Back to Journals » International Journal of General Medicine » Volume 12
Dysphagia in Patients with Sporadic Inclusion Body Myositis: Management Challenges
Authors Mohannak N , Pattison G , Hird K , Needham M
Received 11 September 2019
Accepted for publication 21 November 2019
Published 5 December 2019 Volume 2019:12 Pages 465—474
DOI https://doi.org/10.2147/IJGM.S198031
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Nika Mohannak,1 Gemma Pattison,2 Kathryn Hird,1 Merrilee Needham1,3,4
1School of Medicine, The University of Notre Dame, Fremantle, Western Australia, Australia; 2Department of Speech Pathology, Royal Perth Hospital, Perth, Western Australia, Australia; 3Department of Neurology, Fiona Stanley Hospital, Murdoch, Western Australia, Australia; 4Institute for Immunology and Infectious Diseases, Murdoch University, Murdoch, Western Australia, Australia
Correspondence: Merrilee Needham
Institute for Immunology and Infectious Diseases, Murdoch University, Building 390 Discovery Way, Murdoch, WA 6150, Australia
Tel +61 8 9360 1334
Fax +61 8 9360 1380
Email [email protected]
Abstract: Dysphagia in inclusion body myositis (IBM) is common and associated with increased mortality and morbidity due to aspiration pneumonia, malnutrition and dehydration. There is currently no consensus on treatment of dysphagia in IBM and outcomes are variable depending on timing of intervention, patient preference and available expertise. There is a paucity of research exploring the pathophysiology of dysphagia in IBM and appropriate investigations. Increased knowledge of the aetiopathogenesis is likely to change the approach to treatment as well as improve the quality of life for patients. This review explores the epidemiology and pathophysiology of dysphagia in IBM and the currently available treatment strategies.
Keywords: inflammatory myopathies, swallowing, epidemiology, pathophysiology, treatment, diagnosis
Introduction
Inclusion body myositis (IBM) is the most common acquired muscle disease in Caucasians over 50 years of age.1 The disease is characterised by progressive weakening of selected muscle groups including the quadriceps, long forearm flexors and the muscles of the oropharynx.2 Dysphagia is a frequent and potentially fatal complication of IBM. Dysphagia in IBM patients is linked to medical complications including malnutrition, dehydration, recurrent aspiration pneumonia and decreased quality of life.3,4 Any intervention that improves swallowing function in IBM patients therefore has the capacity to significantly improve quality of life, and potentially reduce the medical complications and mortality associated with IBM.
Although some advances have been made in the treatment of IBM, particularly in the areas of exercise and support of respiratory function, the management of dysphagia remains a somewhat limited area of research. Although the first case of IBM was described in 1967 in a 66-year-old man with chronic polymyositis and mild dysphagia,5 it was not until 1988 when Wintzen and colleagues performed myotomies in patients with IBM that dysphagia as a part of the clinical picture was formally identified.6 Until recently, little attention has been given to dysphagia in IBM. A Cochrane review in 2016 identified only one randomised controlled trial of intervention for dysphagia in IBM and concluded that evidence was lacking for or against any specific intervention for dysphagia.7
Many aspects related to diagnosis and treatment of dysphagia in IBM pose challenges to management. In this review, we address the epidemiology of dysphagia in IBM, the diagnosis of dysphagia in IBM, the latest concepts in the pathophysiology of dysphagia in IBM and the current opinion on the optimal management of dysphagia in IBM. We also discuss the importance of addressing dysphagia, the challenges as well as emerging approaches to treatment.
Epidemiology of Dysphagia in IBM
The reported incidence of dysphagia in IBM is variable, owing in part to the insidious nature of the symptoms as well as patient selection methods (eg, case series, directed questionnaire, clinical evaluation). The overall incidence of dysphagia has been reported to be as low as 40%3,8,9 and as high as 80%.9–11 In an observational study of 40 IBM patients, 40% were found to incidentally have dysphagia at the time of diagnosis.12 However, when a directed questionnaire specifically seeking symptoms of dysphagia was utilised in a cohort with IBM, symptoms were reported in 80%11 In a retrospective study of 526 cases of oropharyngeal dysphagia, muscle disorders accounted for 5.7%, with two thirds of these noted to be due to inflammatory myopathy.13 IBM patients demonstrate the most severe and frequent dysphagia compared to other inflammatory myopathies.14
These prevalence figures are however almost certainly an underestimate.11,15,16 In a study performed by Schrey and his colleagues, dysphagia was either ignored or not recognised and therefore not treated in seven of the 25 patients with IBM.15 Similarly, Cox and his colleagues reported poor correlation between complaints of dysphagia and abnormalities on Videofluroscopic Swallow Studies (VFSS).11 Patients who denied dysphagic symptoms were found to have marked abnormalities on VFSS.11 Murata and his colleges examined two cohorts of patients with IBM: those reporting dysphagia and those reporting no dysphagia.17 In VFSS studies pharyngeal propulsion, defined as cricopharyngeal achalasia, was observed across all of the trial participants reinforcing that dysphagia occurs sub-clinically in IBM patients who may not report swallowing impairments.17
Dysphagia in IBM patients is typically diagnosed late in the course of the disease after limb weakness has been established.18 The poor outcomes of treatment in dysphagia may be attributed to the advanced stage at presentation. Though it is viewed as a late complication of IBM, dysphagia does not appear related to disease severity and can in some cases be a presenting symptom.4,6,9,11,19–22 The incidence of dysphagia as a presenting symptom is as high as 50%4 and as low as 10%12 In one case series dysphagia was the presenting symptom in 15% of cases and predated other manifestations by up to 10 years.3 Recently, Shibata and his colleagues described a case of IBM where dysphagia was the isolated presenting symptom for a period of 5 years prior to the onset of any other muscle weakness.23
While there are marked differences in the reported incidence and timing of presentation of dysphagia, it is evident that dysphagia is a common manifestation of IBM. There is a need for further epidemiological surveys to determine whether the varying differences in incidence and timing of presentation of dysphagia in IBM are related to how they are detected in different clinics, or whether the differences are real and due to genetic or other aetiological factors. It is important for clinicians to consider IBM as a differential diagnosis in patients over 50 years who present with dysphagia that is thought to be due to neuromuscular weakness.
