Back to Journals » Patient Preference and Adherence » Volume 16
Dynamics of Patient-Based Benefit-Risk Assessment of Medicines in Chronic Diseases: A Systematic Review
Authors EL Masri H , McGuire TM, van Driel ML , Benham H, Hollingworth SA
Received 24 May 2022
Accepted for publication 25 August 2022
Published 20 September 2022 Volume 2022:16 Pages 2609—2637
DOI https://doi.org/10.2147/PPA.S375062
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Hiba EL Masri,1 Treasure M McGuire,1– 3 Mieke L van Driel,4 Helen Benham,5,6 Samantha A Hollingworth1
1School of Pharmacy, The University of Queensland, Brisbane, Queensland, Australia; 2Faculty of Health Sciences and Medicine, Bond University, Robina, Queensland, Australia; 3Mater Pharmacy, Mater Health, Brisbane, Queensland, Australia; 4Primary Care Clinical Unit, Faculty of Medicine, The University of Queensland, Brisbane, Australia; 5Faculty of Medicine, The University of Queensland, Brisbane, Queensland, Australia; 6Department of Rheumatology, Princess Alexandra Hospital, Brisbane, Queensland, Australia
Correspondence: Hiba EL Masri, School of Pharmacy, The University of Queensland, 20 Cornwall St, Woolloongabba, Brisbane, Queensland, 4102, Australia, Tel +61 478512234, Email [email protected]
Background: A critical gap exits in understanding the dynamics of patient-based benefit-risk assessment (BRA) of medicines in chronic diseases during the disease journey.
Purpose: To systematically review and synthesize current evidence on the changes of patients’ preferences about the benefits and risks of medicines during their disease journey including the influence of disease duration and severity, and previous treatment experience.
Methods: A systematic review of studies identified in PubMed and Embase, from inception to November 2020, was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement. Articles were eligible if they analyzed adult patient-based BRA of medicines with a chronic disease, based on at least one of the pre-specified dimensions: disease severity, disease duration, or previous treatment experience.
Results: A total of 26,228 articles were identified and 105 were eligible for inclusion. Of these, 85 detected a variation in patient-based BRA of medicines with at least one of the pre-specified criteria. Patients with higher disease severity and more treatment experience have increased risk tolerance. It remains inconclusive whether disease duration directly affects the relative importance of a patient’s preference.
Conclusion: Factors important for patients’ BRA of their medicines during a chronic disease journey vary more with their clinical situation and previous treatment experience than with time since diagnosis. Due to the importance of these factors on patients’ perspectives and potential impact on their decision-making and eventually their clinical outcomes, there is a need for more studies to assess the dynamics of patients’ BRA in every disease.
Keywords: patient preference, choice behavior, decision making, health knowledge, attitudes, practice, attributes, risk tolerance
Introduction
Benefit-risk assessment of medicines (BRA) is primarily an exercise that balances two dimensions: the dimension of benefit which includes not only therapeutic efficacy but also improvement of quality of life, and the dimension of risk which consists of the safety profile of the given medicine and the potential risk of unobserved adverse events anticipated on the basis of the mechanism of action and mode of administration.1 The dimension of cost is also often embedded in this analysis.2 BRA of medicines – based on current evidence – is regularly performed at multiple levels to ensure the judicious and safe use of medicines: at a macro-level in regulatory decisions, at a meso-level in guidelines setting, and at a micro-level in shared-decision making.3 Often, however, expert assessment fails to incorporate patients’ preferences and perceptions that might be incongruous with clinicians’ presumptions and opinions.4 A patient-based BRA can complement the expert evidence-based analysis and therefore enhance patients’ involvement, satisfaction, and ultimately adherence, and clinical outcomes. The concept of a more patient-focused evaluation of medicines has emerged and has gained increasing attention from experts and researchers in the last decade.5
 |
Figure 1 Flowchart of literature search results. |
 |
Figure 2 Inter-relationship model of the dynamics of patient-based BRA of medicines in chronic disease. |
Patient-based BRA of medicines is commonly associated with sociodemographic characteristics6,7 but it is unclear if an individual’s patient-based BRA changes during disease progression. Evidence shows that patients tend to evaluate the benefits and risks of their medicines on a shorter time scale than medical professionals.8 However, they may continue to revise their initial BRA and expectations as a result of eventual iterative trial and evaluation, experiences with unwanted side effects, and improvement or worsening of their condition. Increasing numbers of consecutive treatments and a longer disease duration result in an “experienced patient” and in the setting of a chronic disease this may well influence treatment preferences and benefit risk trade-offs.9
Little is known about the dynamics of patient-based BRA of medicines during chronic disease journeys. We therefore aim to systematically review current evidence on the changes of patients’ preferences about the benefits and risks of their medicines during their disease journey, specifically with longer disease duration, increased disease severity, and treatment experience.
