Back to Journals » Cancer Management and Research » Volume 11
Early and sustained deep molecular response achieved with nilotinib in high Sokal risk chronic myeloid leukemia patients
Authors Zaidi U, Kaleem B, Borhany M , Maqsood S , Fatima N , Sufaida G, Ansari SH, Farzana T, Shamsi TS
Received 3 October 2018
Accepted for publication 17 December 2018
Published 15 February 2019 Volume 2019:11 Pages 1493—1502
DOI https://doi.org/10.2147/CMAR.S181911
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Harikrishna Nakshatri
Uzma Zaidi,1 Bushra Kaleem,2 Munira Borhany,1 Sidra Maqsood,2 Naveena Fatima,2 Gul Sufaida,3 Saqib Hussain Ansari,1 Tasneem Farzana,1 Tahir Sultan Shamsi1
1Department of Clinical Hematology, National Institute of Blood Diseases & Bone Marrow Transplantation, Karachi, Pakistan; 2Department of Clinical Research, National Institute of Blood Diseases & Bone Marrow Transplantation, Karachi, Pakistan; 3Department of Molecular Medicine, National Institute of Blood Diseases & Bone Marrow Transplantation, Karachi, Pakistan
Background: Nilotinib (Tasigna®) is a second-generation tyrosine kinase inhibitor that shows faster and deeper molecular responses (MR) in comparison to Imatinib as initial therapy in chronic phase chronic myeloid leukemia (CML). Efficacy and safety data for nilotinib in the Asian population is scarce, particularly in Pakistan. We aimed to determine the MR to nilotinib and its safety profile in patients with chronic phase CML.
Patients and methods: This observational study was conducted among 173 patients with newly diagnosed CML presenting in the chronic phase. Most patients (50.1%) had a high Sokal score at diagnosis. All patients received nilotinib 600 mg/day. The hematological and molecular responses were assessed at 3 and 6 months respectively and thereafter at 6-monthly intervals. Long-term event free survival (EFS), transformation free survival (TFS), overall survival (OS) and adverse events were observed.
Results: Cumulative incidence of major MR (MMR) was 86% and deep MR (DMR ie MR 4.0 and MR4.5) was 39%. Early MMR and DMR after 6 months of therapy were achieved by 74.9% and 37% of patients, respectively. Two-year EFS, TFS and OS rates for all patients were 91.9%, 92% and 92.3%, respectively. At median follow-up of 24 months, 81% and 49% of patients sustained MMR and DMR, respectively. The main adverse events were weight gain (4.6%) and abdominal pain (4%).
Conclusion: This study showed promising results in terms of achievement of early and sustained DMR in chronic phase CML, therefore, we recommend nilotinib as frontline treatment in Pakistani population.
Keywords: chronic myeloid leukemia, tyrosine kinase inhibitors, nilotinib, molecular response, Sokal Risk Score
Background
Chronic myeloid leukemia (CML) is a clonal myeloproliferative disorder characterized by the presence of breakpoint cluster region-abelson (BCR-ABL) oncoprotein that has markedly enhanced tyrosine kinase activity.1 Treatment outcomes and survival rates for patients with CML in chronic phase have substantially improved with the emergence of tyrosine kinase inhibitors (TKIs).2,3The results of the International Randomized Study of Interferon and STI571 trial, comparing interferon vs imatinib, showed superior response rate and improved progression-free survival in the imatinib group, compared with previous standard therapy. However, long-term follow-up revealed failure to achieve a complete cytogenetic response (CCyR) in 18% of patients, loss of response in ~10%, and intolerance to imatinib in 4%–8%.4
This led to the development of second-generation TKIs (nilotinib, dasatinib, and ponatinib), which are more potent inhibitors of BCR-ABL kinase activity.5 Nilotinib (Tasigna®) was found to be active against most imatinib-resistant mutations of BCR-ABL, except T315I, and induced durable CyRs in ~50% of patients in chronic phase CML when used as second-line therapy.6 Thereafter, nilotinib received US Food and Drug Authority approval for first-line treatment of CML, based on the results of the Phase III, multicenter, open-label, randomized trail Evaluating Nilotinib Efficacy and Safety in Clinical Trials–Newly Diagnosed Patients (ENESTnd), which compared two different doses of nilotinib with standard dose of imatinib. The results of that trial revealed higher rates of major molecular response (MMR) with nilotinib compared with imatinib (71% with nilotinib 300 mg twice daily, 67% with nilotinib 400 mg twice daily, and 44% with imatinib).7,8
