Fahy WA, Naya I. Prevention of clinically important deteriorations in COPD with umeclidinium/vilanterol. Int J Chron Obstruct Pulmon Dis. 2016;11:1413–1424. doi:10.2147/COPD.S101612
27. Anzueto AR, Vogelmeier CF, Kostikas K, et al. The effect of indacaterol/glycopyrronium versus tiotropium or salmeterol/fluticasone on the prevention of clinically important deterioration in COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:1325–1337. doi:10.2147/COPD.S133307
28. Greulich T, Kostikas K, Gaga M, et al. Indacaterol/glycopyrronium reduces the risk of clinically important deterioration after direct switch from baseline therapies in patients with moderate COPD: a post hoc analysis of the CRYSTAL study. Int J Chron Obstruct Pulmon Dis. 2018;13:1229–1237. doi:10.2147/COPD.S159732
29. Ian Naya LT, Mullerova H, Compton C, Jones P. Long-term outcome following first clinically important deterioration in COPD. Eur Respir J. 2016;46:PA304.
30. Make BJ, Eriksson G, Calverley PM, et al. A score to predict short-term risk of COPD exacerbations (SCOPEX). Int J Chron Obstruct Pulmon Dis. 2015;10:201–209. doi:10.2147/COPD.S69589
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.