Methods
We developed a protocol for our review (PROSPERO ID: CRD42020190966) and followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.10
Systematic Literature Search
We performed a systematic search using PubMed and EMBASE databases from inception to 30 November 2020 using a validated generic search strategy to retrieve published data on patient-based BRA of medicines,11 in combination with search terms relevant to chronic diseases and corresponding treatments. We provided the search syntaxes used in PubMed and EMBASE in Supplementary Information 1. We included studies if they analyzed perceptions or preferences of adult patients (>18 years) with a chronic disease about the balance of benefits and risks of their treatment based on stage of the disease, treatment history, other clinical characteristics, or time post-diagnosis. Chronic diseases, also known as noncommunicable diseases, tend to be long lasting conditions with persistent effects.12,13 They are generally the result of a combination of genetic, physiological, environmental and behavioral factors.12 The most reported chronic conditions groups include arthritis, asthma, back pain, cancer, cardiovascular disease, chronic obstructive pulmonary disease, diabetes, chronic kidney disease, mental health conditions and osteoporosis.13
We excluded studies if they predominately discussed adherence, failed to address patients’ perceptions or preferences on the benefits and risks of chronic treatment, addressed public perceptions or preferences on the benefits and risks of preventive treatment, or did not have a sub-group analysis of patient preferences based on at least on one of three pre-specified dimensions: disease severity, disease duration and previous treatment experience. We chose these dimensions as indicators of disease progression in chronic conditions. In fact, long-standing disease duration is a hallmark of chronic conditions.12 Moreover, adapting therapeutic strategies based on disease severity and previous lines of treatment is an overarching principle in the management of chronic diseases.
Data Extraction
Two reviewers (HM and SH) fully reviewed and independently assessed studies for inclusion and extracted data into a spreadsheet. We resolved disagreements by discussion and adjudication with a third reviewer. For each article that met our inclusion criteria, the two reviewers independently extracted the data. We collected information relevant to the STROBE checklist14 and specifically included: authors, year of publication, study country, disease or condition, sample size, target study population plus age and gender, methods used to elicit patient preferences, attributes assessed, and summary of findings.
Quality Assessment
There are no established criteria to assess risk of bias or the methodological quality of patient preference studies15 but some reviewers have adapted existing quality assessment models used for randomized clinical trials or constructed a new tool.16,17 We adopted a checklist constructed by Eiring et al17 consisting of 31 quality criteria within five domains: 1) external validity of the study, 2) quality of construct representation, 3) minimization of the risk of construct-irrelevant variance due to multiple factors such as impairments in the cognitive abilities of the participants, numeracy skills, emotions and prejudices, 4) quality of reporting and analysis, and 5) other aspects that may strengthen or weaken the study. Two reviewers (HM and SH) independently scored all studies and categorized them into high, medium, and low overall quality, with disagreements resolved by consensus (Supplementary Information 2, Table S1).
Data Synthesis and Analysis
A meta-analysis was not appropriate because the included studies would be methodologically and clinically diverse. Therefore, we qualitatively synthesized the results and presented them in narrative and tabular forms to clarify the nature of changes patient-based BRA of medicines with longer disease duration, increased disease severity, and more patient treatment experience. We used our findings to develop a model of the interrelationships and dynamics of patient-based BRA of medicines in chronic disease.
Results
The search returned 26,228 records and we removed 955 duplicate records (using automatic deduplication in Endnote followed by a manual process). We screened the 25,273 remaining articles at title and abstract level; 544 articles were assessed for inclusion. After full text review, 105 eligible articles were included (Figure 1).
Study Characteristics
These articles assessed the variation of patient-based BRA of medicines with at least one of three pre-defined criteria for this systematic review: disease duration, disease severity, and treatment experience. Most articles (n = 78, 74%) investigated the variation of patient-based BRA of medicines with one of these dimensions, 26 articles (25%) investigated the variation of patient preferences with two dimensions; only one article (1%) examined all three (Table 1). Four in five studies (n = 85, 81%) detected a variation in patient-based BRA of medicines with at least one of the three pre-specified dimensions. There was no association between any of the three dimensions and patient preferences of medicines attributes in 20 studies (19%).
 |
Table 1 Description of Studies Included in the Systematic Review |
Most articles (n = 79, 75%) were published between 2010 and 2020, a quarter (n = 25, 24%) between 2000 and 2009, and one article (1%) was published before 2000. Predominately, the studies were conducted in one country (n = 87, 83%), with the majority from North America and Europe. There was a wide range of therapeutic areas, including autoimmune, cardiovascular, and gastrointestinal diseases, diabetes, and cancer (Table 1). All studies conducted their analyses at a specific point of time of the chronic condition, and there were no studies taking multiple BRA measures over an extended period.
