Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 7
Dyslipidemia and cardiovascular disease risk profiles of patients attending an HIV treatment clinic in Harare, Zimbabwe
Authors Zhou D , Kodogo V, Chokuona K, Gomo E, Oektedalen O, Stray-Pedersen B
Received 2 December 2014
Accepted for publication 20 January 2015
Published 13 May 2015 Volume 2015:7 Pages 145—155
DOI https://doi.org/10.2147/HIV.S78523
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Shenghan Lai
Danai Tavonga Zhou,1,2 Vitaris Kodogo,1 Kudzai Fortunate Vongai Chokuona,1 Exnevia Gomo,1 Olav Oektedalen,3 Babill Stray-Pedersen2
1Department of Medical Laboratory Sciences, College of Health Sciences, University of Zimbabwe, Avondale, Zimbabwe; 2Institute of Clinical Medicine, University in Oslo, Oslo University Hospital, Oslo, Norway; 3Department of Infectious Diseases, Oslo University Hospital, Oslo, Norway
Abstract: The chronic inflammation induced by human immunodeficiency virus (HIV) contributes to increased risk of coronary heart disease (CHD) in HIV-infected individuals. HIV-infected patients generally benefit from being treated with antiretroviral drugs, but some antiretroviral agents have side effects, such as dyslipidemia and hyperglycemia. There is general consensus that antiretroviral drugs induce a long-term risk of CHD, although the levels of that risk are somewhat controversial. The intention of this cross-sectional study was to describe the lipid profile and the long-term risk of CHD among HIV-positive outpatients at an HIV treatment clinic in Harare, Zimbabwe. Two hundred and fifteen patients were investigated (females n=165, mean age 39.8 years; males n=50; mean age 42.0 years). Thirty of the individuals were antiretroviral-naïve and 185 had been on antiretroviral therapy (ART) for a mean 3.9±3.4 years. All participants had average lipid and glucose values within normal ranges, but there was a small difference between the ART and ART- for total cholesterol (TC) and high-density lipoprotein (HDL).Those on a combination of D4T or ZDV/NVP/3TC and PI-based ART were on average oldest and had the highest TC levels. Framingham risk showed 1.4% prevalence of high CHD risk within the next ten years. After univariate analysis age, sex, TC/HDL ratio, HDL, economic earnings and systolic BP were associated with medium to high risk of CHD. After multivariate regression analysis and adjusting for age or sex only age, sex and economic earnings were associated with medium to high risk of CHD. There is small risk of developing CHD, during the next decade in HIV infected patients at an HIV treatment clinic in Harare.
Keywords: human immunodeficiency virus, coronary heart disease risk, antiretroviral therapy, dyslipidemia
Introduction
Human immunodeficiency virus (HIV)-infected patients have, like the general population, established cardiovascular risk factors, such as family history, age, male sex, hypertension, hyperlipidemia, and smoking.1,2 HIV-infected individuals have an increased relative risk of coronary heart disease (CHD), acute myocardial infarction, and peripheral vascular disease compared with the population not infected with HIV.3–7 The use of antiretroviral therapy (ART) has shown adverse influences on the lipid profile and is associated with a higher risk of diabetes mellitus and CHD.8–11 In our context, the lipid profiles of low-density lipoprotein (LDL), high-density lipoprotein (HDL), triglycerides, and total cholesterol (TC) are of special interest.
HIV infection has been associated with progression of atherosclerosis.12 Rasheed et al were the first to show a direct effect of HIV on lipid metabolism.13 A South African study performed in ART-naïve blacks measured an association between the degree of inflammation and the degree of dyslipidemia.14 When these patients were followed up 3 years later, there was a higher degree of dyslipidemia in the treatment-naïve group than in the treatment-experienced group. In contrast, another African study found a significant burden of risk factors for CHD among ART-experienced patients than among their ART-naïve counterparts.15
The recommended treatment regimen for HIV today is the combination of three or more antiretroviral drugs to effectively reduce the viral load.16 In Zimbabwe, the ART regimens mostly used are nucleotide reverse transcriptase inhibitors (NRTIs) such as tenofovir (Gilead Sciences, Foster City, CA, USA) in combination with non-nucleoside reverse transcriptase inhibitors (NNRTIs) such as nevirapine (Boehringer Ingelheim, Ridgefield, CT, USA) or in combination with protease inhibitors such as lopinavir and ritonavir, together known as Kaletra/Alluvia (Abbott Laboratories, North Chicago, IL, USA).17,18 There is conflicting evidence from studies performed in both African and Western countries as to whether ART induces dyslipidemia and thereby the risk of CHD.19,20
A study of patients in a randomized controlled trial (Development of Antiretroviral Therapy in Africa, DART) in Zimbabwe and Uganda reported modest lipid elevations in African patients switching from first-line ART to predominantly ritonavir-boosted lopinavir + NNRTI-based second-line regimens after 48 weeks.21 However, there is still a paucity of data describing both the state of lipid profiles and cardiovascular risk profiles in the Zimbabwean HIV-positive setting. As both HIV infection and ART regimens might be associated with cardiovascular disease risk, we sought to determine lipid alterations and CHD risk profiles in two groups of HIV-infected patients, one on treatment with ART and the other ART-naïve.