Pathophysiology of Dysphagia in IBM
Characterising the pathophysiology of dysphagia in IBM is desirable for diagnostic purposes as well as targeting specific management. There is a paucity of research investigating the pathophysiology of dysphagia specific to IBM. More often it has been included as part of broader inflammatory myopathy studies. Whilst there is significant symptom overlap, patients with IBM demonstrate more severe and frequent dysphagia11,14 and it is associated with poorer outcomes and higher risk of aspiration pneumonia.4,6,11,14,17,24,25 This is further complicated by subclinical dysphagic processes and non-recognition by clinicians and patients, hindering early detection and intervention.11 Having a clear understanding of the aetiopathogenesis of dysphagia specific to IBM will result in improved understanding of physiology and targeted approach to detection and management, in turn, improved patient outcomes.
Initially, it was thought that the underlying aetiopathogenesis of dysphagia in IBM was due to spasm of the upper esophageal sphincter (UES) as a result of hyperplasia or hypertrophy of the cricopharyngeus muscle.25–27 Subsequent studies indicated that reduced UES opening is a constant clinical feature.4,11,14,17,26,28–30 Various causes may account for this in IBM including fibrosis of cricopharyngeal muscle,31 suprahyoid muscle weakness,14 diminished descending bolus forces30 and impaired relaxation or spasm of UES.31 One study using combination VFSS and manometry indicated both suprahyoid and pharyngeal muscle weakness were responsible for impaired UES opening in IBM rather than cricopharyngeal spasm as previously suggested.14 With our current understanding of IBM and the effect on skeletal muscle perhaps this makes more sense.
Reduced UES opening can lead to pyriform fossa stasis30 being reported by the patient as the sensation of food stuck in the throat, resulting in the need to swallow repeatedly.9,11,28 Additionally impaired pharyngeal muscle contraction,11,17,29,30 pharyngeal weakness,14,17,28,29 decreased epiglottic deflection,17,29 impaired laryngeal elevation,4,11,14,31,32 reduced tongue control and poor base of tongue retraction28 have also been implicated in dysphagia in IBM. However, a recent study using MRI imaging of 20 patients did not report impaired laryngeal elevation.10
Evidence of aspiration on VFSS is high in IBM with figures ranging from 35% to 58%.11,15,28 This variability regarding the timing, amount and source of aspiration warrants further investigation. In two studies involving 23 and 35 patients with IBM evidence of aspiration was reported in 35% and 53%, respectively.11 A more recent study of 25 cases reported aspiration in 58% of patients and frank aspiration pneumonias in 25% of patients.15 Despite the number of analysed cases roughly being the same amongst these three studies, there is a notable difference in the reporting of aspiration and figures are most likely to be within 50–60%.
The majority of dysphagia in IBM research has focussed on the oro-pharyngeal phase of swallowing. However, one study of 4 patients highlighted oesophageal striated muscle involvement leading to oesophageal dysphagia characterised by a reduction of upper esophageal peristalsis and low UES pressures.33 This area also warrants further investigation.
On a histopathological level, like in the limb muscles, the cricopharyngeal muscle biopsies have both an inflammatory response and degenerative changes including rimmed vacuoles.13,25–27 It has been suggested that inflammation contributes to reduced compliance of the sphincter and therefore impedes the opening of the UES.11,26,29 It is interesting to note that whilst the histology between the cricopharyngeal muscle and limb muscles are identical, the pathological outcome of the cricopharyngeal muscle is hyperplasia and hypertrophy rather than atrophy as observed in the limb muscles. There is a need for more research to investigate the underlying reasons behind this phenomenon.
The definitive pathophysiology of dysphagia in IBM is yet to be elucidated. Understanding the pathogenesis of the disordered swallow in IBM will, in turn, help to identify more suitable targets for treatment of dysphagia.34 Collectively, it is evident that pathophysiology lies significantly but not solely within the oro-pharyngeal stage of swallowing.
Diagnosis of Dysphagia in IBM
The diagnosis of dysphagia can be difficult as the clinical course of IBM varies between patients including site of onset, rate of progression of weakness and extent of muscle weakness and atrophy. Dysphagia is a manifestation of the systemic disease and not a disease isolated to the oropharynx, although as stated above, it may start in this muscle group. Dysphagia in IBM may manifest as a feeling of stasis, it is reported as a need to swallow repeatedly, a need to wash solids down with liquids, regurgitation, or choking.28,32,35 There is often a delay in the diagnosis of dysphagia in IBM,13 and there a number of reasons for this.
Due to the insidious nature of their disease, IBM patients often become accustomed to minor difficulties swallowing over many years/months, and other issues seem more functionally important, so therefore unless prompted, they do not report it unless it is severe and progressive. Cox et al suggested that the two most sensitive questions to detect dysphagia history in IBM are11,15 “Does food get stuck in your throat?” and “Do you have to swallow repeatedly to get rid of food?”11
In addition, patients may have marked swallowing abnormalities on investigation but be asymptomatic.17 Incomplete opening of the UES and pharyngeal propulsion defined as cricopharyngeal achalasia occurs early without warning signs and was observed in all IBM patients, even those who did not report any difficulties with their swallowing function in the Murata et al study.17 The underlying process of this dysfunction may be due to inflammatory involvement of the cricopharyngeus and pharyngeal constrictor muscles early in the disease.