68% (n = 71) of studies were of medium quality, 24% (n = 25) were high, and 8% (n = 9) were of low quality (Supplementary Information 1). High-quality studies typically had a detailed and efficient process to construct attributes and levels, as well as a high effort to minimize the risk of irrelevant variance, by piloting the study or sequencing the questions. 95% of studies were rated high in the quality of reporting and analysis, particularly for the analysis of pre-specified measures and patients’ subgroups.
The number of participants in the included studies varied between 11 and 14,033 and two-thirds of the studies (n = 66, 63%) had between 101 and 500 participants with eight studies (7%) including more than 1000 participants (Table 1). Participants were predominantly female with 35 studies having less than 50% female participants. In most studies (94%) the targeted population were outpatients; only three studies had a mixed cohort of inpatients and outpatients, and three studies did not report these details. There were many recruitment approaches and settings, and some studies adopted more than one approach to achieve the targeted sample size and ensure a representative group of patients. The approaches encompassed recruitment via patient and consumer panels, research agencies, patient registries and databases, patient societies and local groups, and in clinics, specialty centres, and hospitals. Almost half of the studies (n = 50, 48%) reported the response rate, which varied between 7% and 100%.
All studies included a well-defined study question and conducted pre-specified analyses; 22 studies (21%) combined two or more methodologies (Table 1). The analyses were predominantly quantitative; only two studies were qualitative and eight had a mixed method approach. The strategies to elicit patient preferences for their treatment attributes included: discrete choice experiment, other conjoint analysis method, standard gamble, time trade-off, willingness to pay, best-worst scenario, survey or questionnaire, interview, and other methods. The attributes most frequently investigated were outcome-related attributes (n = 98, 93%), mainly efficacy and safety, as well as process-related attributes (n = 68, 65%), including mode of administration and frequency and timing of dosage. Cost-related attributes were assessed in 34 studies (32%).
Disease Duration
Twenty-three studies (22% of total included studies) addressed the variation of patient-based BRA of medicines with disease duration (Table 2): 8 studies (35% of subset) found that with a longer disease duration, patients tend to accept a higher risk of potential side effects and/or higher cost in trade of higher efficacy whereas three studies (13% of subset) reported the opposite. Twelve studies (52% of subset) did not detect any variation in patient preferences with disease duration.
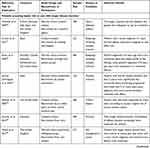 | 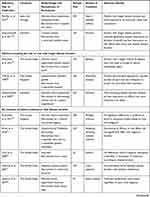 |  |
Table 2 Studies Assessing the Variations of Patient-Based BRA of Medicines with Disease Duration |
Disease Severity
Fifty-one studies (49% of total included studies) measured the impact of disease severity on patient-based BRA of medicines (Table 3). Overall, 29 studies (57% of subset) reported patients were more willing to accept a higher risk of treatment-related side effects or a higher cost of treatment when they had more severe symptoms, more disease damage, or a higher risk for disease progression. Thirteen studies (25% of subset) reported a greater risk aversion and a reduced importance for efficacy with disease progression whilst the reminder (n = 9, 17% of subset) found no variation of patient-based BRA with disease severity.
 |  |  |  |  |  |  |
Table 3 Studies Assessing the Variations of Patient-Based BRA of Medicines with Disease Severity |
Treatment Experience
Fifty-eight studies (55% of total included studies) examined the dynamics of the evolution of patient-based BRA of medicines with previous treatment experiences (Table 4): 37 studies (64% of subset) reported an increased patient acceptance of risks, cost, or inconvenience with treatment experience, 10 studies (17% of subset) reported a decreased patient tolerance of risks, cost, or inconvenience with treatment history while 11 studies (19% of subset) found no association.