The Sokal risk scoring system is widely used to stratify risk in CML patients at baseline to predict the response to treatment and prognosis. Most of the studies have shown that at diagnosis, two-thirds of patients with chronic phase CML were in the low Sokal risk group. In a study by Cortes et al, in which nilotinib was used as frontline therapy in chronic phase CML, 70% of patients had a low Sokal risk score at diagnosis.9
Pakistan is a developing country and it has always been difficult to provide optimal health care to patients because of limited health resources. In Pakistan, imatinib and nilotinib are the only TKIs available for use. In most areas, imatinib is still being used as first-line treatment, with 65%–70% of patients achieving CCyR. This is believed to be the first study of CML patients from all over Pakistan to report the molecular response (MR) to nilotinib as front-line therapy in high, intermediate, and low Sokal risk patients. The aim of this study was to highlight the benefit of achieving early and sustained deep MRs (DMRs) with nilotinib, which are needed to achieve treatment-free remission and reduce the economic burden on health authorities. We also observed the number of adverse events with nilotinib and the improvement in overall survival (OS) and outcome of CML in our population.
Patients and methods
Patients
This was an observational study conducted from March 2011 to June 2017. The study was approved by the Institutional Review Board of the National Institute of Blood Diseases and Bone Marrow Transplantation (NIBD-RD-70/15–2011). Informed written consent for participation in the study was obtained from all patients. We included patients aged ≥18 years, newly diagnosed with chronic phase of CML by bone marrow biopsy and detection of Philadelphia-positive chromosomes by conventional karyotyping/fluorescent in situ hybridization or positive BCR-ABL result from PCR (using Ipsogen BCR-ABL1 Mbcr RGQ RT-PCR Kit with detection limit of 0.001% on International Scale [IS]) in peripheral blood or bone marrow aspirate. Sokal risk score was calculated in all patients at baseline, who were categorized into low-, intermediate-, and high-risk groups. Patients who had received hydroxyurea for cytoreduction were also included in the study. Women of child-bearing age were counseled for strict contraception during treatment and a negative pregnancy test was obtained before commencing nilotinib therapy. However, patients with severe cardiac dysfunction and Eastern Cooperative Oncology Group performance status ≥2 were excluded because of the risk of cardiotoxicity.
Treatment and response definitions
All patients received nilotinib 300 mg twice daily orally, 1 hour before meals. Baseline spleen size was recorded in all patients. Baseline laboratory data included complete peripheral blood counts, hepatic and renal profiles, and levels of lactate dehydrogenase and serum calcium, phosphorous and uric acid. Complete Blood Count was done in all patients initially every 2 weeks and then monthly. Molecular monitoring of BCR-ABL transcripts was performed in the molecular department of our institute at 6-monthly intervals, contrary to European LeukemiaNet (ELN) recommendations of 3-monthly monitoring to minimize the financial burden on patients and health authorities. Ultrasensitive CML Monitoring Xpert® BCR-ABL Ultra was used, which has a detection limit of ≤0.001%, equivalent to five log reduction of IS, as defined later. Treatment was continued in all patients without dose modification until there was any sign of disease progression or loss of response. Adverse events were recorded at each visit and graded according to National Cancer Institute Common Toxicity Criteria version 4.0.10 Treatment was interrupted transiently if the neutrophil and platelet count fell below 0.5×109/L and 40×109/L, respectively. TKI domain mutational analysis was performed using amplification-refractory mutation system–PCR in patients who were unresponsive to treatment.
Operational definitions
Response to treatment was assessed according to ELN recommendations 2013.11 Complete hematological response (CHR) was defined as normalization of peripheral blood counts, absence of immature granulocytes, basophilia, and blast cells, and disappearance of palpable splenomegaly at 3 months of treatment. MMR was defined as a BCR-ABL/ABL transcript ratio <0.1%. DMR (MR 4 and MR 4.5) was defined as BCR-ABL/ABL transcript ratio <0.01% and <0.0032% on IS, respectively. The terms of optimal response, warning, and treatment failure were used to categorize patients as per ELN recommendations. Loss of response or resistance to nilotinib at any time during treatment was considered as treatment failure, and patients were tested for BCR-ABL tyrosine kinase domain mutations and clonal evolution by conventional karyotyping.
