Back to Journals » Clinical Ophthalmology » Volume 16
Early Canadian Real-World Experience with Brolucizumab in Anti-Vascular Endothelial Growth Factor-Experienced Patients with Neovascular Age-Related Macular Degeneration: A Retrospective Chart Review
Authors Giunta M, Gauvin Meunier LP, Nixon D, Steeves J, Noble J
Received 26 May 2022
Accepted for publication 18 August 2022
Published 30 August 2022 Volume 2022:16 Pages 2885—2894
DOI https://doi.org/10.2147/OPTH.S376199
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Michel Giunta,1,2 Louis-Pierre Gauvin Meunier,3 Donald Nixon,4,5 Jeff Steeves,6 Jason Noble7,8
1GOGiunta Ophtalmologie, Sherbrooke, QC, Canada; 2Department of Surgery, Centre Hospitalier Universitaire de Sherbrooke, Sherbrooke, QC, Canada; 3Institut de l’Œil des Laurentides, Boisbriand, QC, Canada; 4Northern Ontario School of Medicine, Barrie, ON, Canada; 5TriMed Eye Centre, Barrie, ON, Canada; 6Department of Ophthalmology and Visual Sciences, Dalhousie University, Rothesay, NB, Canada; 7Department of Ophthalmology and Vision Sciences, University of Toronto, Toronto, ON, Canada; 8Sunnybrook Health Sciences Centre, Toronto, ON, Canada
Correspondence: Michel Giunta, GOGiunta Ophtalmologie, 20, 12e Avenue S., #200, Sherbrooke, Québec, J1G 2V4, Canada, Tel +1 819 563 6889, Email [email protected]
Purpose: To assess early real-world outcomes with brolucizumab in Canadian patients with neovascular age-related macular degeneration (nAMD) for which they previously received ≥ 1 anti-vascular endothelial growth factor (anti-VEGF) agent(s).
Patients and Methods: This multisite, real-world, retrospective chart review included data from a consecutive sample of 73 patients who received brolucizumab for nAMD after treatment with ≥ 1 other anti-VEGF agents. The principal reasons for switching to brolucizumab were to extend the treatment interval (51.6% of patients) and to treat persistent macular fluid (34.2%). The primary outcomes were best-corrected visual acuity (BCVA) and the incidence rates of intraocular inflammation (IOI), retinal vasculitis (RV), and retinal vascular occlusion (RVO). Secondary outcomes included central retinal thickness (CRT), injection interval, and presence of intraretinal and subretinal fluid (IRF and SRF). All parameters were measured at baseline until the last treatment visit between April 27, 2020, and August 31, 2021.
Results: Over a mean follow-up of 28 weeks, a nonsignificant mean improvement in BCVA was identified (4.3 [standard deviation (SD) 8.3] letters; P=0.057), with 47.9% experiencing a gain of ≥ 5 letters. IOI was detected in 3 patients (4.1%), one of whom also developed RV and RVO (1.4%), which is consistent with existing brolucizumab data. Significant reductions were observed in mean CRT (− 36.6 μm [SD 56.1 μm]; P=0.0002) and presence of any macular fluid (56.1% [SD 5.6%]; P< 0.001), IRF (66.6% [SD 6.3%]; P< 0.001), and SRF (62.7% [SD 6.3%]; P< 0.001). The mean injection interval increased significantly by 2.1 weeks (SD 2.7; P< 0.001).
Conclusion: In the first real-world Canadian analysis, brolucizumab was associated with improvements in functional outcomes in treatment-experienced patients, consistent with other real-world studies. The incidence of IOI, RV, and RVO were in line with the post hoc safety analysis of HAWK and HARRIER data.