Materials and methods
Recruitment of participants and data collection
This cross-sectional observational study was carried out at an HIV treatment clinic in Harare, Zimbabwe. HIV-infected patients attending the clinic for scheduled visits on selected days between March and August 2013 were sequentially approached. The patients were informed about the study, along with its risks and benefits. Those who qualified and were willing to participate gave written consent before being interviewed and measured for weight, height, and blood pressure (BP). A questionnaire-guided interview was used to collect demographic and clinical data such as age, sex, marital status, health status, clinical history, and family history. The participants were then bled using venipuncture to obtain 5 mL blood samples in one fluoride tube and one plain tube per participant. Blood samples were separated into fluoride plasma and plain serum within 12 hours of collection. Samples were stored at −80°C until assay. Plasma and serum samples were thawed at room temperature before assaying for glucose (fluoride plasma) and lipids (plain serum).
Inclusion and exclusion criteria
HIV-infected patients attending the HIV treatment clinic on a regular basis were included. ART-experienced patients were defined as patients who reported prior use of three antiretroviral drugs, whereas ART-naïve patients had never taken antiretroviral drugs. Male and female HIV-infected patients aged 18 years or older were included. Patients with documented history of diabetes, cardiovascular disease, hypertension, and dyslipidemia before being infected with HIV were excluded.
Sample size calculation
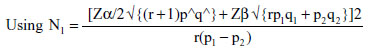
where p1 (test group) =43.4% and p2 (control group) =15.9%, q1=56.6% and q2=84.1%; ratio of ART experienced: ART-naïve used in the study =5:1, hence r=5; N1= minimum sample size for the ART-naïve control group; N2=5×N1 is the minimum sample size for the ART-experienced test group; Zα/2=1.96 (95% confidence interval); Zβ =0.84 (power of study).
The minimum sample size was 186, (31 ART-naïve, 155 ART-experienced).
Quantitative determination of lipids and glucose
Quantization was carried out using a BS120 Mindray® machine (Nanshan, People’s Republic of China), for which the test principles are described as follows. Lipids (TC, HDL, LDL) are measured enzymatically in serum in a series of coupled reactions that hydrolyze cholesteryl esters and oxidize the 3-OH group of cholesterol.22,23 One of the reaction byproducts, H2O2, is measured quantitatively in a peroxidase-catalyzed reaction that produces a color. Absorbance is measured at 500 nm. The color intensity is proportional to the cholesterol concentration.22,23 Plasma glucose was determined using the glucose oxidase method, where the enzyme (glucose oxidase) catalyzes the oxidation of glucose to gluconic acid.24
Cardiovascular risk values
Elevated BP was defined according to the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III), where the systolic BP is ≥140 mmHg and/or the diastolic BP is >90 mmHg.25 The NCEP ATP III guidelines were also used to define dyslipidemia and hyperglycemia, ie, elevated TC is defined as >200 mg/dL, elevated LDL as >100 mg/dL, depressed HDL as <40 mg/dL, and elevated glucose as >110 mg/dL.25 Obesity was defined by body mass index (BMI), using the World Health Organization classification.26 BMI was stratified into categories as follows: <15, very severely underweight; 15–16, severely underweight; 16.1–18.5, underweight; 18.6–24.9, normal; 25–29.9, overweight; 30–35, moderately obese; and 36–40, severely obese.27 Overall CHD risk was calculated using age, sex, TC, HDL, smoking history, and systolic BP on the US National Health Institute Framingham On-line calculator.28 The Framingham calculator applies NCEP ATP III guidelines as follows: older age (being older than 55 years for women and older than 45 years for men); elevated TC, >200 mg/dL; decreased HDL, <40 mg/dL; and elevated systolic BP, >140 mmHg.28 Those on treatment for hypertension were categorized as having a slightly higher risk of CHD in the next 10 years than those of the same sex and age, by the Framingham risk calculator.28 Framingham risk scores were then used to define risk of coronary artery disease or heart attack as low (less than 10% risk), moderate (10%–20% risk), or high (more than 20% risk).