 |  |  |  |  |  |  |
Table 4 Studies Assessing the Variations of Patient-Based BRA of Medicines with Treatment Experience |
Narrative Synthesis
Our findings suggest that patient preferences may not have a clear association with disease duration. Half of the studies addressing the variation of patient-based BRA of medicines with disease duration (52%) reported no association between risk acceptance and disease duration,18–29 with fewer studies (35%) reported a higher tolerance for risk with more years since diagnosis30–38 whilst 13% reporting the opposite with more risk aversion with longer disease duration.39–41 There is a clearer association between patient preferences and disease severity with more than half of the studies (57%) identified in this category reported an increased risk tolerance with progressing disease severity36,39–66 whilst 17% of these studies found no association.18,20,23,29,67–71 There was a discernable association between patient treatment experience and increased risk tolerance (64%).31–33,35,37,38,45,48,72–100 Efficacy-related attributes as well as willingness-to-pay for more efficacious treatment gained more importance for patients with increasing experience with medicines.33,35,45,72,74,79,89,93,96,99 Safety-related attributes had more weight for treatment-naïve patients, but the importance diminished for patients with more treatment experience as they became more risk-tolerant.33,73,79,80,88,90,98–100 Process-related attributes, and particularly acceptance of injectable medications, changed considerably with treatment experience. Patients with more exposure to treatment were less concerned about the convenience of treatment and more open to using different formulations and routes of administration.33,78,81,82,87 Patients who had used injectable medicines placed less importance on mode of administration and convenience and were more willing to accept self-injectable treatments than patients who had not used these prior.32,37,38,75–77,80,83–86,91,92,94,95,97 However, not expectantly, previous experience of side effects was associated with patients becoming more risk averse.64,101–108 A model depicting the inter-relationship and dynamic impact of disease severity, disease duration and treatment experience on patients’ preferences and risk tolerance in chronic disease is represented in Figure 2.
Discussion
We identified 105 studies that investigated patient preferences of medicines’ attributes in a vast range of chronic conditions and explored preferences across three dimensions of disease duration, disease severity, and treatment experience. Most studies (81%) reported variations in patient preferences with one or more dimensions and only 19% found no association. The findings suggest that patient treatment experience, positive or negative, and disease severity are dominant factors that influence the dynamics of patient-based BRA of medicines. Disease duration seems to be a weaker contributor to these dynamics. In fact, time since diagnosis, when considered as an independent direct factor, provides increasing opportunities of preference reinforcement. However, in chronic disease, it is most often that with time patients may experience worsening of symptoms, more lines of treatments, and side effects.107 This may suggest that disease duration also provides circular reinforcement of the dominant factors influencing the dynamics of patient preferences.
Patients have an increasing risk tolerance and a greater willingness-to-pay with treatment experience during their disease journey.31–33,35,37,38,45,48,72–100 This may be explained by the impact of previous treatments on patients’ preferences.109 Although treatment-naïve patients are relatively more risk averse than treatment-experienced patients,79,99,100 the latter who had previously endured side effects become less risk tolerant.101,103,107,108 This is in line with the concept distinguishing patients’ perceptions ex-ante (prior to an event/anticipated) and ex-post (after the event/experienced),110 when a direct experience of a serious adverse event may alter how patients assess the BRA of their medicines. They may overemphasize risk and overestimate the severity of potential side effects.111 For example, patients with multiple myeloma who had previously experienced severe or life-threatening side effects put more importance on low toxicity than on progression-free survival.107
Another salient result is the increased acceptance of injectable treatments, notably self-administration, among patients who had already used this mode of administration. For example, insulin-naive patients are more averse to taking subcutaneous insulin in the future76 whereas insulin‐treated patients placed less importance on mode of administration.75 Abu Hassan et al found that negative concerns about the use of insulin such as self-injection, needle phobia, inconvenience, and embarrassment are significantly higher in insulin-naïve diabetic patients than in experienced insulin-user diabetic patients.112 This is confirmed by the increased use of subcutaneous injectable devices, driven by increased users’ satisfaction with respect to convenience, ergonomics, and portability.113
Moreover, we found that patients with higher disease severity,42,50,52 more pronounced symptoms,46 or increased disease damage49 placed higher importance on efficacy and less importance on the safety profile and cost. Indeed, patients may tolerate more severe potential side effects when their disease progression negatively affects their quality of life. For example, patients with inflammatory bowel disease develop a greater acceptance for potential risks of treatment when their condition worsens, in a desperate search for a cure.114 It remains inconclusive how disease duration, as an independent factor, alters patient preferences. The contrast across these dimensions suggests that factors important for patients’ assessment of benefits and risks of their medicines during a chronic disease journey will vary more with their clinical situation and previous treatment experience than with time since their diagnosis.