Keywords: best-corrected visual acuity, central retinal thickness, injection interval, intraocular inflammation, retinal vascular occlusion
Introduction
Neovascular age-related macular degeneration (nAMD) is one of the most common causes of severe and irreversible vision loss.1–3 As its name implies, the prevalence and severity of nAMD increase significantly with advancing age.3–6 Anti-vascular endothelial growth factor (anti-VEGF) agents remain the standard of care for patients with nAMD.7,8
The current management of nAMD requires frequent and ongoing intravitreal anti-VEGF injection and can represent a heavy burden for patients, caregivers, physicians, and the healthcare system.9,10 Treatment burden is an important factor driving real-world anti-VEGF undertreatment,11–14 which in turn has been shown to result in inferior clinical outcomes. A multinational real-world study, which included Canada, identified reductions in anti-VEGF injection visits from the first year of therapy (mean 8.6 visits and 5.0 injections) to the second year (mean 4.9 visits and 2.2 injections).11 Mean gain in visual acuity (VA) declined from +2.4 letters in the first year to +0.6 letters in the second year. The principal burden associated with anti-VEGF therapy is the need for frequent treatment and monitoring, which represents considerable loss of patient and caregiver time and leads to reduced work productivity, activity impairment, and increased indirect costs.15–18 Patient anxiety related to their intravitreal injection was also found to be an important burden in a European study, with 93% of 131 patients reporting anxiety, including 54% for ≥2 days prior to their appointment.17 Patient-reported effects of this anxiety included inability to relax (58%), disrupted sleep (26%), reduced concentration (13%), and headache and/or nausea (10%). The most common desired improvements to the injection treatment regimen were fewer injections (42%) and fewer appointments to attend (22%) while achieving the same treatment results.
The Phase 3 HAWK and HARRIER trials found that brolucizumab was noninferior to active-control aflibercept in improving visual function at a longer treatment interval (every 12 weeks [q12w] versus q8w, respectively) in an anti-VEGF-naive population.19 Reduction in central retinal thickness (CRT) and anatomic retinal fluid outcomes favored brolucizumab over aflibercept. Based on these results, brolucizumab was approved by Health Canada on March 12, 2020, for the management of patients with nAMD. Although overall safety with brolucizumab was similar to aflibercept, incidence of intraocular inflammation (IOI) – including uveitis, iritis, and endophthalmitis – was higher in the 3-mg and 6-mg brolucizumab arms.19 In response to post-marketing reports in the United States of cases of IOI after brolucizumab administration, including occlusive retinal vasculitis,20 a post hoc independent Safety Review Committee analysis of HAWK and HARRIER data determined a 4.6% incidence of definite/probable IOI, 3.3% IOI and retinal vasculitis (RV), and 2.1% IOI, RV, and retinal vascular occlusion (RVO).21 According to the post-marketing data, the rates per 10,000 brolucizumab injections are 5.6 for RV, 3.2 for RVO, and 6.7 for both.22
The present study provides the first real-world Canadian data from a retrospective case analysis of patients who were switched from another anti-VEGF agent to brolucizumab. The objectives were to describe safety and efficacy outcomes with this agent in early Canadian clinical practice and to contribute to the growing real-world evidence for this agent.
Materials and Methods
This study is a real-world, multisite, retrospective chart review of patients treated with brolucizumab for nAMD after previous anti-VEGF therapy. The study was approved by an Institutional Review Board (Advarra; Reference ID: Pro00050966) and was conducted in adherence to the tenets of the Declaration of Helsinki.23 Patients provided written informed consent to use their data, and patient data were deidentified by the investigators before inclusion in the study database.
Data Source
Data were gathered from 5 Canadian ophthalmology clinics: 2 in Ontario, 2 in Quebec, and 1 in New Brunswick. The authors input case data between April 27, 2020, and August 31, 2021, pertaining to baseline characteristics, previous anti-VEGF therapy, brolucizumab treatment outcomes at each visit, and adverse event (AE) details into a prespecified Excel® sheet. An independent retina specialist conducted an objective assessment of the data before it was shared with the statistician.
Study Population
For each investigator, all patients with nAMD who satisfied the inclusion/exclusion criteria were identified using electronic medical records or injection logbooks. The study population consisted of patients in Canada ≥50 years of age who were diagnosed with nAMD in the study eye, received ≥1 previous anti-VEGF agent, and for whom brolucizumab was prescribed as a switch by the treating physician according to the current prescribing information. Reasons for switching to brolucizumab were captured as part of the baseline patient characteristics. Full inclusion/exclusion criteria are listed in Supplemental Table S1.
Outcome Measures
The primary outcomes were the change in mean best-corrected VA (BCVA) from baseline to last visit, as measured in Early Treatment Diabetic Retinopathy Study (ETDRS) letters and incidence of IOI, RV, and/or RVO. The primary safety outcome reflected the safety signal identified in brolucizumab during HAWK and HARRIER and in post-marketing clinical experience.