Statistical analysis
Data were entered into and analyzed using Statistical Package for Social Sciences version 21 (IBM Statistics, Armonk, NY, USA) and Stata version 13 (StataCorp, College Station, TX, USA) software. Shapiro-Wilk test (P<0.05) and visual inspection of histograms, normal Q-Q plots, and box-plots were used to check dependent variables for normality.29 Continuous data were described as the mean ± standard deviation in descriptive statistics and analyzed for differences using t-tests, with the level of significance set at P<0.05. Skewed data were reported as medians (interquartile ranges) and compared using k median tests. Categorical variables were compared using contingency tables and Pearson’s chi-square tests and/or Fisher’s exact tests. Known risk factors, such as age, sex, lipid and glucose levels, BMI, economic income, systolic BP, diastolic BP, history of ART, and duration on ART, were tested for association with risk of future cardiovascular disease using univariate and multivariate logistic regression calculation.
Results
Demographics of participants
A total of 300 patients were targeted for inclusion, but only 235 patients were accessed during the study. Of the patients approached at the clinic during their regular visits, 13 declined for various reasons and two did not qualify (one was diabetic and one was below the age of 18 years). Five patients gave consent but were not bled for all the samples needed for the study due to needle discomfort, excessive bleeding, or collapsed veins.
Finally, 215 HIV-infected adults had complete data. Of these, 30 (14.0%) were ART-naïve (Table 1). The mean age for all participants was 40.13±10.14 years; 165 were female (mean age 39.8 years) and 23.3% (n=50) were male (mean age 40.2 years). Patients were selected for participation because they did not have current life-threatening illnesses. Almost two thirds (n=145) of participants had been diagnosed with HIV due to appearance of AIDS-defining illnesses such as tuberculosis (60%), diarrhea and unexplained weight loss (20%), or other opportunistic infections (20%). About one-third (n=60) were diagnosed after undergoing voluntary testing without clinical symptoms. This might explain the difficulty in getting a high number of ART-naïve patients in this study as more than two thirds of patients started ART soon after initial diagnosis due to their illness. The majority of participants were non-smokers (95.8%, n=206) and most were married. Those who had a history of elevated BP in the preceding 6 months were classified as hypertensive (n=76, 35.5%). Forty-four (58%) of these hypertensive participants were diagnosed with high BP for the first time at the point of inclusion into the study, whilst 42% (n=32) of the hypertensive participants were on antihypertensive medical treatment upon inclusion.
  | Table 3 Results from multivariate regression analysis |
Our HIV treatment clinic mainly serves low-income patients. Of all the 215 participants, 44.7% (n=96) were employed, with mean earnings of USD 154.30±214.14 per month, and approximately 50% of all participants (n=103) earned less than USD 50 per month.
As shown in Figure 1, the participants originated from different parts of Zimbabwe, coming mostly from provinces near Harare and Mutare, whilst 8.4% originated from neighboring Sub-Saharan regional countries (mainly Malawi and Mozambique). This geographic heterogeneity is apparent across all urban centers of Zimbabwe, as people move across the country in search of opportunities and jobs.
  | Figure 1 Provinces of origin of participants. |
Diagnosis and treatment history
Participants had been diagnosed for a mean 5.5±4.1 years prior to the study, 38% had been diagnosed within 3–5 years, with 24% of them having been diagnosed less than 2 years or 6–10 years, respectively, before data collection; only 14% of participants had been diagnosed for more than 10 years before the study. ART-experienced participants had been on ART for a mean duration of 3.9±3.4 years. There was a positive correlation between years since diagnosis and years since beginning ART (Figure 2).