Investigations of Dysphagia in IBM
Dysphagia can be diagnosed by clinical history and confirmed instrumentally by VFSS and flexible endoscopic evaluation of swallowing (FEES). Manometry and MRI imaging are currently not used in routine clinical practice but may have the potential to aid diagnosis. Clinical history with specific questioning, using Cox et al11 screening questions above, may identify difficulty with swallowing but instrumental analysis is required to characterise the temporal disruption of swallowing coordination and identify the underlying mechanisms involved. Instrumental analysis is considered the gold standard in the diagnosis of dysphagia and should be considered in all IBM patients whose clinical history suggests some swallowing difficulties (refer to Figure 1).
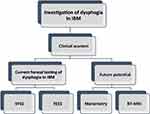 |
Figure 1 Investigation paradigm in IBM. |
VFSS adequately assesses all phases of the swallow and allows the anatomy and physiology of patient’s oropharyngeal dysphagia to be defined.36 It is an adequate assessment for swallowing function, penetration and aspiration risk. The most common VFSS abnormalities in IBM reported by Oh and his colleagues4 include impaired tongue base retraction, cricopharyngeal dysfunction, decreased laryngeal elevation and pharyngeal pooling. These findings have been reproducible in multiple studies looking at dysphagia in IBM11,14,17,32,37 and therefore yield reliability. Videofluroscopic abnormalities are common in IBM patients and were noted to be as high as 72% in the Langdon et al paper.14 The recent introduction of objective measures and normative data for VFSS16 will ensure more specific data regarding progression rates and a move towards dysphagia staging in IBM. The major disadvantage of videofluoroscopy imaging is radiation exposure.38
In FEES, a laryngoscope is passed transnasally to the hypopharynx to view the larynx and pharynx.36 Food and drinks are dyed to aid visualization of the bolus.36 FEES allows an assessment of the anatomy, secretions and pharyngeal phase of the swallow.16,30 Vallecular and hypopharyngeal stasis of both secretions and bolus can be observed and rated accordingly.16 Both VFSS and FEES can diagnose aspiration and penetration reliably. The FEES registry study39 showed that FEES was safely and efficaciously used in a large cohort of patients with different diagnosis, among them 29 patients with myopathies.39
Manometry can quantify the strength of pharyngeal contraction, the completeness of UES relaxation, and the relative timing between these two events. According to Oh et al28 pharyngoesophageal manometry findings included low amplitude pharyngeal constrictor contraction (75%), normal resting tone and relaxation of the UES (82%), and diminished inferior oesophageal sphincter pressure (42%).28 These manometry findings have been reproducible in multiple studies.13,14,17 Concurrent use of pharyngeal manometry with video fluoroscopy can further delineate underlying pathology and direct treatment modalities.14
Recent literature focuses on the use of novel real-time MRI (RT-MRI) which is as reliable as VFSS and FEES for evaluating swallowing in IBM patients.38,40 A study conducted by Carstens and his colleagues using the RT-MRI on 22 patients found that IBM patients were more comfortable with RT-MRI than VFSS or FEES imaging modalities.38,40 An advantage of the RT-MRI is that it provides additional information such as quantitative functional and morphologic pathologies, which are not provided by VFSS or FEES methods38,40 and there is no radiation exposure.
Instrumental assessment is currently considered the gold standard in the diagnosis of dysphagia and should be considered in all IBM patients whose clinical history suggest some swallowing difficulties. VFSS is reasonable as an initial assessment and will assess for swallowing dysfunction, aspiration risk and penetration events. The use of manometry as a diagnostic tool following VFSS analysis allows the underlying mechanism of the dysphagia to be delineated. Information such as pressure response of UES, timing and strength of pharyngeal contraction, UES relaxation and the relationship between these events from manometry will, in turn, direct the appropriate treatment modality (particularly if considering an invasive treatment option). Following the promising recent research, there is potential for this to change in the future with RT-MRI imaging to be utilised as both a screening and diagnostic tool as it does not rely on X-ray exposure and allows direct visualisation of all the swallowing muscles.
There is a need for both increased reporting from patients and increased specific questioning from clinicians. This will help identify the symptoms at an earlier stage, promote earlier intervention and awareness and lead to further studies on treatments, and in turn a consensus on the most appropriate treatment. Conservative treatments may be effective if diagnosed in the early phase and therefore early diagnosis of dysphagia is paramount.11,15,41
Treatment Strategies for Dysphagia in IBM
Currently, treatment options for IBM can be broadly categorized into non-invasive and invasive, refer to Figure 2.
 |
Figure 2 Summary of current treatment strategies for dysphagia in IBM patients. |
Non-Invasive Treatment Options for Dysphagia in IBM
Non-Invasive treatment options for dysphagia secondary to IBM include compensatory as well as medical approaches. Exercise therapy is the only proven therapy to delay the progression of IBM in limb muscles.42–45 A case report by Malandraki and colleagues outlines a lingual strengthening program that was suggested to be effective in maintaining disease-related lingual strength loss and swallowing.46 The Mendelsohn manoeuvre is a compensatory manoeuvre associated with peak pharyngeal contraction, contraction duration and longer bolus transit time.4 It increases the extent and duration of laryngeal elevation and thereby increases the duration and width of UES opening47. The Mendelsohn manoeuvre was useful for helping some IBM patients maintain a stable weight and continue eating without aspiration28,48 but is likely to be more helpful early in the disease course while there is still some remaining muscle function. However, many patients find the Mendelsohn manoeuvre difficult to complete and therefore compliance is an issue.7,49 The insidious decline in swallowing function and delayed presentation of dysphagia in many IBM patients may mean that it is already too late for conservative management by the time it is detected.