Survival outcomes
Event-free survival (EFS) was calculated from the first dose of nilotinib to the first event of loss of CHR, MMR, or CyR (if available), dose interruption because of adverse effects/non-availability of drug, evidence of disease progression, development of TKI resistance or death for any reason during therapy. Transformation-free survival (TFS) was calculated from the first dose of nilotinib to the first documentation of disease transformation into accelerated or blast phase. OS was calculated from the first dose of nilotinib to the date of death or last follow-up.
Statistical analysis
SPSS version 20.0 was used to calculate the frequency of qualitative variable ie, gender, and the mean, median, and SD of quantitative variables, such as age, blast cells, basophils, hemoglobin, platelets, and white blood cells. A Sokal calculator was used to categorize patients into low-, intermediate- and high-risk groups. OS, EFS, and TFS probabilities were estimated by the Kaplan–Meier method.
Ethics approval and consent to participate
All procedures performed in the study involving human participants were in accordance with the ethical standards of the Institutional Review Board of National Institute of Blood Diseases and Bone Marrow Transplantation and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors. Informed consent was obtained from all the individual participants included in the study.
Results
One hundred and seventy-three newly diagnosed patients with chronic phase CML received nilotinib between March 2011 and June 2017. The baseline characteristics of the patients are shown in Table 1. The median age of patients was 38 years and male to female ratio was 1.25:1. The median time from diagnosis to start of treatment was 1 month (range 1–23 months). The median follow-up time was 24 months (range 0.7–79 months). Eighty-eight (51.4%) patients had high Sokal risk score at baseline. Almost all patients received hydroxyurea for cytoreduction before starting nilotinib. For all patients, data points were available at diagnosis and then at every 6 months till their last follow-up. Baseline biochemical profile of patients is shown in Table 2.
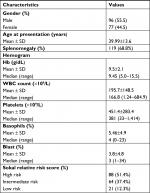  | Table 1 Baseline characteristics of patients with CML-CP Abbreviation: CML-CP, chronic myeloid leukemia in chronic phase. |
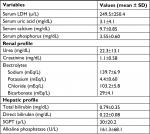  | Table 2 Biochemical parameters of patients with CML-CP Abbreviations: CML-CP, chronic myeloid leukemia in chronic phase; LDH, lactate dehydrogenase; SGPT, serum glutamic pyruvic transaminase. |
CHR was achieved by 163 (95.3%) of patients at 3 months after starting therapy. Six of eight (4.6%) patients who failed to achieve CHR had a high Sokal risk score. One patient died after 3 months of follow-up from a non-CML-related cause, while one patient was excluded from the study because she had conceived. Four patients remained in chronic phase and continued nilotinib therapy, and three of them achieved CHR at 7 months. The remaining patients never achieved CHR.
One hundred and seventy-one patients were evaluable for MR. Overall, 147 (86%) of patients achieved MMR. Six months after starting nilotinib, 126 (73.9%) of patients achieved MMR, while 63 (37%) of them showed DMR. Among the high Sokal risk group, 61 (69.3%) and 31 (35.2%) of patients achieved MMR and DMR, respectively, at 6 months (Table 3). Response durability was assessed in all patients over a median follow-up of 24 months (range: 0.7–79 months). Overall, 138 (81%) and 84 (49%) of patients were able to sustain MMR and DMR, respectively, while 72 (81.8%) and 45(51.5%) of patients in the high Sokal risk group sustained MMR and DMR, respectively, at 24 months of treatment.