The secondary outcomes of this study focused on whether the treatment advantages associated with brolucizumab administration in HAWK and HARRIER are observed in a Canadian real-world clinical setting.
- Mean change in injection interval and mean number of injections from baseline to last visit
- Mean change in injection interval was calculated as the difference in the mean intervals between the baseline and second brolucizumab injections and between the last 2 injections included in the analysis
- Proportions of patients who experienced ≥5-, ≥10-, and ≥15-letter improvements and ≥5-, ≥10-, and ≥15-letter losses from baseline to last visit
- Mean change in CRT from baseline to last visit
- Proportion of patients with ≥15-μm improvement in CRT from baseline to last visit
- Mean change in the presence of macular fluid from baseline to last visit
Baseline was defined as the date of the first brolucizumab injection.
Statistical Analysis
Descriptive statistics for demographics, disease history, and baseline characteristics are presented for the eligible patients. Categorical variables (eg, sex and race) are presented as the number and percentage of patients in each category. Continuous variables (eg, BCVA) are summarized by the mean, standard deviation (SD), median, and interquartile range (IQR). Descriptive statistics are provided for brolucizumab injections. Intervals between injections and the total length of time on brolucizumab treatment are summarized using descriptive statistics. The number of injections received is presented in frequency tables. No power calculation was performed for this patient sample.
Regression analysis was conducted to determine potential correlations between the number of brolucizumab doses and BCVA or CRT and injection interval and BCVA or CRT. Only data extracted from the patient charts by the treating physician were analyzed. No imputing of missing values was performed.
Differences between groups were evaluated by Student’s t-test (unpaired). For data with non-normal distribution, Mann–Whitney U-test was applied. All tests were 2-sided. Statistical significance was considered present at levels >95% (P<0.05). Statistical analysis was performed using STATA statistical software, version 13.
Results
Data were received for 81 patients from 5 physician sites over a mean of 28 weeks; 73 met the inclusion/exclusion criteria (Supplemental Figure S1). Patient baseline characteristics are presented in Table 1 and Table 2 lists the nAMD treatment history and baseline clinical values. As expected in this population, a high proportion had ≥1 serious comorbidities. Most patients were switched to brolucizumab after their initial anti-VEGF option, and nearly two-thirds were switched to extend the treatment interval. The mean (SD) treatment interval with the patients’ previous anti-VEGF at the time of switch was 4.5 (1.0) weeks.
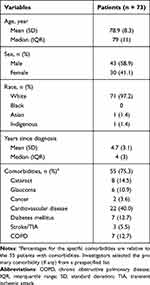 |
Table 1 Baseline Patient Characteristics |
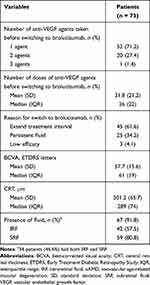 |
Table 2 nAMD Treatment History and Baseline Clinical Values |
BCVA
Over the course of their brolucizumab treatment, patients experienced a numerical mean improvement in their BCVA of 4.3 (SD 8.3) letters compared with their previous anti-VEGF response (Figure 1; Supplemental Figure S2). Significantly more patients gained ≥5 letters (35 [47.9%]) than lost ≥5 letters (8 [11.0%]; SD 5.8; 95% confidence interval [CI] 23.4 to 50.5; P<0.001) (Figure 2). A probable correlation was identified between BCVA and the number of brolucizumab doses given (coefficient −0.85 [standard error (SE) 0.42]; 95% CI −1.69 to −0.01; P=0.05).
 |
Figure 1 Mean change in BCVA. Abbreviations: BCVA, best-corrected visual acuity; CI, confidence interval; ETDRS, Early Treatment Diabetic Retinopathy Study; SD, standard deviation. |
 |
Figure 2 BCVA gains and losses. Abbreviation: BCVA, best-corrected visual acuity. |
Adverse Events
Five cases of IOI were detected in 3 patients (4.1%). Two patients experienced anterior and posterior IOI; 1 case was found to be moderate and the other was severe. IOI was mild in the patient with posterior-only inflammation.