There are no established pharmacological treatments for dysphagia in IBM, although multiple studies have investigated the use of intravenous immunoglobulin (IVIG). The use of IVIG has been reported to coincide with some improvement of dysphagia.31,50–56 A randomised controlled trial by Dalakas et al53 showed an improvement in the swallowing function in IVIG randomised IBM patients compared with placebo.53 Similarly, Dobloug and his colleagues found short-term beneficial effects in severe dysphagia following IVIG treatment in a selected number of IBM patients.54 In a case series, Murata and his colleagues described a cohort that were previously unable to eat half solid meals improved after IVIG administration.31 The treatment efficacy, however, lasted 2 months.31 Cherin et al followed four IBM patients with dysphagia who were treated with IVIG and they reported an improvement of swallowing after the third treatment.50
Studies have also investigated the use of subcutaneous immunoglobulin to achieve long-lasting stabilization of swallowing. A case report described the use of subcutaneous immunoglobulin as beneficial on an IBM patient.57 The improvement in swallowing following subcutaneous immunoglobulin was also supported by the results of a case series by Cherin and colleagues who demonstrated improvement in muscle strength and resolution of dysphagia symptoms in six cases.33
A commonality of all the studies that investigated the use of immunoglobulin is that the patient cohort had moderate or severe IBM and the beneficial effect was reported in the short-term. Future studies should include patients with early (newly diagnosed) IBM as this group might be more responsive to immunoglobulin treatment than those with advanced disease. There is still no consensus globally on the role of immunoglobulin for the treatment of dysphagia in IBM and there is a need for further high quality longer trials of immunoglobulin, and the development of novel therapies to help treat this disabling complication of IBM.
Invasive Treatment Options for Dysphagia in IBM
Currently, invasive options are the most effective in treating dysphagia in IBM and are utilised when severe weight loss and malnutrition are apparent.58
Balloon dilation of the pharyngoesophageal segment decreases cricopharyngeal retraction and therefore keeps the UES open. It is simple, minimally invasive and low-cost. In a study of three IBM patients with dysphagia, 3 months of balloon dilation therapy used in conjunction with IVIG resulted in patients previously unable to eat regular meals, eating regular meals for at least one-year post-therapy.31 These results contrast to Oh and his colleagues who performed a retrospective review of 26 patients and found that dilations were performed in one-quarter of the patients and that two-thirds did not note any benefit despite a number of repeated procedures.28
Botulinum toxin injection to the UES/cricopharyngeus muscle was found to be effective in alleviating dysphagia and reducing the rate of aspiration.4,15,24,28,58,59 Initially trialed by Schneider and his colleagues in 1994,58 botulinum toxin was found to improve swallowing in all but 2 of 7 patients with cricopharyngeus muscle dysfunction. Following this study, Liu et al59 described two IBM patients who experienced beneficial effects on their dysphagia with durations of 6.4 to 8 months post-injection.59 Similarly, Di Pede et al combined botulinum toxin injections with rehabilitation and found improvement of swallowing in three IBM patients.24 Botulinum toxin was beneficial in Schrey et al study whereby repetitive injections of BoNT-A alleviated dysphagia in 12 patients.15 The injections not only showed symptomatic improvement in all 12 patients but completely eliminated aspiration in seven of the 12 patients who demonstrated aspiration pre-the injection.15 Preliminary results from a recent abstract also demonstrate the beneficial effect of botulinum toxin injections in IBM patients.60 Botulinum toxin injection is associated with improved quality of life according to a study by Kelly and his colleagues.61
Conversely, Oh et al4 described two IBM patients for whom botulinum toxin injection was not effective at alleviating dysphagia. The doses of the toxin and the injection technique, however, were not reported.4,28 An overall disadvantage of the botulinum toxin is that it requires repeated administration and the potential complication of hoarseness or an exacerbation of dysphagia rather than relief62 due to the diffusion of botulinum toxin to the adjacent proximal pharynx.
The combination of balloon dilation and BoNT-A, however, has been suggested to improve dysphagia over the long term. In a retrospective study by Parres in 201563 patients who had balloon dilations were compared to patients who had dilation with concurrent Botox injection into the cricopharyngeus. The combination of dilation and BoNT-A resulted in transient worsening of dysphagia followed by improvement lasting 4–5 months.63 Larger studies are needed to investigate whether the combination of the two approaches can augment one another.