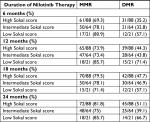  | Table 3 Categorization of molecular response based on Sokal score Abbreviations: DMR, deep molecular responses; MMR, major molecular responses |
Response rates according to ELN criteria 2013 are shown in Table 4. Optimal response at 12, 24, 48, and 72 months was achieved in 122 (71.1%), 121 (70.9%), 127 (74.1%), and 78 (45.4%) of patients, respectively. Treatment failure by ELN definition at 12, 24, 48, and 72 months occurred in 18(10.5%), 32 (18.8%), 24 (13.9%), and 35 (20.5%) of patients, respectively.
  | Table 4 Response assessment according to ELN recommendations 2013 Abbreviation: ELN, European LeukemiaNet. |
Six of 171 patients transformed to the accelerated phase at 12, 14, 19, 24, 28, and 33 months of therapy and four to blast phase at 12, 17, 18, and 27 months. The characteristics of patients with disease progression are described in Table 5. All six patients in accelerated phase continued nilotinib therapy, and four of them responded to escalated dose of nilotinib and subsequently reverted to chronic phase. Patients in blast phase discontinued nilotinib therapy.
  | Table 5 Characteristics of patients with disease progression Note: *statistical significance. |
TKI domain mutational analysis revealed a total of 34 out of 46 patients with mutations and the analysis was performed in those who either failed to achieve an optimal response or those who progressed into accelerated or blast phase. Of them, T315I (n=19), E255K (n=6), G250E (n=3), Y253H (n=3), M351T (n=2), and E255V (n=1) mutations were commonly observed. Among 34 patients, those with T315I mutation were switched to alternate treatment due to non-availability of ponatinib in the country. Patients harboring E255K/V, Y253H, and M351T mutations were switched to dasatinib. The G250E mutation positive patients were switched to high dose of imatinib. These patients remained in the chronic phase of disease. Clonal evolution was not detected in any of the study patients. The estimated EFS, TFS, and OS rates for the whole group were 91.9% (Figure 1), 92% (Figure 2), and 92.3% (Figure 3), respectively, at 24 months.
  | Figure 1 EFS in CML patients treated with nilotinib as first-line therapy distributed on the basis of Sokal risk score. Abbreviations: CML, chronic myeloid leukemia; EFS, event-free survival. |
  | Figure 3 Overall survival in CML patients treated with nilotinib as first-line therapy distributed on the basis of Sokal risk score. Abbreviation: CML, chronic myeloid leukemia. |
The adverse events commonly observed were weight gain in 8 (4.6%) patients and abdominal pain in 7 (4%) (Figure 4A and B). A small proportion of our patients, ie, 2 (1.15%), had deranged hepatic profile that was managed by minor dose reduction. Severe neutropenia and thrombocytopenia occurred in only 8 (5%) and 7 (4%) patients respectively, and they were corrected by intermittent dose interruptions for 1–2 weeks (Figure 4B). None of the patients required treatment withdrawal due to hematological or systemic adverse events.
  | Figure 4 Non-hematological (A) and hematological (B) adverse event profile of patients on nilotinib therapy. |
Discussion
Compared with imatinib, second-generation TKIs are now recognized to induce earlier and deeper cytogenetic and molecular responses.12 Results of 5 years of follow-up of patients receiving nilotinib in the ENESTnd trial had higher rates of CyR, DMR, and OS compared with those with imatinib.13,14 The Dasatinib versus Imatinib study (DASISION) in treatment-naive CML patients, with dasatinib as frontline therapy, also reported the positive impact of attaining early and deep MRs on patient outcomes.15
In Pakistan, the choice of TKIs is limited to imatinib and nilotinib. There is an increasing need to establish the efficacy and safety of nilotinib in our population to provide optimal care at minimal cost, and improve survival and outcome at the same time. In this study, we observed that a higher number of patients receiving standard dose nilotinib achieved the desired MR within appropriate timelines defined in international guidelines. Since no local data on molecular monitoring are available in Pakistan, we compared our results with the ENESTnd trial. We found that a significant proportion of patients achieved early and deeper MRs and sustained these over a median follow-up of 24 months.
Previously, a prospective study conducted at another center in Pakistan, including 304 consecutive patients with chronic phase CML treated with imatinib, reported superior and sturdier long-term CyRs. Estimated 5-year EFS and OS of all patients were 79% and 86%, respectively. CCyR was achieved in 206 (67.8%) patients.16 Comparing our results with this imatinib study, it is evident that nilotinib produced better OS and TFS, when used in a frontline setting. Primary endpoints of both these studies were different, that is, CyR in the imatinib study compared with MR in our study.