  | Figure 2 Positive correlation (regression analysis r2=0.5768, P=0.0000) between years since diagnosis and years of antiretroviral treatment. |
Laboratory parameters
The ART-experienced group as well as the ART-naïve group had normal average values for TC, HDL, LDL, and glucose based on NCEP ATP III guidelines. The majority of patients (97%, n=208) had a low risk of CHD as indicated by Framingham risk scores less than 10%, and only four (1.9%) had moderate risk, ie, Framingham risk scores of 10%–20%. There was a 1.4% (n=3) prevalence of participants with a Framingham risk score higher than 20%, indicating a high risk of CHD. Table 1 shows the comparison of demographic, clinical, and biochemical parameters related to ART history. There was a significant difference between ART-experienced and ART-naïve participants for mean years since diagnosis, median TC, and median HDL, but no significant differences for distribution by sex, economic earnings, BMI, median systolic and diastolic BP, median TC/HDL ratio, median LDL, median BMI, mean random blood glucose, and Framingham risk scores. There was a significant difference in prevalence of history of tuberculosis, with those on ART more likely to have suffered from tuberculosis at some time in the past (odds ratio 3.25, confidence interval 1.06–13.27), suggesting that these patients had been more immune-compromised in the past.
Body mass index of participants
There was no significant difference in mean BMI between the ART-naïve and ART-experienced participants, although all 14 underweight patients were ART-experienced. However, there was no significant difference in the prevalence of overweight by ART use (odds ratio 1.395, 95% confidence interval 0.591–3.498, P=0.416).
Antiretroviral therapy regimens
Figure 3 shows the ART regimens that were used by the participants. The majority of participants (60.5%, n=130) in our study were on a triple combination of tenofovir, nevirapine, and lamivudine (GlaxoSmithKline, Brentford, London, UK), 23 (21.1%) were on a tenofovir, efavirenz (Bristol-Myers Squibb, New York City, NY, USA), and lamivudine regimen, and the rest were on either stavudine (Bristol-Myers Squibb) or zidovudine (ViiV Healthcare, Brentford, London, UK) in combination with nevirapine and lamivudine or a protease inhibitor-based second-line therapy.
When patients were stratified by different ART combinations and compared with ART-naïve patients, the distribution of BMI, random blood glucose, LDL, and TC/HDL ratio was all the same. On the other hand, the distribution of age, TC, and HDL was significantly different across categories of ART combinations when compared with the ART-naïve group (Table 4). Those on protease inhibitor-based second-line treatment had on average the highest levels of TC and those on stavudine-based or zidovudine-based first line had the highest levels of HDL.
There was a significant difference across the different combinations for age, with those on a combination of stavudine or zidovudine/nevirapine/lamivudine and protease inhibitor-based therapy being on average oldest (Table 2). There was a significant difference across the ART groups for TC, while there was no significant difference across the groups for HDL, LDL, diastolic BP, systolic BP, BMI, or random blood glucose.
Framingham risk scores
There was a low (1.4%) prevalence of high risk for future cardiovascular disease when participants were evaluated by Framingham risk scores (Figure 4). The risk of cardiovascular disease ranged from <1% (n=145, 67.4% of participants) to >20% (n=3, 1.4%). There was no difference in the prevalence of medium to high risk of CHD across all categories of ART (P=0.134) and no difference when comparing ART-experienced and ART-naïve groups (P>0.05, Tables 1 and 4). When tested using univariate analysis, the following were associated with medium to high risk of CHD: age, sex, TC/HDL ratio, HDL, economic earnings, and systolic BP (Table 2). However, after multivariate regression analysis and adjusting for age and sex, there was only a statistically significant association between age, sex, and economic earnings with a medium to high risk of CHD (Table 3).
  | Figure 4 Distribution of future CHD risk using Framingham risk scores amongst the participants. |
Discussion
This is the first study describing both the lipid profile and the calculated risk of future cardiovascular disease in HIV-positive Zimbabwean individuals treated with different ART regimens. Although our results indicate a low risk of long-term cardiovascular disease, ART is associated with some changes in lipid metabolism to be taken notice of. When considering serum TC and LDL levels, lipid profiles seemed more atherogenic in the antiretroviral-treated participants compared with their therapy-naïve counterparts. On the other hand, when classified by HDL or TC/HDL ratio, the ART-naïve participants seem to be at higher risk.
Previous clinical studies have shown that protease inhibitor- based ART is especially associated with highly atherogenic lipid levels, and this peaks 2–3 years after initiating ART.9,30,31 For example, the Data Collection on Adverse Events of Anti-HIV Drugs (DAD) study group showed evidence of multiple risk factors for cardiovascular disease, including dyslipidemia among HIV-infected persons on NRTIs and protease inhibitors, with an increased risk in older patients; 1.4% of patients in that study had a previous history of cardiovascular disease and 51.5% were cigarette smokers.32
In contrast, in our study, less than 5% of the participants had been on a protease inhibitor-based regimen, while more than 80% were on an NNRTI-containing regimen with a mean exposure time of 3.5 years, indicating that even NNRTI-based ART regimens influence lipid metabolism unfavorably, as shown in previous cohorts and clinical trials.33,34 Most studies have shown that the level of HDL cholesterol is lower in non-treated HIV patients than in ART-exposed patients, in accordance with our results.35,36 The DAD study showed a higher risk of cardiovascular disease risk in older patients, ie, 25% of DAD patients were over 50 years of age.32 Our study population was made up of relatively young adults, with a mean age of 40.3±10.1 years and only 17% of participants being older than 50 years.