The studies revealed a range of strategies to elicit patient preferences. Conjoint analysis methods (especially discrete choice experiments) were the most frequently used, but there were 15 different methods employed in the studies reviewed. This mirrors the overall upward trend observed in the use of patient preferences elicitation methods over the last decade.115 There is currently no comprehensive comparison of these emerging methods, but increasing publications are providing guidance to select the most appropriate approach for a given application.116–118
What are the implications for discussing benefits and risks of medicines with patients, at different points along their disease journey? Treatment paradigms and recommendations are shifting to earlier and more aggressive treatments. For example, in rheumatoid arthritis there is a “window of opportunity” in the first three months of disease onset to prevent damage occurring.119 Our results suggest that patients will be more risk averse and concerned during this phase, although they will become more risk tolerant and put higher importance on efficacy with more experience with treatment or when their symptoms become more severe. It is critical that patients and clinicians adequately understand that individual BRA may change.
Understanding the dynamics of patient-based BRA is also important when considering patient preferences in regulatory decisions. Having patients directly involved in the decision-making process or using evidence derived from patients in empirical studies should be routinely utilised as part of the evidence considered.4,120 Such input must be balanced and derived from cohorts of patients at different points of their disease journey and with different levels of exposure to treatments.
Despite the current evidence of the dynamics of patient-based BRA of medicines during the disease journey, only 105 out of 544 identified in the title and abstract screening had sub-group analyses based on disease duration, disease severity, or treatment experience. Due to the importance of these dimensions on patient preferences and potential impact on patients’ decision-making and clinical outcomes, there is a need for more studies to assess changes: larger studies that may be statistically powered for such sub-group analyses; the use of different methodologies; or longitudinal studies.
Strengths and Limitations
This is the first study, to our knowledge, to systematically review evidence of the dynamics of patient-based BRA of medicines in chronic diseases. The strengths of our review include the registered protocol, a validated search strategy, pre-specified eligibility criteria, and duplicate screening and data extraction.
This review has several limitations. Given the methodological and clinical heterogeneity of included studies, it was not possible to draw robust conclusions or conduct a meta-analysis. Therefore, we considered a narrative synthesis to be the most suitable format. We note that such a review is subject to a higher bias than a quantitative systematic review.121 However, the strong and consistent trends across the varied methods and wide range of chronic diseases studied support our proposed dynamic BRA model.
This review encompassed studies from various chronic conditions, with substantial differences in the burden of the disease on the patients as well as the efficacy-safety profile of suggested treatments. Moreover, studies included were not longitudinal. They assessed patients’ BRA of their medicines at one point of their disease journey when there may be other unidentified individual factors impacting patients’ perspectives.
Conclusion
This study identified and reviewed a large body of literature regarding the dynamics of patient-based BRA of medicines during the disease journey in chronic conditions. We conclude that factors impacting patients’ risk tolerance vary more with their disease severity and previous treatment experience than with time since diagnosis. These findings may be utilized to provide context for patient centered clinical decision-making around the use of medicines in chronic disease.
Key Points for Decision Makers
- Patient assessment of benefits and risks of medicines in chronic conditions is likely to evolve during the disease journey
- Patients with increased treatment experience tend to become more risk tolerant
- Patients with experience in self-injectables have a higher acceptance for this mode of administration
- Patients with increased disease severity are willing to accept higher risks to achieve improved clinical outcomes
- Patients with experiences of side effects may become more risk averse
Acknowledgments
The authors would like to thank Christine Dalais for her valuable contribution in preparing the list of search terms relevant to chronic diseases and corresponding treatments.
Author Contributions
All authors made a significant contribution to the review reported, whether that is in the conception, design, execution, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Curtin F, Schulz P. Assessing the benefit: risk ratio of a drug–randomized and naturalistic evidence. Dialogues Clin Neurosci. 2011;13(2):183–190. doi:10.31887/DCNS.2011.13.2/fcurtin
2. Eichler HG, Abadie E, Raine JM, et al. Safe drugs and the cost of good intentions. N Engl J Med. 2009;360(14):1378–1380. doi:10.1056/NEJMp0900092
3. Muhlbacher AC, Juhnke C. Patient preferences versus physicians’ judgement: does it make a difference in healthcare decision making? Appl Health Econ Health Policy. 2013;11(3):163–180. doi:10.1007/s40258-013-0023-3
4. Muhlbacher AC, Juhnke C, Beyer AR, et al. Patient-focused benefit-risk analysis to inform regulatory decisions: the European Union perspective. Value Health. 2016;19(6):734–740. doi:10.1016/j.jval.2016.04.006
5. van Til JA, Ijzerman MJ. Why should regulators consider using patient preferences in benefit-risk assessment? Pharmacoeconomics. 2014;32(1):1–4. doi:10.1007/s40273-013-0118-6
6. Bewtra M, Johnson FR. Assessing patient preferences for treatment options and process of care in inflammatory bowel disease: a critical review of quantitative data. Patient. 2013;6(4):241–255. doi:10.1007/s40271-013-0031-2
7. Durand C, Eldoma M, Marshall DA, et al. Patient preferences for disease modifying anti-rheumatic drug treatment in rheumatoid arthritis: a systematic review. J Rheumatol. 2020;47(2):176–187. doi:10.3899/jrheum.181165
8. Dohnhammar U, Reeve J, Walley T. Patients’ expectations of medicines – a review and qualitative synthesis. Health Expect. 2016;19(2):179–193. doi:10.1111/hex.12345
9. Kromer C, Peitsch WK, Herr R, et al. Treatment preferences for biologicals in psoriasis: experienced patients appreciate sustainability. JDDG. 2017;15(2):189–200.
10. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097
11. El Masri H, McGuire T, Dalais C, et al. Patient-based benefit-risk assessment of medicines: development, refinement, and validation of a content search strategy to retrieve relevant studies. J Med Libr Assoc. 2022;110(2):185–204. doi:10.5195/jmla.2022.1306
12. World Health Organization. Noncommunicable diseases; 2021. Available from: https://www.who.int/en/news-room/fact-sheets/detail/noncommunicable-diseases.
13. Australian Institute of Health and Welfare. Chronic disease; 2021. Available from: https://www.aihw.gov.au/reports-data/health-conditions-disability-deaths/chronic-disease/overview.
14. von Elm E, Altman DG, Egger M, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4(10):e296. doi:10.1371/journal.pmed.0040296
15. Yu T, Enkh-Amgalan N, Zorigt G. Methods to perform systematic reviews of patient preferences: a literature survey. BMC Med Res Methodol. 2017;17(1):166. doi:10.1186/s12874-017-0448-8
16. Purnell TS, Joy S, Little E, et al. Patient preferences for noninsulin diabetes medications: a systematic review. Diabetes Care. 2014;37(7):2055–2062. doi:10.2337/dc13-2527
17. Eiring Ø, Landmark BF, Aas E, et al. What matters to patients? A systematic review of preferences for medication-associated outcomes in mental disorders. BMJ Open. 2015;5(4):e007848. doi:10.1136/bmjopen-2015-007848
18. Bottomley C, Lloyd A, Bennett G, et al. A discrete choice experiment to determine UK patient preference for attributes of disease modifying treatments in Multiple Sclerosis. J Med Econ. 2017;20(8):863–870. doi:10.1080/13696998.2017.1336099
19. Bruce JM, Bruce AS, Lynch S, et al. Probability discounting of treatment decisions in multiple sclerosis: associations with disease knowledge, neuropsychiatric status, and adherence. Psychopharmacology. 2018;235(11):3303–3313. doi:10.1007/s00213-018-5037-y
20. Choi TN, Westermann H, Sayles W, et al. Beliefs about asthma medications: patients perceive both benefits and drawbacks. J Asthma. 2008;45(5):409–414. doi:10.1080/02770900801971834
21. Fraenkel L, Bodardus S, Wittnik DR. Understanding patient preferences for the treatment of lupus nephritis with adaptive conjoint analysis. Med Care. 2001;39(11):1203–1216. doi:10.1097/00005650-200111000-00007
22. Gelhorn HL, Balantac Z, Ambrose CS, et al. Patient and physician preferences for attributes of biologic medications for severe asthma. Patient Prefer Adherence. 2019;13:1253–1268. doi:10.2147/PPA.S198953
23. Johnson FR, Van Houtven G, Ozdemir S, et al. Multiple sclerosis patients’ benefit-risk preferences: serious adverse event risks versus treatment efficacy. J Neurol. 2009;256(4):554–562. doi:10.1007/s00415-009-0084-2
24. Lewis HB, Schroeder M, Gunsoy NB, et al. Evaluating patient preferences of maintenance therapy for the treatment of chronic obstructive pulmonary disease: a discrete choice experiment in the UK, USA and Germany. Int J Chron Obstruct Pulmon Dis. 2020;15:595–604. doi:10.2147/COPD.S221980
25. Rigopoulos D, Ioannides D, Chaidemenos G, et al.. Patient preference study for different characteristics of systemic psoriasis treatments (Protimisis). Dermatol Ther. 2018;31(3). doi:10.1111/dth.12592
26. Scarpato S, Antivalle M, Favalli EG, et al. Patient preferences in the choice of anti-TNF therapies in rheumatoid arthritis. Results from a questionnaire survey (RIVIERA study). Rheumatology. 2010;49(2):289–294. doi:10.1093/rheumatology/kep354
27. Tada Y, Ishii K, Kimura J, et al. Patient preference for biologic treatments of psoriasis in Japan. J Dermatol. 2019;46(6):466–477. doi:10.1111/1346-8138.14870