The impact of attaining early MRs on OS is so far unclear. Long-term follow-up of patients will predict the beneficial effect on survival. Recent trials have suggested that patients who demonstrate stable DMRs may be considered for cessation of treatment in a clinical trial setting.17–19
Sokal scoring is the most widely used risk scoring system to predict the prognosis of patients with CML at baseline. It was developed in 1984 and was intended for use with patients treated with interferon and hydroxyurea.20 However, its applicability in the TKI era has been studied by various groups, but the results pertaining to its authenticity are contradictory.21 Kantarjian et al suggested that nilotinib could improve CCyR and MMR rates at 24 months across all Sokal risk groups in the ENESTnd study.8 We calculated the Sokal score of all patients at baseline and found predominantly high-risk patients in our study group. High Sokal score was attributable to the presence of thrombocytosis at initial presentation in most patients. This differs from the published literature. In a large series of patients studied by Hasford et al, high Sokal score was reported in 24% patients only.22 A Japanese study evaluated the prognostic significance of different scoring systems and reported lower numbers of patients with a high-risk Sokal score (18.6%).23
CML is reported in older age groups worldwide. The median age of our patients at diagnosis was 38 years; this is in accordance with other studies showing that CML is manifested at an earlier age in Asians because of specific genetic polymorphisms.24 Bansal et al from India reported a median age of 38 years in newly diagnosed CML patients.25 Two previous studies published from Pakistan have reported median ages of 38 and 37 years.16,26
In our study, the rate of disease transformation to accelerated or blast phase was not high despite a large proportion of patients with high Sokal risk score. The risk factors for disease transformation in our patients were mainly failure to achieve CHR and MMR within desired timelines. This was despite being compliant with the standard dose of nilotinib and acquisition of TKI mutations at different times during treatment. One of the five patients who transformed to blast phase had transient treatment interruption because of financial constraints. The patient remained unresponsive after restarting nilotinib, developed T315I mutation, and eventually evolved into blast phase.
Resistance to TKI is a major impediment in gaining optimal response to treatment and disease progression in CML patients. Point mutations in the BCR-ABL kinase domain can lead to TKI resistance and are responsible for treatment failure in many cases.27 Clinically, relevant mutations associated with nilotinib are E255K/V, F359V/C, and T315I.28 T315I mutation was the most commonly found mutation in our patients, followed by E255K. Twenty of 36 patients remained in the chronic phase of CML despite the presence of the mutations. Only few of these patients could be offered the third-generation TKIs because of the limited availability of these drugs in Pakistan. We did not find any significant association of age, high white blood cell count, and blast percentage with disease progression and transformation in our study group.
The safety and tolerability of nilotinib has been studied in various clinical trials. The adverse events reported with nilotinib are infrequent and usually tolerable. Long-term use in western populations is associated with cardiovascular events and renal and gastrointestinal disturbances, hence it is not recommended for use in patients with severe cardiac or renal dysfunction.29,30 ELN has recently published a detailed review of the long-term adverse effects of TKIs, according to which, second-generation TKIs are associated with unexpected harmful effects in patients, some of which may be reversible.31 Therefore, it is imperative to monitor patients closely to minimize any undesirable effects of the drug. Nilotinib seemed to be well tolerated in our patients. The most common adverse events reported were weight gain and abdominal pain. Drug-induced myelosuppression was minimal and mostly noticed during the first 6 months of treatment. Grade 3 or 4 neutropenia and thrombocytopenia were observed in a small number of patients during the early phase of treatment and were managed with transient treatment interruption and dose reduction. No significant elevation in liver enzymes was found in our patients as reported previously by Cortes9 and no cardiovascular event was reported. Cardiotoxicity with nilotinib is usually reported with higher doses (800 mg),31 this could be one of the main reasons of low cardiac adverse events observed in our patients as majority was compliant and tolerant to treatment and achieved desirable MRs at standard (600 mg) dose. The occurrence of CML in relatively younger age group of patients, with good performance status and insignificant co-morbidities and a reduced environmental exposure to risk factors, such as smoking and alcoholism due to religious prohibition could be the other reasons attributable to reduced hepatotoxicity and cardiotoxicity observed in our patients. Overall, nilotinib demonstrated a good safety profile with no unexpected systemic adverse events during treatment.