RV and RVO were identified in 1 patient (1.4%) who reported sudden significant visual change with little to no pain 8 weeks after his sixth brolucizumab dose. This patient also experienced severe anterior and posterior IOI. Physical examination at slit lamp identified moderate anterior-chamber (AC) and vitreous inflammation causing a hazy view of the posterior pole, dilated veins akin to central RVO, and infarcts to the superior hemifield from arterial occlusion. Retinal edema was confirmed on optical coherence tomography and fundus photography (Figures 3A and B). VA had declined to counting fingers. Brolucizumab was discontinued, and the patient was started on oral prednisone 60 mg daily for 6 days, then tapered for 2 weeks, and prednisolone 1% drops every hour while awake for 3 days, tapered to q2h for 10 days and then qid for 2 months. On day 3, he had improved to 20/200 and after 2 months was 20/80 (baseline 20/40); however, inferior hemifield visual-field loss persists from the superior occlusive event. The AC inflammation resolved within weeks and the RVO within a month. His macula has remained dry for 5 months.
Brolucizumab was discontinued in a second patient further to AEs. VA at the time of discontinuation was 50 letters, which was a gain of 8 letters from baseline and −1 letter from the previous injection (fourth) visit 3 weeks earlier. Brolucizumab was not discontinued in the patient who experienced moderate anterior and posterior IOI; this patient had gained 4 letters from baseline at the last recorded visit.
CRT
Patients experienced a significant mean reduction in their CRT of 36.6 μm (SD 56.1 μm) from baseline to last injection visit (Figure 4; Supplemental Figure S3). More than 60% of patients (n = 44) experienced a ≥15-μm improvement in CRT over this period. A correlation between CRT and the number of doses given was unlikely (coefficient 1.82 [SE 2.94]; 95% CI −4.04 to 7.67; P=0.54).
 |
Figure 4 Mean change in CRT. Abbreviations: CRT, central retinal thickness; CI, confidence interval; SD, standard deviation. |
Injection Interval
The mean injection interval increased significantly by 2.1 weeks (SD 2.7; 95% CI: 1.5 to 2.7; P<0.001) from baseline (4.7 [SD 2.3] weeks) to last visit (6.8 [SD 2.8] weeks). During this time, patients received a mean of 6.0 (SD 2.3) injections. No significant correlation was found between injection interval and either BCVA (coefficient 0.053 [SE 0.37]; 95% CI −0.68 to −0.78; P=0.89) or CRT (coefficient 2.14 [SE 2.48]; 95% CI −2.80 to 7.08; P=0.39).
Macular Fluid
The overall proportion of patients with any macular fluid decreased from 91.8% at baseline to 40.3% at last visit, representing a 56.1% reduction (SD 5.6; 95% CI 39.2 to 64.9; P<0.001). Presence of intraretinal and subretinal fluid (IRF and SRF) were reduced by 66.6% (57.5% to 19.4%; SD 6.3 95% CI 47.4 to 76.5; P<0.001) and 62.7% (80.8% to 30.6%; SD 6.3 95% CI 35.8 to 63.7; P<0.001), respectively.
Discussion
This Canadian retrospective case study provides a real-world analysis of the safety and efficacy outcomes in early post-approval use of brolucizumab in patients with ≥1 previous anti-VEGF agents for their nAMD. An increase in mean BCVA and reduction in mean CRT were identified from baseline initiation of brolucizumab to the last visit, with the latter achieving statistical significance. Nearly half of patients gained ≥5 letters, while only 11% lost ≥5 letters. A significantly longer mean injection interval and reduced presence of macular fluid were also noted, which were the principal reasons for switching to brolucizumab.