The most invasive treatment method is cricopharyngeal myotomy. It has been reported to provide relief for a large percentage of patients.12,29,64–66 Around 60% of patients with IBM who undergo myotomy report benefit.2,28 The myotomy divides the cricopharyngeus muscle and redistributes pressure such that bolus propulsion out of the pharynx requires less pressure.67 It is effective albeit a non-reversible method and is not suitable for all IBM patients. Langdon and her colleagues suggest that myotomy is only appropriate treatment for dysphagia based on the aetiology of the swallow.14 They specify that in patients where laryngeal excursion, intrabolus pressure, tongue and pharyngeal propulsion are adequate but the UES fails to relax are the most likely benefit from a myotomy.14 Conversely, IBM patients that have abnormal hyolaryngeal excursion or a hyporeflexic sphincter will not benefit from a cricopharyngeal myotomy.14 Another patient group that cricopharyngeal myotomy is not suitable for is those with hiatus hernia. A case report illustrated an IBM patient with dysphagia and incidental finding of hiatus hernia. Treatment of the dysphagia with a cricopharyngeal myotomy, subsequently resulted in aspiration pneumonia and assisted ventilation post-operation.68
Another invasive option to treat the burden of eating and malnutrition associated with severe dysphagia in IBM with the use of a percutaneous endoscopic gastrostomy (PEG) feeding tube. PEG requires an invasive endoscopic procedure with insertion of the feeding tube through the anterior abdominal wall, an operation which occasionally can be complicated by bleeding, peritonitis or perforation of other abdominal organs.69 In the Oh et al study PEG placement occurred in about one-quarter of IBM patients without clear effect on outcome due to the severity of the disease.28 The PEG placement does not always replace oral intake or eliminate the risk of aspiration. The 6 patients that received a PEG placement in the Oh et al study died during the course of the study due to aspiration.28 The role of the PEG tube in IBM is unclear at this stage and PEG seems to be used as a last resort for refractory dysphagia in IBM.21,56,63 There is a need for studies that investigate its use earlier in the disease course.
In summary, the current evidence suggests that minimally invasive and invasive methods are more effective long term than non-invasive in terms of improving swallowing as an endpoint. Minimally invasive methods should be considered first-line to improve swallowing in IBM patients with moderate-severe dysphagia. If dysphagia is not alleviated by balloon dilation or botox or a combination of the two, the patient may benefit from a more invasive method, such as cricopharyngeal myotomy. The benefits of treating debilitating and severe dysphagia outweigh the risks of the invasive methods as the treatment is likely to significantly improve the quality of life of the patient.
Significance of Addressing Dysphagia in IBM Patients
Swallowing is an essential function of the body and important for human life and socialization. An inability to swallow therefore has significant effects on social, emotional and medical aspects of IBM patients.
IBM patients who have dysphagia isolate themselves more, avoid eating out with other people due to embarrassment and the fact they may require more assistance during meals.70 There are adverse effects of dysphagia on self-esteem, socialization and enjoyment of life.70 The quality of life of IBM patients with dysphagia is significantly impaired and they become more socially isolated.
Complications of dysphagia include aspiration leading to chest infection and pneumonia, malnutrition, dehydration and increased length of hospital stay and re-admission to hospital.71 Pneumonia especially is a frequent complication of, occurring in all patients diagnosed with dysphagia secondary to IBM.28,72 A strong relationship has been established between laryngeal penetration/aspiration and pneumonia.73 As a result, dysphagia is directly associated with an increased risk of aspiration pneumonia and death.72,73 Further, nutritional deficits in protein increase the breakdown of muscle, thus contributing to the progression of this disease.
Conclusions and Future Directions
Due to the complex and insidious nature of IBM, the diagnosis and management of dysphagia is challenging and more research is required to further characterise and manage dysphagia in patients with IBM. Detailed imaging and functional studies may yield more information on the underlying mechanisms in the musculature of the oropharynx and lead to more specific therapies, and better-designed clinical trials to test these.
There is a need to develop more effective forms of treatment that are non-invasive in nature and that will delay or stop the progression of dysphagia, particularly when introduced early in the course of the disease. A commonality between all current treatment modalities is that they are most effective when dysphagia is diagnosed early. Therefore, a strong emphasis should be placed on the screening, diagnosis and investigation of dysphagia during the early stages of the disease – ideally at diagnosis because the delay-to diagnosis remains around 5 years.3,12,74–76 Most IBM patients are unaware of the potential sinister significance of dysphagia as a symptom or the implications of dysphagia on their health, wellbeing and social life. Some IBM patients accept dysphagia as an untreatable part of aging and the disease process and therefore more emphasis should be placed on educating patients so they know to report early when it is only a minor issue, as this will form a vital part of the overall treatment strategy. Clinicians should systematically screen for swallowing function at each appointment using the questions suggested by Cox et al.11
The ultimate treatment goal of dysphagia in IBM patients is to prevent aspiration and its related consequences, maintain nutrition and hydration and to improve quality of life for patients.
Acknowledgements
We thank Lisa Majteles, Head of Department of Speech Pathology at Fiona Stanley Hospital, Perth WA for her comments on the manuscript.
Disclosure
All authors of this review article report no conflicts of interest in this work.