Our study had some limitations. First, for economic reasons, molecular monitoring of BCR-ABL transcripts was performed only at 6-monthly intervals in all patients. Second, CyR was not evaluated, which was also mainly due to financial constraints.
Conclusion
Frontline treatment of CML with nilotinib in Pakistani population, with most being in the high Sokal risk group, achieved early and durable DMRs leading to considerably improved outcome. Long-term treatment with nilotinib was well tolerated. Nilotinib should be considered as first-line treatment throughout the country because of its better tolerability and induction of deeper MR.
Acknowledgments
All the patients, their families, doctors, nurses, and laboratory staff are being acknowledged for all the immense support that was provided throughout this study. We thank Cathel Kerr, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript. The abstract of this paper was submitted for the EHA Conference 2018 in the category of poster presentation.
Author contributions
UZ and MB contributed in development of the main idea of the study, literature search, and manuscript writing. BK performed mutational and statistical analyses. MB, SHA, and TF contributed in the critical review of the manuscript. SM and NF performed the data collection and statistical analysis. GS performed the molecular testing. TSS critically reviewed and approved the final manuscript. All authors contributed toward data analysis, drafting and revising the paper, gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Ren R. Mechanisms of BCR-ABL in the pathogenesis of chronic myelogenous leukaemia. Nat Rev Cancer. 2005;5(3):172–183. | ||
Ic H. Current management of chronic myeloid leukemia with tyrosine kinase inhibitors. Turkish J Hematol. 2013;30:247–255. | ||
Ferdinand R. Mitchell S A, Batson S, Tumur I. Treatments for chronic myeloid leukemia: a qualitative systematic review. J Blood Med. 2012;3:51–76. | ||
Hochhaus A, O’Brien SG, Guilhot F, et al; IRIS Investigators. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia. 2009;23(6):1054–1061. | ||
Kantarjian HM, Giles F, Gattermann N, et al. Nilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is effective in patients with Philadelphia chromosome positive chronic myelogenous leukemia in chronic phase following imatinib resistance and intolerance. Blood. 2007;110(10):3540–3546. | ||
Kantarjian H, Giles F, Wunderle L, et al. Nilotinib in imatinib-resistant cml and Philadelphia chromosome-positive ALL. N Engl J Med. 2006;354(24):2542–2551. | ||
Rosti G, Palandri F, Castagnetti F, et al. Nilotinib for the frontline treatment of Ph(+) chronic myeloid leukemia. Blood. 2009;114(24):4933–4938. | ||
Kantarjian HM, Hochhaus A, Saglio G, et al. Nilotinib versus imatinib for the treatment of patients with newly diagnosed chronic phase, Philadelphia chromosome-positive, chronic myeloid leukaemia: 24-month minimum follow-up of the phase 3 randomised ENESTnd trial. Lancet Oncol. 2011;12(9):841–851. | ||
Cortes JE, Jones D, O’Brien S, et al. Nilotinib as front-line treatment for patients with chronic myeloid leukemia in early chronic phase. J Clin Oncol. 2010;28(3):392–397. | ||
Cancer Institute N. Common terminology criteria for adverse events (CTCAE) Version 4.0; 2009. Available from: http://www.hrc.govt.nz/sites/default/files/CTCAE%20manual%20-%20DMCC.pdf. Accessed June14, 2010. | ||
Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122(6):872–884. | ||
Jain P, Kantarjian H, Alattar ML. Analysis of long term responses and their impact on outcomes in patients with chronic phase CML treated with four different TKI modalities – analysis of 5 prospective clinical trials. Lancet Haematol. 2015;2(3):e118–e128. | ||
Hughes TP, Coutre PD, Jootar SLRA. ENESTnd 5-year follow-up: continued benefit of frontline nilotinib (NIL)compared with imatinib (IM) in patients (PTS) with chronic myeloid leukemia in chronic phase (CML-CP). Haematologica. 2014;99(s1):236–237. | ||