Several real-world retrospective analyses of brolucizumab in treatment-experienced patients with nAMD have been published. No change in BCVA was observed in the BREW study (+0 letters; N = 42 eyes; the United States [US]),24 the SHIFT study (+0.03 logarithm of the minimum angle of resolution [logMAR]; N = 63 eyes; Germany),25 and the analyses of Enriquez et al (−0.8 letters; N = 166 treatment-experienced eyes representing 96.5% of the total study population; US)26 and Walter et al (−0.5 letters; N = 530 eyes; US);27 however, the BRAILLE (India) and REBA studies (Germany and India) found significant improvement: approximately 10 letters (0.91 ± 0.49 logMAR at baseline to 0.73 ± 0.51 logMAR at final visit; P<0.00001) in BRAILLE (N = 74 eyes)28 and +10.4 (SD 4.8; P=0.014) letters in REBA (N = 80 eyes).29
Most studies identified significant reductions in CRT. Our results (−36.6 µm; 12.2% reduction) were similar to the improvements in the BREW (−51 µm [16.2%]), SHIFT (−66.8 µm [16.3%]), Enriquez et al (−26.9 µm [9.1%]), and Walter et al (−40.4 µm [12.1%]) studies.24–27 Larger reductions were observed in the BRAILLE (−130.9 µm [31.8%]) and REBA (−185.7 µm [38.4%]) studies;28,29 however, mean baseline CRTs for these latter patient populations were 411.0 µm and 483.2 µm, respectively. The notable exception was the SHIFT study, in which the relatively modest reduction was from a mean baseline CRT of 409.4 µm.25 Hussain et al found significant reductions in central subfield thickness in the study eyes of patients with nAMD who were switched to brolucizumab from aflibercept (340 µm to 305 µm [10.2%]; P<0.001; n = 48) and from bevacizumab (401 µm to 325 µm [19.0%]; P=0.009; n = 10) after a mean of 6 weeks post-initial brolucizumab injection.30
Few studies reported on the change to treatment interval over the study period. In the REBA study, extension to 12-week dosing was achieved in 31.2% of eyes, and 68.8% of eyes were maintained at 8 weeks.29 The mean treatment interval was extended from 6.3 to 6.8 weeks (+0.5 weeks; P=0.001) in the study by Walters et al27 Rave et al were able to significantly extend the average brolucizumab treatment interval from 33.8 to 60.4 days (P<0.001).31 Extensions of ≥7 days were achieved by 106 (81.5%) of patients. Mean changes in VA and central foveal thickness from baseline to final injection did not reach statistical significance. It should be noted that treatment intervals reflect the individual investigator’s clinical judgement. The Canadian product monographs for the 3 licenced anti-VEGF agents – ranibizumab, aflibercept, and brolucizumab – specify an initial treatment interval of every 4 weeks (monthly for ranibizumab).32–34 After 3 doses, the monographs for aflibercept and brolucizumab allow for the extension of dosing intervals based on the stability of VA and the absence of disease activity.
These studies also supported our findings that brolucizumab reduced IRF and SRF; however, the variance in reported outcomes precludes a more detailed comparison between our study and the current literature.
Our safety results (4.1% IOI and 1.4% IOI and RVO) are consistent with the findings of the Safety Review Committee review of the HAWK and HARRIER data.21 The BREW, BRAILLE, and REBA studies reported no cases of IOI or RVO,24,28,29 and the SHIFT study had no cases of RVO despite 7 patients (11.1%) developing an IOI.25 Enriquez et al reported 14 cases of IOI in 13 patients (8.6%) and 1 associated RVO (0.7%).26 In the study by Walter et al, the incidence of IOI was 4.8% with 1 case (0.2%) of RVO.27 In the REBEL study, which included both treatment-naïve and -experienced patients (N = 282 eyes), 10 patients (3.5%) developed an IOI with 1 case (0.4%) of occlusive vasculitis.35 More than half (54.4%) of patients discontinued brolucizumab use, 86% of whom did so out of concern for the risk of AEs. A second retrospective study involving a mix of treatment-naïve (n = 10 eyes) and -experienced (n = 89 eyes) patients with nAMD identified 8 cases of IOI over an average of 81 days after initial brolucizumab injection.36 The incidence of IOI was 30.0% in treatment-naive eyes and 5.6% in treatment-experienced eyes (P<0.01). Two eyes experienced RV, losing 3 and 10 letters.