In contrast with the DAD study, the Strategies for Management of Antiretroviral Therapy (SMART) study found no associations between rate of cardiovascular disease and cumulative or recent use of NRTIs (zidovudine, stavudine, or lamivudine). However, recent use of specific NRTIs (abacavir and didanosine) was associated with an increased rate of cardiovascular disease, compared with those with no recent use of the two drugs.37 After adjustment for predicted 10-year risk of CHD, recent use of both didanosine and abacavir remained associated with increased rates of cardiovascular disease.37 Of note is that the SMART study population had a high rate of cardiac risk factors at baseline, with 39% of participants being current smokers, 36% having ischemic changes on their baseline electrocardiograms, 19% taking antihypertensive agents, and 18% being on lipid-lowering drugs.37
Interestingly, an African study performed in newly identified HIV-positive individuals showed that ART improved the dyslipidemic profile found before starting ART.14 In another study carried out in HIV-infected urban dwellers in Nigeria, the lipid profiles were followed for a period of 15 months in individuals on ART and in treatment-negative asymptomatic HIV-infected patients.38 When compared with apparently healthy HIV-negative individuals, TC, HDL, LDL, and triglyceride levels in the ART-experienced subjects were not significantly different, while the levels of TC and HDL were significantly lower in the HIV-positive antiretroviral-naïve versus ART-experienced patients.38 The low levels of HDL observed in antiretroviral-naïve patients seem to be a recurring theme in most studies, including ours, but whether ART contributes to increased cardiovascular risk remains uncertain. The mechanisms of how HIV infection and ART induce these lipid abnormalities are still unknown. Chronic immune activation caused by HIV has been proposed, an activation that persists even after successful treatment with ART.39 Even if ART induces lipid abnormalities that are classic risk factors for the development of atherosclerosis, ART is of tremendous importance for survival and reduction of morbidities in HIV-positive patients. These patients have longer survival, but are more threatened by cardiovascular disease.
A Nigerian study conducted by Muhammad et al in 100 ART-experienced and 100 ART-naïve patients found a significantly higher burden of some risk factors for cardiovascular disease (hypertension, hypercholesterolemia, obesity, and metabolic syndrome) among ART-treated HIV patients than in their ART-naïve counterparts.15 Interestingly, in our study, antihypertensive medication was started in 17% of the ART-experienced participants compared with 2% of the ART-naïve participants (P<0.001). Prior results exploring the effect of ART on BP are conflicting.40,41 Previous studies have shown a correlation between sustained hypertension and a nadir CD4 cell count <200 cells/μL, as well as with duration of ART, with an especially high proportion of hypertensive HIV patients having a nadir cell count <50 cells/μL.42–44 We have no measurements regarding immunodeficiency in our study, but the patients on ART would be expected to have had more advanced immunodeficiency (and hence the lowest nadir CD4 cell counts) in the past than the ART-naïve patients.
The participants in our study had a low risk of developing cardiovascular disease for the next 10 years when evaluated by Framingham risk score. We found risk scores ranging from <1% (67.5% of patients) to >20% (1.4% of patients); all the higher risk patients (n=3) were found among participants on ART (one on nevirapine and two on protease inhibitors). This is far lower than reported in Europe and developed countries, but is in agreement with studies done elsewhere in Africa.45–49 In our study, the lipid values were within normal range, but TC and HDL were both higher in the ART-positive group, constituting 85% of all participants. Of note is that the majority of patients in our study were young adults (mean age 40.3 years, with 17% [n=37] being aged older than 50 years), with 75% being women and 96% being non-smokers, contributing to a low risk of future cardiovascular disease. Smoking has been independently associated with cardiovascular disease in HIV-positive patients, and the risk decreases after smoking cessation.50 We found no differences in the prevalence of cardiovascular disease risk across the different types of ART, and there were no differences when comparing ART-experienced patients with ART-naïve patients (Tables 1 and 4). Interestingly, 70% of the ART-positive patients were treated with lamivudine in combination with tenofovir and non-nucleoside antiretroviral drugs, which has not been associated with an increased risk of myocardial infarction, in contrast with some protease inhibitors and other NRTIs.51 Multivariate regression analysis (for age, sex, TC/HDL ratio, systolic BP, economic earnings) found an association between higher risk of CHD and monthly earnings, which is a new finding for African countries. Not surprisingly, it tells us that the long-term complications of HIV are influenced by socioeconomic factors.