Larson RA, Kim D-W, Issaragrilsil S. Efficacy and safety of nilotinib (NIL) vs imatinib (IM) in patients (pts) with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP): long-term follow-up (f/u) of ENESTnd. Blood. 2014;124(21):4541. | ||
Cortes JE, Jones D, O’Brien S, et al. Results of dasatinib therapy in patients with early chronic-phase chronic myeloid leukemia. J Clin Oncol. 2010;28(3):398–404. | ||
Aziz Z, Iqbal J, Akram M, Saeed S. Treatment of chronic myeloid leukemia in the imatinib era. Cancer. 2007;109(6):1138–1145. | ||
Saußele S, Richter J, Hochhaus A, Mahon FX. The concept of treatment-free remission in chronic myeloid leukemia. Leukemia. 2016;30(8):1638–1647. | ||
Nicolini FE, Noël M-P, Escoffre M. Preliminary report of the STIM2 study: a multicenter stop imatinib trial for chronic phase chronic myeloid leukemia de novo patients on imatinib. Blood. 2013;122(21):654. | ||
Takahashi N, Kyo T, Maeda Y, et al. Discontinuation of imatinib in Japanese patients with chronic myeloid leukemia. Haematologica. 2012;97(6):903–906. | ||
Oyekunle AA, Osho PO, Aneke JC, Salawu L, Durosinmi MA. The predictive value of the Sokal and Hasford scoring systems in chronic myeloid leukaemia in the imatinib era. J Hematol Malig. 2012;2(2):25–32. | ||
Yahng SA, Jang EJ, Choi SY, Lee SE, Kim SH, Kim DW. Prognostic discrimination for early chronic phase chronic myeloid leukemia in imatinib era: comparison of Sokal, Euro, and EUTOS scores in Korean population. Int J Hematol. 2014;100(2):132–140. | ||
Hasford J, Baccarani M, Hoffmann V, et al. Predicting complete cytogenetic response and subsequent progression-free survival in 2060 patients with CML on imatinib treatment: the EUTOS score. Blood. 2011;118(3):686–692. | ||
Yamamoto E, Fujisawa S, Hagihara M, et al. European treatment and outcome study score does not predict imatinib treatment response and outcome in chronic myeloid leukemia patients. Cancer Sci. 2014;105(1):105–109. | ||
Kuntegowdanahalli LC, Kanakasetty GB, Thanky AH, et al. Prognostic and predictive implications of Sokal, Euro and EUTOS scores in chronic myeloid leukaemia in the imatinib era-experience from a tertiary oncology centre in Southern India. Ecancermedicalscience. 2016;10:679. | ||
Bansal S, Prabhash K, Parikh P. Chronic myeloid leukemia data from India. Indian J Med Paediatr Oncol. 2013;34(3):154–158. | ||
Usman M, Syed NN, Kakepoto GN, Adil SN, Khurshid M. Chronic phase chronic myeloid leukemia: response of imatinib mesylate and significance of Sokal score, age and disease duration in predicting the hematological and cytogenetic response. J Assoc Physicians India. 2007;55:103–107. | ||
Soverini S, Gnani A, Colarossi S, et al. Philadelphia-positive patients who already harbor imatinib-resistant Bcr-Abl kinase domain mutations have a higher likelihood of developing additional mutations associated with resistance to second- or third-line tyrosine kinase inhibitors. Blood. 2009;114(10):2168–2171. | ||
Branford S, Melo JV, Hughes TP. Selecting optimal second-line tyrosine kinase inhibitor therapy for chronic myeloid leukemia patients after imatinib failure: does the BCR-ABL mutation status really matter? Blood. 2009;114(27):5426–5435. | ||
Rea D. Management of adverse events associated with tyrosine kinase inhibitors in chronic myeloid leukemia. Ann Hematol. 2015;94(Suppl 2):149–158. | ||
Rosti G, Castagnetti F, Gugliotta G, Palandri F, Baccarani M. Physician’s guide to the clinical management of adverse events on nilotinib therapy for the treatment of CML. Cancer Treat Rev. 2012;38(3):241–248. | ||
Steegmann JL, Baccarani M, Breccia M, et al. European LeukemiaNet recommendations for the management and avoidance of adverse events of treatment in chronic myeloid leukaemia. Leukemia. 2016;30(8):1648–1671. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