References
1. Needham M, Corbett A, Day T, Christiansen F, Fabian V, Mastaglia FL. Prevalence of sporadic inclusion body myositis and factors contributing to delayed diagnosis. J Clin Neurosci. 2008;15(12):1350–1353. doi:10.1016/j.jocn.2008.01.011
2. Houser SM, Calabrese LH, Strome M. Dysphagia in patients with inclusion body myositis. Laryngoscope. 1998;108(7):1001–1005. doi:10.1097/00005537-199807000-00009
3. Dimachkie MM, Barohn RJ. Inclusion body myositis. Curr Neurol Neurosci Rep. 2013;13(1):321. doi:10.1007/s11910-012-0321-4
4. Oh TH, Brumfield KA, Hoskin TL, Stolp KA, Murray JA, Bassford JR. Dysphagia in inflammatory myopathy: clinical characteristics, treatment strategies, and outcome in 62 patients. Mayo Clinic Proc. 2007;82(4):441–447. doi:10.4065/82.4.441
5. Chou SM. Myxovirus-like structures in a case of human chronic polymyositis. Science (New York, NY). 1967;158(3807):1453–1455. doi:10.1126/science.158.3807.1453
6. Wintzen AR, Bots GT, de Bakker HM, Hulshof JH, Padberg GW. Dysphagia in inclusion body myositis. J Neurol Neurosurg Psychiatry. 1988;51(12):1542–1545. doi:10.1136/jnnp.51.12.1542
7. Jones K, Pitceathly RD, Rose MR, et al. Interventions for dysphagia in long-term, progressive muscle disease. Cochrane Database Syst Rev. 2016;2:Cd004303. doi:10.1002/14651858.CD004158.pub3
8. Amato AA, Barohn RJ. Inclusion body myositis: old and new concepts. J Neurol Neurosurg Psychiatry. 2009;80(11):1186–1193. doi:10.1136/jnnp.2009.173823
9. Mulcahy KP, Langdon PC, Mastaglia F. Dysphagia in inflammatory myopathy: self-report, incidence, and prevalence. Dysphagia. 2012;27(1):64–69. doi:10.1007/s00455-011-9338-0
10. Olthoff A, Carstens PO, Zhang S, et al. Evaluation of dysphagia by novel real-time MRI. Neurology. 2016;87(20):2132–2138. doi:10.1212/WNL.0000000000003337
11. Cox FM, Verschuuren JJ, Verbist BM, Niks EH, Wintzen AR, Badrising UA. Detecting dysphagia in inclusion body myositis. J Neurol. 2009;256(12):2009–2013. doi:10.1007/s00415-009-5229-9
12. Lotz BP, Engel AG, Nishino H, Stevens JC, Litchy WJ. Inclusion body myositis. Observations in 40 patients. Brain. 1989;112(Pt 3):727–747. doi:10.1093/brain/112.3.727
13. Williams RB, Grehan MJ, Hersch M, Andre J, Cook IJ. Biomechanics, diagnosis, and treatment outcome in inflammatory myopathy presenting as oropharyngeal dysphagia. Gut. 2003;52(4):471–478. doi:10.1136/gut.52.4.471
14. Langdon PC, Mulcahy K, Shepherd KL, Low VH, Mastaglia FL. Pharyngeal dysphagia in inflammatory muscle diseases resulting from impaired suprahyoid musculature. Dysphagia. 2012;27(3):408–417. doi:10.1007/s00455-011-9384-7
15. Schrey A, Airas L, Jokela M, Pulkkinen J. Botulinum toxin alleviates dysphagia of patients with inclusion body myositis. J Neurol Sci. 2017;380:142–147. doi:10.1016/j.jns.2017.07.031
16. Ko EH, Rubin AD. Dysphagia due to inclusion body myositis: case presentation and review of the literature. Ann Otol Rhinol Laryngol. 2014;123(9):605–608. doi:10.1177/0003489414525588
17. Murata KY, Kouda K, Tajima F, Kondo T. A dysphagia study in patients with sporadic inclusion body myositis (s-IBM). Neurol Sci. 2012;33(4):765–770. doi:10.1007/s10072-011-0814-y
18. Suzuki N, Mori-Yoshimura M, Yamashita S, et al. Multicenter questionnaire survey for sporadic inclusion body myositis in Japan. Orphanet J Rare Dis. 2016;11(1):146. doi:10.1186/s13023-016-0524-x
19. Riminton DS, Chambers ST, Parkin PJ, Pollock M, Donaldson IM. Inclusion body myositis presenting solely as dysphagia. Neurology. 1993;43(6):1241–1243. doi:10.1212/WNL.43.6.1241
20. Badrising UA, Maat-Schieman MLC, van Houwelingen JC, et al. Inclusion body myositis. J Neurol. 2005;252(12):1448–1454. doi:10.1007/s00415-005-0884-y
21. Menon K, Rotstein L. Inclusion body myositis presenting as severe dysphagia. Intern Med J. 2015;45(S3):16–17. doi:10.1111/imj.12590
22. Molberg O, Dobloug C. Epidemiology of sporadic inclusion body myositis. Curr Opin Rheumatol. 2016;28(6):657–660. doi:10.1097/BOR.0000000000000327
23. Shibata S, Izumi R, Hara T, et al. Five-year history of dysphagia as a sole initial symptom in inclusion body myositis. J Neurol Sci. 2017;381:325–327. doi:10.1016/j.jns.2017.09.014
24. Di Pede C, Masiero S, Bonsangue V, Ragona RM, Del Felice A. Botulinum toxin and rehabilitation treatment in inclusion body myositis for severe oropharyngeal dysphagia. Neurol Sci. 2016;37(10):1743–1745. doi:10.1007/s10072-016-2586-x
25. Danon MJ, Friedman M. Inclusion body myositis associated with progressive dysphagia: treatment with cricopharyngeal myotomy. Can J Neurol Sci. 1989;16(4):436–438. doi:10.1017/S031716710002953X
26. Bachmann G, Streppel M, Krug B, Neuen-Jacob E. Cricopharyngeal muscle hypertrophy associated with florid myositis. Dysphagia. 2001;16(4):244–248. doi:10.1007/s00455-001-0082-8
27. Shapiro J, Martin S, DeGirolami U, Goyal R. Inflammatory myopathy causing pharyngeal dysphagia: a new entity. Ann Otol Rhinol Laryngol. 1996;105(5):331–335. doi:10.1177/000348949610500501
28. Oh TH, Brumfield KA, Hoskin TL, Kasperbauer JL, Basford JR. Dysphagia in inclusion body myositis: clinical features, management, and clinical outcome. Am J Phys Med Rehabil. 2008;87(11):883–889. doi:10.1097/PHM.0b013e31818a50e2
29. Darrow DH, Hoffman HT, Barnes GJ, Wiley CA. Management of dysphagia in inclusion body myositis. Arch Otolaryngol Head Neck Surg. 1992;118(3):313–317. doi:10.1001/archotol.1992.01880030103021