Strengths and limits of the study
This study was well powered, and the participants were carefully selected in a randomly manner for participation and were regularly monitored for adherence. However, the study is influenced by the inherent weakness of an observational cross-sectional study design, selection bias (with more females than males due to differences in the health-seeking behaviors of men and women in our setting), lack of HIV-negative controls, and lack of locally generated reference ranges for comparison. Due to lack of CD4 cells and viral load values, each patient’s health status was not properly defined, with no HIV staging. The groups of participants were generally small, especially the patient group without ART. The small sample size increases the risk of type II statistical errors. Another major weakness stems from the use of Framingham risk score in HIV-infected patients. It has been proposed that the Framingham risk score may underestimate the 10-year risk in some subgroups and overestimate this risk in others, depending on risk factors and geographic origin.52 This warrants further research, for example, using the HIV-specific cardiovascular risk calculator engineered by the DAD study that leaves out antihypertensive therapy but adds in five other factors to the equation, being number of years taking indinavir or lopinavir, current treatment with indinavir, lopinavir, or abacavir, previous smoking, diabetes, and a family history of cardiovascular disease.52
Conclusion
Our main finding is that the risk of future cardiovascular disease is low among outpatients at a Zimbabwean HIV treatment clinic, although there were the worrying aspects of high atherogenic lipid profiles and hypertension. There is still a need to control plasma lipid levels and BP in HIV-positive patients in an attempt to reduce the long-term cardiovascular risk, which is upcoming in HIV-positive patients.
Acknowledgments
The authors are grateful to: the Letten Foundation, Norway, for funding this research; the academic, technical staff, and students of the University of Zimbabwe, College of Health of Sciences, Department of Medical Laboratory Sciences; Kudzaishe Mateveke, University of Zimbabwe, College of Health of Sciences Research Support Centre for support during the statistical analysis; and to the staff and patients of Newlands Clinic.
Author contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data, drafting the article, or revising it critically for important intellectual content, gave their approval of the version to be published, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosure
The authors report no conflicts of interest in this work.
References
Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–2512. | |
Ford ES, Greenwald JH, Richterman AG, et al. Traditional risk factors and D-dimer predict incident cardiovascular disease events in chronic HIV infection. AIDS. 2010;24:1509–1517. | |
Currier JS, Taylor A, Boyd F, et al. Coronary heart disease in HIV-infected individuals. J Acquir Immune Defic Syndr. 2003;33:506–512. | |
Obel N, Thomsen HF, Kronborg G, et al. Ischemic heart disease in HIV-infected and HIV-uninfected individuals: a population-based cohort study. Clin Infect Dis. 2007;44:1625–1631. | |
Lang S, Mary-Krause M, Cotte L, et al. Increased risk of myocardial infarction in HIV-infected patients in France, relative to the general population. AIDS. 2010;24:1228–1230. | |
Durand M, Sheehy O, Baril JG, Lelorier J, Tremblay CL. Association between HIV infection, antiretroviral therapy, and risk of acute myocardial infarction: a cohort and nested case-control study using Quebec’s public health insurance database. J Acquir Immune Defic Syndr. 2011;57:245–253. | |
Periard D, Cavassini M, Taffe P, et al. High prevalence of peripheral arterial disease in HIV-infected persons. Clin Infect Dis. 2008;46:761–767. | |
Pere D, Ignacio SL, Ramon T, et al. Dyslipidemia and cardiovascular disease risk factors management in HIV-1-infected subjects treated with HAART in the Spanish VACH cohort. Open AIDS J. 2008;2:26–28. | |
Riddler SA, Li X, Chu H, et al. Longitudinal changes in serum lipids among HIV-infected men on highly active antiretroviral therapy. HIV Med. 2007;8:280–287. | |