A few studies have examined the benefit of anti-VEGF switchback, ie, switching from one agent to another and then back to the original. Despreaux et al (N = 47 eyes in 45 patients) found that switching from initial ranibizumab to aflibercept significantly improved mean BCVA from the initial switch to 3 months after the switchback (66.7 to 68.2 letters; P=0.02); however, statistical significance was lost in a subanalysis of eyes that received ≥3 aflibercept injections.37 Gains of ≥5 letters were achieved by 27.7% of eyes. In a study by Slean et al, 21 eyes (19 patients) with recurrent macular fluid were switched from either bevacizumab or ranibizumab to aflibercept and back again.38 Median central macular thickness improved significantly from initial therapy to aflibercept (317 µm to 285 µm; P=0.034), worsened while on aflibercept (296 µm; P=0.080), and improved on return to the original anti-VEGF (283 µm; P=0.016). Total macular fluid volume was reduced in the first switch to aflibercept (2.56 mm3 to 2.44 mm3; P=0.080), increased with aflibercept use (3.18 mm3; P=0.019), and decreased with the switchback (2.11 mm3; P=0.016). A small (N = 11 patients) study by Koike et al found that slight improvements in logMAR VA (0.22 to 0.24; P=0.62) and CRT (306.8 µm to 256.1 µm; P=0.13) were achieved with switches from aflibercept to ranibizumab for 3 monthly doses and back to aflibercept.39 Extensions in injection intervals were possible in 4 patients (36.4%; 6.7 weeks to 9.3 weeks; P=0.96). No switchback studies have been conducted with brolucizumab.
Study Limitations
This study has several limitations, including its real-world retrospective nature and inherent variance in the assessment and treatment approaches of each individual investigator. This latter factor has the strongest potential to affect outcomes such as treatment interval, which is determined by each investigator’s clinical discretion. The small sample size limits the conclusiveness of our findings and the absence of a control group precludes direct comparison with other populations and study results. Also, the ongoing COVID-19 pandemic may have hampered regular clinical care.
Conclusion
This Canadian retrospective analysis of the efficacy and safety of brolucizumab after switching from ≥1 anti-VEGF agents was consistent with other real-world studies, demonstrating improvements in both BCVA and CRT after switching. Furthermore, significant improvements in injection intervals and decreased macular fluid were observed. Finally, the incidence rates of IOI and RVO were reflective of the values determined in a post hoc safety analysis of HAWK and HARRIER data. Patients with serious AEs were successfully treated with only topical or topical and systemic steroids.
Acknowledgments
The authors wish to acknowledge James Whelan, MD, FRCSC, for his independent oversight of the development of this study and manuscript, Jose Joao Mansure, PhD, CCRP, for his statistical analysis, and Jeff Alexander, SNELL Medical Communication, for his medical writing assistance.
Funding
This study was funded by Novartis Pharmaceuticals Canada Inc.
Disclosure
- Dr. Michel Giunta discloses that he has served on advisory boards for Abbott, Alcon, and Novartis. His institution has received research grants from Allergan, Bayer AG, Chengdu Kanghong Biotech, Hoffmann-La Roche, and Novartis. He has received honoraria as a speaker from Abbott, Alcon, Bausch & Lomb, Bayer AG, and Novartis.
- Dr. Louis-Pierre Gauvin Meunier discloses that he has served on advisory boards for Alcon, Allergan, Bausch & Lomb, Bayer AG, Johnson & Johnson, and Novartis. He has received honoraria as a speaker from Allergan, Bayer AG, Johnson & Johnson, and Novartis, and as a consultant for Novartis. He reports personal fees from SNELL Medical Communication Inc.
- Dr. Donald Nixon discloses that he has served on advisory boards for Bayer AG, Novartis, Johnson & Johnson, and Sentrex. He has received research grants from Bayer AG, Novartis, Johnson & Johnson, and Sentrex. He has received honoraria as a speaker from Bayer AG, Novartis, Johnson & Johnson, and Sentrex. He receives patent payments from Oculus.
- Dr. Jeff Steeves discloses that he has received honoraria as a speaker from Novartis.
- Dr. Jason Noble discloses that he has served on advisory boards for Bayer and Novartis.
References
1. Li JQ, Welchowski T, Schmid M, Mauschitz MM, Holz FG, Finger RP. Prevalence and incidence of age-related macular degeneration in Europe: a systematic review and meta-analysis. Br J Ophthalmol. 2020;104:1077–1084. doi:10.1136/bjophthalmol-2019-314422
2. Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106–e116. doi:10.1016/S2214-109X(13)70145-1
3. National Eye Institute. Age-related macular degeneration (AMD) data and statistics. Available from: https://www.nei.nih.gov/learn-about-eye-health/resources-for-health-educators/eye-health-data-and-statistics/age-related-macular-degeneration-amd-data-and-statistics.