30. Leonard R, Kendall K Dysphagia assessment and treatment planning: a team approach; 2019.
31. Murata KY, Kouda K, Tajima F, Kondo T. Balloon dilation in sporadic inclusion body myositis patients with Dysphagia. Clin Med Insights Case Rep. 2013;6:1–7. doi:10.4137/CCRep.S10200
32. Felice KJ, North WA. Inclusion body myositis in Connecticut: observations in 35 patients during an 8-year period. Medicine. 2001;80(5):320–327. doi:10.1097/00005792-200109000-00006
33. Cherin P, Delain J-C, de Jaeger C, Crave J-C. Subcutaneous immunoglobulin use in inclusion body myositis: a review of 6 cases. Case Rep Neurol. 2015;7(3):227–232. doi:10.1159/000441490
34. Greenberg SA. Inclusion body myositis: clinical features and pathogenesis. Nat Rev Rheumatol. 2019;15(5):257–272. doi:10.1038/s41584-019-0186-x
35. Buchholz DW, Neumann S. Dysphagia in patients with inclusion body myositis. Dysphagia. 1999;14(3):187.
36. Leslie P, Carding PN, Wilson JA. Investigation and management of chronic dysphagia. BMJ (Clinical Research Ed). 2003;326(7386):433–436. doi:10.1136/bmj.326.7386.433
37. Olthoff A, Carstens P-O, Zhang S, et al. Evaluation of dysphagia by novel real-time MRI. Neurology. 2016;87(20):2132. doi:10.1212/WNL.0000000000003337
38. Carstens P-O, Zhang S, Olthoff A, et al. Real-time MRI for evaluation of dysphagia in inclusion body myositis (IBM) (P2.015). Neurology. 2015;84(14 Supplement):
39. Dziewas R, auf dem Brinke M, Birkmann U, et al. Safety and clinical impact of FEES – results of the FEES-registry. Neurol Res Pract. 2019;1(1):16. doi:10.1186/s42466-019-0021-5
40. Carstens PO, Zhang S, Olthoff A, et al. G.P.66: real-time MRI for the evaluation of dysphagia in inclusion body myositis (IBM). Neuromuscular Disord. 2014;24(9):814. doi:10.1016/j.nmd.2014.06.080
41. Lawson Mahowald M. The benefits and limitations of a physical training program in patients with inflammatory myositis. Curr Rheumatol Rep. 2001;3(4):317–324. doi:10.1007/s11926-001-0036-z
42. Arnardottir S, Alexanderson H, Lundberg IE, Borg K. Sporadic inclusion body myositis: pilot study on the effects of a home exercise program on muscle function, histopathology and inflammatory reaction. J Rehabil Med. 2003;35(1):31–35. doi:10.1080/16501970306110
43. Alexanderson H. Exercise in myositis. Curr Treatm Opt Rheumatol. 2018;4(4):289–298. doi:10.1007/s40674-018-0113-3
44. Alexanderson H. Exercise in inflammatory myopathies, including inclusion body myositis. Curr Rheumatol Rep. 2012;14(3):244–251. doi:10.1007/s11926-012-0248-4
45. Johnson LG, Collier KE, Edwards DJ, et al. Improvement in aerobic capacity after an exercise program in sporadic inclusion body myositis. J Clin Neuromuscul Dis. 2009;10(4):178–184. doi:10.1097/CND.0b013e3181a23c86
46. Malandraki GA, Kaufman A, Hind J, et al. The effects of lingual intervention in a patient with inclusion body myositis and Sjogren’s syndrome: a longitudinal case study. Arch Phys Med Rehabil. 2012;93(8):1469–1475. doi:10.1016/j.apmr.2012.02.010
47. Langmore SE, Miller RM. Behavioral treatment for adults with oropharyngeal dysphagia. Arch Phys Med Rehabil. 1994;75(10):1154–1160. doi:10.1016/0003-9993(94)90094-9
48. Maugars YM, Berthelot JM, Abbas AA, Mussini JM, Nguyen JM, Prost AM. Long-term prognosis of 69 patients with dermatomyositis or polymyositis. Clin Exp Rheumatol. 1996;14(3):263–274.
49. Low J, Wyles C, Wilkinson T, Sainsbury R. The effect of compliance on clinical outcomes for patients with dysphagia on videofluoroscopy. Dysphagia. 2001;16(2):123–127. doi:10.1007/s004550011002
50. Cherin P, Pelletier S, Teixeira A, et al. Intravenous immunoglobulin for dysphagia of inclusion body myositis. Neurology. 2002;58(2):326. doi:10.1212/WNL.58.2.326
51. Dalakas MC. Controlled studies with high-dose intravenous immunoglobulin in the treatment of dermatomyositis, inclusion body myositis, and polymyositis. Neurology. 1998;51(6 Suppl 5):S37–45. doi:10.1212/WNL.51.6_Suppl_5.S37
52. Walter MC, Lochmuller H, Toepfer M, et al. High-dose immunoglobulin therapy in sporadic inclusion body myositis: a double-blind, placebo-controlled study. J Neurol. 2000;247(1):22–28. doi:10.1007/s004150050005
53. Dalakas MC, Sonies B, Dambrosia J, Sekul E, Cupler E, Sivakumar K. Treatment of inclusion-body myositis with IVIg: a double-blind, placebo-controlled study. Neurology. 1997;48(3):712–716. doi:10.1212/WNL.48.3.712
54. Dobloug C, Walle-Hansen R, Gran JT, Molberg O. Long-term follow-up of sporadic inclusion body myositis treated with intravenous immunoglobulin: a retrospective study of 16 patients. Clin Exp Rheumatol. 2012;30(6):838–842.