Brown TT, Cole SR, Li X, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS Cohort Study. Arch Intern Med. 2005;165:1179–1184. | |
Islam FM, Wu J, Jansson J, Wilson DP. Relative risk of cardiovascular disease among people living with HIV: a systematic review and meta-analysis. HIV Med. 2012;13:453–468. | |
Hsue PY, Scherzer R, Hunt PW, et al. Carotid intima-media thickness progression in HIV-infected adults occurs preferentially at the carotid bifurcation and is predicted by inflammation. J Am Heart Assoc. 2012;1: pii jah3-e000422. | |
Rasheed S, Yan JS, Lau A, Chan AS. HIV replication enhances production of free fatty acids, low density lipoproteins and many key proteins involved in lipid metabolism: a proteomics study. PLoS One. 2008;3: e3003. | |
Fourie CMT, Van Rooyen JM, Schutte AE. HIV infection and cardiovascular risk in black South Africans. Cardiovasc J Afr. 2011;22(3):117–119. | |
Muhammad S, Sani MU, Okeahialam BN. Cardiovascular disease risk factors among HIV-infected Nigerians receiving highly active antiretroviral therapy. Niger Med J. 2013;54:185–190. | |
Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Available at http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed January 15, 2015. | |
National Drug and Therapeutics Policy Advisory Committee. Guidelines for Antiretroviral Therapy in Zimbabwe. Harare, Zimbabwe: Ministry of Health and Child Welfare; 2005. | |
Mudzinge D, Nyazika TK, Chisango TJ, Zhou DT. Differences in serum levels of magnesium, phosphate, and albumin for HAART-experienced and HAART-naïve female patients attending Parirenyatwa Opportunistic Infections Clinic in Harare, Zimbabwe. ISRN AIDS J. 2013;2013:383214. | |
Egaña-Gorroño L, Martínez E, Cormand B, Escribà T, Gatell J, Arnedo M. Impact of genetic factors on dyslipidemia in HIV-infected patients starting antiretroviral therapy. AIDS J. 2013;27:529–538. | |
Pefura Yone EW, Betyoumin AF, Kengne AP, Kaze Folefack FJ, Ngogang J. First-line antiretroviral therapy and dyslipidemia in people living with HIV-1 in Cameroon: a cross-sectional study. AIDS Res Ther. 2011;8:33. | |
Gomo ZA, Hakim JG, Reid A, et al. Impact of second-line antiretroviral regimens on lipid profiles in an African setting: the DART trial sub-study. AIDS Res Ther. 2014;11:32. | |
Centers for Disease Control and Prevention. Total cholesterol, direct HDL, precipitated HDL, triglycerides, and LDL NHANES 2003–2004. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/l13_c_met_lipids.pdf. Accessed January 25, 2015. | |
Bachorik PS, Albers JJ. Precipitation methods for quantification of lipoproteins. In: Albers JJ, Segrest JP, editors. Methods In Enzymology. Orlando, FL, USA: Academic Press; 1986. | |
Howanitz PJ, Howanitz JH. In: Henry JB, editor. Clinical Diagnosis and Management by Laboratory Methods. 17th ed. Philadelphia, PA, USA: WB Saunders; 1984. | |
National Cholesterol Education Program (NCEP) Expert Panel On Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2004;106:3143–3421. | |
World Health Organization. Global database on body mass index. Available from: http://apps.who.int/bmi/index.jsp?introPage=intro_3.html. Accessed January 25, 2015. | |
Seidell JC, Flegal KM. Assessing obesity: classification and epidemiology. Br Med Bull. 1997;53:238–252. | |
US Department of Health and Human Services, National Heart, Lung and Blood Institute. Risk assessment tool for estimating 10-year risk of having a heart attack. Available from: http://cvdrisk.nhlbi.nih.gov/. Accessed January 25, 2015. | |
Shapiro SS, Wilk MD. An analysis of variance tests for normality (complete samples). Biometrika. 1965;52:591–611. | |
Walmsley S, Bernstein B, King M, et al. Lopinavir-ritonavir versus nelfinavir for the initial treatment of HIV infection. N Engl J Med. 2002;346:2039–2046. | |
Carr A, Samaras K, Thorisdottir A, Kaufmann GR, Chisholm DJ, Cooper DA. Diagnosis, prediction, and natural course of HIV-1 protease-inhibitor-associated lipodystrophy, hyperlipidemia, and diabetes mellitus: a cohort study. Lancet. 1999;353:2093–2099. | |
Friis-Møller N, Weber R, Reiss P, et al. Cardiovascular disease risk factors in HIV patients – association with antiretroviral therapy, results from the DAD study. AIDS. 2003;17:1179–1193. | |