28. Turk D, Boeri M, Abraham L, et al. Patient preferences for osteoarthritis pain and chronic low back pain treatments in the United States: a discrete-choice experiment. Osteoarthritis Cartilage. 2020;28(9):1202–1213. doi:10.1016/j.joca.2020.06.006
29. Wong YN, Egleston BL, Sachdeva K, et al. Cancer patients’ trade-offs among efficacy, toxicity, and out-of-pocket cost in the curative and noncurative setting. Med Care. 2013;51(9):838–845. doi:10.1097/MLR.0b013e31829faffd
30. Aristides M, Weston AR, FitzGerald P, et al. Patient preference and willingness-to-pay for Humalog Mix25 relative to Humulin 30/70: a multicountry application of a discrete choice experiment. Value Health. 2004;7(4):442–454. doi:10.1111/j.1524-4733.2004.74007.x
31. Arroyo R, Sempere AP, Ruiz-Beato E, et al. Conjoint analysis to understand preferences of patients with multiple sclerosis for disease-modifying therapy attributes in Spain: a cross-sectional observational study. BMJ Open. 2017;7(3):e014433. doi:10.1136/bmjopen-2016-014433
32. Bauer B, Brockmeier B, Devonshire V, et al. An international discrete choice experiment assessing patients’ preferences for disease-modifying therapy attributes in multiple sclerosis. Neurodegener Dis Manag. 2020;10(6):369–382. doi:10.2217/nmt-2020-0034
33. Garcia-Dominguez JM, Muñoz D, Comellas M, et al. Patient preferences for treatment of multiple sclerosis with disease-modifying therapies: a discrete choice experiment. Patient Prefer Adherence. 2016;10:1945–1956. doi:10.2147/PPA.S114619
34. Johnson FR, Ozdemir S, Mansfield C, et al. Crohn’s disease patients’ risk-benefit preferences: serious adverse event risks versus treatment efficacy. Gastroenterology. 2007;133(3):769–779. doi:10.1053/j.gastro.2007.04.075
35. Kromer C, Schaarschmidt ML, Schmieder A, et al. Patient preferences for treatment of psoriasis with biologicals: a discrete choice experiment. PLoS One. 2015;10(6):e0129120. doi:10.1371/journal.pone.0129120
36. Meads DM, O’Dwyer JL, Hulme CT, et al. Patient preferences for pain management in advanced cancer: results from a discrete choice experiment. Patient. 2017;10(5):643–651. doi:10.1007/s40271-017-0236-x
37. Morillas C, Feliciano R, Catalina PF, et al. Patients‘ and physicians‘ preferences for type 2 diabetes mellitus treatments in Spain and Portugal: a discrete choice experiment.. Patient Prefer Adherence. 2015;9:1443–1458. doi:10.2147/PPA.S88022
38. Schaarschmidt ML, Umar N, Schmieder A, et al. Patient preferences for psoriasis treatments: impact of treatment experience. J Eur Acad Dermatol Venereol. 2013;27(2):187–198. doi:10.1111/j.1468-3083.2011.04440.x
39. Manjunath R, Yang JC, Ettinger AB. Patients’ preferences for treatment outcomes of add-on antiepileptic drugs: a conjoint analysis. Epilepsy Behav. 2012;24(4):474–479. doi:10.1016/j.yebeh.2012.05.020
40. O’Brien BJ, Elswood J, Calin A. Willingness to accept risk in the treatment of rheumatic disease. J Epidemiol Community Health. 1990;44(3):249–252. doi:10.1136/jech.44.3.249
41. Schaarschmidt ML, Herr R, Gutknecht M, et al. Patients’ and physicians’ preferences for systemic psoriasis treatments: a nationwide comparative discrete choice experiment (PsoCompare). Acta Derm Venereol. 2018;98(2):200–205. doi:10.2340/00015555-2834
42. Alcusky M, Lee S, Lau G, et al. Dermatologist and patient preferences in choosing treatments for moderate to severe psoriasis. Dermatol Ther. 2017;7(4):463–483. doi:10.1007/s13555-017-0205-2
43. Athavale A, Gooch K, Walker D, et al. A patient-reported, non-interventional, cross-sectional discrete choice experiment to determine treatment attribute preferences in treatment-naïve overactive bladder patients in the US. Patient Prefer Adherence. 2018;12:2139–2152. doi:10.2147/PPA.S178668