4. Klein R, Chou C-F, Klein BEK, et al. Prevalence of age-related macular degeneration in the US population. Arch Ophthalmol. 2011;129:75–80. doi:10.1001/archophthalmol.2010.318
5. Klein R, Klein BEK, Knudtson MD, Meuer SM, Swift M, Gangnon RE. Fifteen-year cumulative incidence of age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology. 2007;114:253–262. doi:10.1016/j.ophtha.2006.10.040
6. Friedman DS, O’Colmain BJ, Munoz B, et al.; Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572.
7. American Academy of Ophthalmology. Age-related macular degeneration preferred practice pattern®; 2019. Available from: https://www.aao.org/preferred-practice-pattern/age-related-macular-degeneration-ppp.
8. Canadian Agency for Drugs and Technologies in Health. CADTH therapeutic review: anti-vascular endothelial growth factor drugs for the treatment of retinal conditions — recommendations report; 2016. Available from: https://www.cadth.ca/sites/default/files/pdf/TR0009_Anti-VEGFs_Recs_Report.pdf.
9. Wolf A, Kampik A. Efficacy of treatment with ranibizumab in patients with wet age-related macular degeneration in routine clinical care: data from the COMPASS health services research. Graefes Arch Clin Exp Ophthalmol. 2014;252:647–655. doi:10.1007/s00417-013-2562-6
10. Boulanger-Scemama E, Querques G, About F, et al. Ranibizumab for exudative age-related macular degeneration: a five year study of adherence to follow-up in a real-life setting. J Fr Ophtalmol. 2015;38:620–627. doi:10.1016/j.jfo.2014.11.015
11. Mehtà H, Tufail A, Daien V, et al. Real-world outcomes in patients with neovascular age-related macular degeneration treated with intravitreal vascular endothelial growth factor inhibitors. Prog Retin Eye Res. 2018;65:127–146. doi:10.1016/j.preteyeres.2017.12.002
12. Ciulla TA, Hussain RM, Pollack JS, Williams D. Visual acuity outcomes and anti-vascular endothelial growth factor therapy intensity in neovascular age-related macular degeneration patients: a real-world analysis of 49 485 eyes. Ophthalmol Retina. 2020;4:19–30. doi:10.1016/j.oret.2019.05.017
13. Ozkaya A, Alkin Z, Togac M, Ahmet S, Perente I, Taskapili M. Five-year outcomes of ranibizumab in neovascular age-related macular degeneration: real life clinical experience. Korean J Ophthalmol. 2017;31:424–430. doi:10.3341/kjo.2016.0125
14. Holz FG, Tadayoni R, Beatty S, et al. Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br J Ophthalmol. 2015;99:220–226. doi:10.1136/bjophthalmol-2014-305327
15. Pina Marin B, Gajate Paniagua NM, Gómez-Baldó R, Gallego-Pinazo R; AMD-MANAGE Investigators. Burden of disease assessment in patients with neovascular age-related macular degeneration in Spain: results of the AMD-MANAGE study. Eur J Ophthalmol. 2022;32:385–394. doi:10.1177/11206721211001716
16. Spooner KL, Mhlanga CT, Hong TH, Broadhead GK, Chang AA. The burden of neovascular age-related macular degeneration: a patient’s perspective. Clin Ophthalmol. 2018;12:2483–2491. doi:10.2147/OPTH.S185052
17. Sivaprasad S, Oyetunde S. Impact of injection therapy on retinal patients with diabetic macular edema or retinal vein occlusion. Clin Ophthalmol. 2016;10:939–946. doi:10.2147/OPTH.S100168
18. Jaffe DH, Chan W, Bezlyak V, Skelly A. The economic and humanistic burden of patients in receipt of current available therapies for neovascular age-related macular degeneration. J Comp Eff Res. 2018;7:1125–1132. doi:10.2217/cer-2018-0058
19. Dugel PU, Koh A, Ogura Y, et al.; HAWK and HARRIER Study Investigators. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 127;2020:72–84. doi:10.1016/j.ophtha.2019.04.017
20. American Society of Retina Specialists. Member update: Novartis-appointed safety review committee reports initial brolucizumab findings; 2020. Available from: https://www.asrs.org/clinical/clinical-updates/4013/Member-Update-Novartis-Appointed-Safety-Review-Committee-Reports-Initial-Broluci.