Fontas E, Van Leth F, Sabin CA, et al. Lipid profiles in HIV-infected patients receiving combination antiretroviral therapy: are different antiretroviral drugs associated with different lipid profiles? J Infect Dis. 2004;189:1056–1074. | |
Glass TR, Ungsedhapand C, Wolbers M, et al. Prevalence of risk factors for cardiovascular disease in HIV-infected patients over time: the Swiss HIV Cohort Study. HIV Med. 2006;7:404–410. | |
Van Der Valk M, Kastelein JJ, Murphy RL, et al. Nevirapine-containing antiretroviral therapy in HIV-1 infected patients results in an anti-atherogenic lipid profile. AIDS. 2001;15:2407–2414. | |
Domingo P, Suarez-Lozano I, Teira R, et al. Dyslipidemia and cardiovascular disease risk factor management in HIV-1-infected subjects treated with HAART in the Spanish VACH cohort. Open AIDS J. 2008;2:26–38. | |
Strategies for Management of Antiretroviral Therapy (SMART) Study Group; El-Sadr WM, Lundgren J, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. | |
Oduola T, Akinbolade AA, Oladokun LO, et al. Lipid profiles in people living with HIV/AIDS on ARV therapy in an urban area of Osun State, Nigeria. World J Med Sci. 2009;4:18–21. | |
Valdez H, Connick E, Smith KY, et al. Limited immune restoration after 3 years suppression of HIV-1 replication in patients with moderately advanced disease. AIDS. 2002;16:1859–1866. | |
Peck RN, Shedafa R, Kalluvya S, et al. Hypertension, kidney disease, HIV and antiretroviral therapy among Tanzanian adults: a cross-sectional study. BMC Med. 2014;12:125. | |
Bergersen BM, Sandvik L, Bruun JN, et al. Elevated Framingham risk score in HIV-positive patients on highly active antiretroviral therapy: results from a Norwegian study of 721 subjects. Eur J Clin Microbiol Infect Dis. 2004;23:625–630. | |
Mateen FJ, Kanters S, Kalyesubulad R, et al. Hypertension prevalence and Framingham risk score stratification in a large HIV-positive cohort in Uganda. J Hypertens. 2013;31:1372–1378. | |
Namusisi O, Sekandi JN, Kasasa S, et al. Risk factors for non-communicable diseases in rural Uganda: a pilot surveillance project among diabetes patients at a referral hospital clinic. Pan Afr Med J. 2011;10:47. | |
Marma AK, Berry JD, Ning H, et al. Distribution of 10-year and lifetime predicted risks for cardiovascular disease in US adults: findings from the National Health and Nutrition Examination Survey 2003 to 2008. Circ Cardiovasc Qual Outcomes. 2010;3:8–14. | |
Jeemon P, Prabhakaran D, Huffman MD, et al; on Behalf of the Sentinel Surveillance in Industrial Populations Study Group. Distribution of 10-year and lifetime predicted risk for cardiovascular disease in the Indian Sentinel Surveillance Study population (cross-sectional survey results). BMJ Open. 2011;1:E000068. | |
Manner IW, Trøseid M, Oektedalen O, Baekken M, Os I. Low nadir CD4 cell count predicts sustained hypertension in HIV-infected individuals. J Clin Hypertens. 2013;15:101–106. | |
Baekken M, Os I, Sandvik L, Oektedalen O. Hypertension in an urban HIV-positive population compared with the general population: influence of combination antiretroviral therapy. J Hypertens. 2008;26:2126–2133. | |
Medina-Torne S, Ganesan A, Barahona I, Crum-Cianflone NF. Hypertension is common among HIV-infected persons, but not associated with HAART. J Int Assoc Physicians AIDS Care. 2012;11:20–25. | |
Khalsa A, Karim R, Mack WJ, et al. Correlates of prevalent hypertension in a large cohort of HIV-infected women: Women`s Interagency HIV Study. AIDS. 2007;21:2539–2541. | |
Petoumenos K, Worm S, Reiss P, et al; D:A:D Study Group. Rates of cardiovascular disease following smoking cessation in patients with HIV infection: results from the D:A:D study. HIV Med. 2011;12:412–421. | |
Worm SW, Sabin C, Weber R, et al. Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from 3 major drug classes: the data collection on adverse events of anti-HIV drugs (D:A:D) study. J Infect Dis. 2010;201:318–330. | |
Mascolini M. When and how to screen for cardiovascular disease risk in people with human immunodeficiency virus. Available from: http://www.centerforaids.org/pdfs/rita0613.pdf. Accessed January 25, 2015. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.