55. Wilson FC, Ytterberg SR, St Sauver JL, Reed AM. Epidemiology of sporadic inclusion body myositis and polymyositis in Olmsted County, Minnesota. J Rheumatol. 2008;35(3):445–447.
56. Suzuki N, Mori-Yoshimura M, Yamashita S, et al. The updated retrospective questionnaire study of sporadic inclusion body myositis in Japan. Orphanet J Rare Dis. 2019;14(1):155. doi:10.1186/s13023-019-1122-5
57. Pars K, Garde N, Skripuletz T, Pul R, Dengler R, Stangel M. Subcutaneous immunoglobulin treatment of inclusion-body myositis stabilizes dysphagia. Muscle Nerve. 2013;48(5):838–839. doi:10.1002/mus.v48.5
58. Schneider I, Thumfart WF, Pototschnig C, Eckel HE. Treatment of dysfunction of the cricopharyngeal muscle with botulinum A toxin: introduction of a new, noninvasive method. Ann Otol Rhinol Laryngol. 1994;103(1):31–35. doi:10.1177/000348949410300105
59. Liu LW, Tarnopolsky M, Armstrong D. Injection of botulinum toxin A to the upper esophageal sphincter for oropharyngeal dysphagia in two patients with inclusion body myositis. Can J Gastroenterol. 2004;18(6):397–399. doi:10.1155/2004/360537
60. Witting N, Daugaard D, Prytz S, Vissing J. Effects of botulinum toxin injections in the cricopharyngeal muscle of OPMD and IBM myopathies with dysphagia. Neuromuscular Disord. 2017;27:S203. doi:10.1016/j.nmd.2017.06.396
61. Kelly EA, Koszewski IJ, Jaradeh SS, Merati AL, Blumin JH, Bock JM. Botulinum toxin injection for the treatment of upper esophageal sphincter dysfunction. Ann Otol Rhinol Laryngol. 2013;122(2):100–108. doi:10.1177/000348941312200205
62. Shaw DW, Cook IJ, Gabb M, et al. Influence of normal aging on oral-pharyngeal and upper esophageal sphincter function during swallowing. Am J Physiol. 1995;268(3 Pt 1):G389–396. doi:10.1152/ajpgi.1995.268.3.G389
63. Parres C. Pharyngoesophageal dilation and botulinum toxin therapy for dysphagia in patients with inclusion body myositis (P3.157). Neurology. 2015;84(14 Supplement):
64. Verma A, Bradley WG, Adesina AM, Sofferman R, Pendlebury WW. Inclusion body myositis with cricopharyngeus muscle involvement and severe dysphagia. Muscle Nerve. 1991;14(5):470–473. doi:10.1002/(ISSN)1097-4598
65. Litchy WJ, Engel AG. Inclusion body myositis with cricopharyngeus muscle involvement and severe dysphagia. Muscle Nerve. 1992;15(1):115.
66. Breithaupt M, Schmidt J. Update on treatment of inclusion body myositis. Curr Rheumatol Rep. 2013;15(5):329. doi:10.1007/s11926-013-0329-z
67. Bonavina L, Khan NA, DeMeester TR. Pharyngoesophageal dysfunctions: the role of cricopharyngeal myotomy. Arch Surg. 1985;120(5):541–549. doi:10.1001/archsurg.1985.01390290023004
68. Sanei-Moghaddam A, Kumar S, Jani P, Brierley C. Cricopharyngeal myotomy for cricopharyngeus stricture in an inclusion body myositis patient with hiatus hernia: a learning experience. BMJ Case Rep. 2013;2013:bcr2012008058. doi:10.1136/bcr-2012-008058
69. Nicholson FB, Korman MG, Richardson MA. Percutaneous endoscopic gastrostomy: a review of indications, complications and outcome. J Gastroenterol Hepatol. 2000;15(1):21–25. doi:10.1046/j.1440-1746.2000.02004.x
70. Ekberg O, Hamdy S, Woisard V, Wuttge-Hannig A, Ortega P. Social and psychological burden of dysphagia: its impact on diagnosis and treatment. Dysphagia. 2002;17(2):139–146. doi:10.1007/s00455-001-0113-5
71. Attrill S, White S, Murray J, Hammond S, Doeltgen S. Impact of oropharyngeal dysphagia on healthcare cost and length of stay in hospital: a systematic review. BMC Health Serv Res. 2018;18(1):594. doi:10.1186/s12913-018-3376-3
72. Price MA, Barghout V, Benveniste O, et al. Mortality and causes of death in patients with sporadic inclusion body myositis: survey study based on the clinical experience of specialists in Australia, Europe and the USA. J Neuromuscular Dis. 2016;3(1):67–75. doi:10.3233/JND-150138
73. Karkos PD, Papouliakos S, Karkos CD, Theochari EG. Current evaluation of the dysphagic patient. Hippokratia. 2009;13(3):141–146.
74. Badrising UA, Maat-Schieman M, van Duinen SG, et al. Epidemiology of inclusion body myositis in the Netherlands: a nationwide study. Neurology. 2000;55(9):1385–1387. doi:10.1212/WNL.55.9.1385
75. Lindberg C, Persson LI, Bjorkander J, Oldfors A. Inclusion body myositis: clinical, morphological, physiological and laboratory findings in 18 cases. Acta Neurol Scand. 1994;89(2):123–131. doi:10.1111/j.1600-0404.1994.tb01647.x
76. Sayers ME, Chou SM, Calabrese LH. Inclusion body myositis: analysis of 32 cases. J Rheumatol. 1992;19(9):1385–1389.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