21. Monés J, Srivastava SK, Jaffe GJ, et al. Risk of inflammation, retinal vasculitis, and retinal occlusion-related events with brolucizumab: post hoc review of HAWK and HARRIER. Ophthalmology. 2021;128:1050–1059. doi:10.1016/j.ophtha.2020.11.011
22. Novartis. Post-marketing data in patients with wet AMD. Available from: https://www.brolucizumab.info/Post-marketing-data.
23. World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi:10.1001/jama.2013.281053
24. Sharma A, Kumar N, Parachuri N, et al. Brolucizumab—early real-world experience: BREW study. Eye. 2021;35:1045–1047. doi:10.1038/s41433-020-1111-x
25. Bulirsch LM, Saßmannshausen M, Nadal J, Liegl R, Thiele S, Golz FG. Short-term real-world outcomes following intravitreal brolucizumab for neovascular AMD: SHIFT study. Br J Ophthalmol. 2021;106:1288–1294. doi:10.1136/bjophthalmol-2020-318672
26. Enriquez AB, Baumal CR, Crane AM, et al. Early experience with brolucizumab treatment of neovascular age-related macular degeneration. JAMA Ophthalmol. 2021;139:441–448. doi:10.1001/jamaophthalmol.2020.7085
27. Walter SD, Saba NJ Efficacy and durability of brolucizumab in patients being transitioned from prior anti-VEGF therapy.
28. Chakraborty D, Maiti A, Sheth JU, et al. Brolucizumab in neovascular age-related macular degeneration – Indian real-world experience: the BRAILLE study. Clin Ophthalmol. 2021;15:3787–3795. doi:10.2147/OPTH.S328160
29. Bilgic A, Kodjikian L, March de Ribot F, et al. Real-world experience with brolucizumab in wet age-related macular degeneration: the REBA study. J Clin Med. 2021;10:2758. doi:10.3390/jcm10132758
30. Hussain RM, Neal A, Yannuzzi NA, et al. Real world experience of brolucizumab for persistent macular fluid in neovascular age-related macular degeneration after prior anti-VEGF treatments.
31. Rave V, Sharma K, Wagner A, Kapoor K. Real-world analysis of brolucizumab in neovascular AMD. Invest Ophthalmol Vis Sci. 2021;62:431.
32. Novartis Pharmaceuticals Canada Inc. Lucentis® (ranibizumab injection) product monograph; 2021.
33. Bayer Inc. Eylea® (aflibercept injection) Product Monograph; 2022.
34. Novartis Pharmaceuticals Canada Inc. Beovu® (brolucizumab injection) product monograph; 2020.
35. Aziz AA, Khanani AM, London N, et al. Real world efficacy and safety of brolucizumab in neovascular AMD: the REBEL study. Invest Ophthalmol Vis Sci. 2021;62(8):451.
36. Hamou SJ, Raimondo CS, Weber P, Woods BC. A retrospective study on the use of brolucizumab for the treatment of NVAMD: a 1-year private practice experience. Invest Ophthalmol Vis Sci. 2021;62:286.
37. Despreaux R, Cohen SY, Semoun O, et al. Short-term results of switchback from aflibercept to ranibizumab in neovascular age-related macular degeneration in clinical practice. Graefes Arch Clin Exp Ophthalmol. 2016;254(4):639–644. doi:10.1007/s00417-015-3084-1
38. Slean GR, Hemarat K, Khurana RN, Stewart JM. Conversion back to bevacizumab or ranibizumab for recurrent neovascular activity with aflibercept in age-related macular degeneration: a case series. Int J Retina Vitreous. 2016;2:2. doi:10.1186/s40942-016-0028-9
39. Koike N, Otsuji T, Tsumura A, et al. Results of switchback from ranibizumab to aflibercept in patients with exudative age-related macular degeneration. Clin Ophthalmol. 2019;13:1247–1251. doi:10.2147/OPTH.S206910
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