44. Brooks A, Langer J, Tervonen T, et al. Patient preferences for GLP-1 receptor agonist treatment of type 2 diabetes mellitus in Japan: a discrete choice experiment. Diabetes Ther. 2019;10(2):735–749. doi:10.1007/s13300-019-0591-9
45. Bruce JM, Jarmolowicz DP, Lynch S, et al. How patients with multiple sclerosis weigh treatment risks and benefits. Health Psychol. 2018;37(7):680–690. doi:10.1037/hea0000626
46. Chapman SC, Horne R, Chater A, et al. Patients’ perspectives on antiepileptic medication: relationships between beliefs about medicines and adherence among patients with epilepsy in UK primary care. Epilepsy Behav. 2014;31:312–320. doi:10.1016/j.yebeh.2013.10.016
47. de Bekker-Grob EW, Essink-Bot ML, Meerding WJ, et al. Patients’ preferences for osteoporosis drug treatment: a discrete choice experiment. Osteoporos Int. 2008;19(7):1029–1037. doi:10.1007/s00198-007-0535-5
48. Fayad F, Ziade NR, Merheb G, et al. Patient preferences for rheumatoid arthritis treatments: results from the national cross-sectional LERACS study. Patient Prefer Adherence. 2018;12:1619–1625. doi:10.2147/PPA.S168738
49. Fox RJ, Salter A, Alster JM, et al. Risk tolerance to MS therapies: survey results from the NARCOMS registry. Mult Scler Relat Disord. 2015;4(3):241–249. doi:10.1016/j.msard.2015.03.003
50. Fraenkel L, Chodkowski D, Lim J, et al. Patients’ preferences for treatment of hepatitis C. Med Decis Making. 2010;30(1):45–57. doi:10.1177/0272989X09341588
51. Fraenkel L, Gulanski B, Wittink D. Patient willingness to take teriparatide. Patient Educ Couns. 2007;65(2):237–244. doi:10.1016/j.pec.2006.08.004
52. Fu AZ, Graves KD, Jensen RE, et al. Patient preference and decision-making for initiating metastatic colorectal cancer medical treatment. J Cancer Res Clin Oncol. 2016;142(3):699–706. doi:10.1007/s00432-015-2073-4
53. Gallagher RM, Kunkel R. Migraine medication attributes important for patient compliance: concerns about side effects may delay treatment. Headache. 2003;43(1):36–43. doi:10.1046/j.1526-4610.2003.03006.x
54. Gray JR, Leung E, Scales J. Treatment of ulcerative colitis from the patient’s perspective: a survey of preferences and satisfaction with therapy. Aliment Pharmacol Ther. 2009;29(10):1114–1120. doi:10.1111/j.1365-2036.2009.03972.x
55. Hauber AB, Mohamed AF, Gonzalez JM, et al. Benefit-risk tradeoff preferences for chronic hand eczema treatments. J Dermatolog Treat. 2017;28(1):40–46. doi:10.1080/09546634.2016.1177161
56. Hauber AB, Mohamed AF, Johnson FR, et al. Treatment preferences and medication adherence of people with Type 2 diabetes using oral glucose‐lowering agents. Diabet Med. 2009;26(4):416–424. doi:10.1111/j.1464-5491.2009.02696.x
57. Hiligsmann M, Dellaert BG, Dirksen CD, et al. Patients’ preferences for anti-osteoporosis drug treatment: a cross-European discrete choice experiment. Rheumatology. 2017;56(7):1167–1176. doi:10.1093/rheumatology/kex071
58. Hodgkins P, Swinburn P, Solomon D, et al. Patient preferences for first-line oral treatment for mild-to-moderate ulcerative colitis: a discrete-choice experiment. Patient. 2012;5(1):33–44. doi:10.2165/11595390-000000000-00000
59. Howell M, Wong G, Rose J, et al. Patient preferences for outcomes after kidney transplantation: a best-worst scaling survey. Transplantation. 2017;101(11):2765–2773. doi:10.1097/TP.0000000000001793
60. Johnson FR, Hauber AB, Ozdemir S, et al. Quantifying women’s stated benefit-risk trade-off preferences for IBS treatment outcomes. Value Health. 2010;13(4):418–423. doi:10.1111/j.1524-4733.2010.00694.x
61. Kløjgaard ME, Manniche C, Pedersen LB, et al. Patient preferences for treatment of low back pain-a discrete choice experiment. Value Health. 2014;17(4):390–396. doi:10.1016/j.jval.2014.01.005
62. Lacy BE, Yu J, Crowell MD. Medication risk-taking behavior in functional dyspepsia patients. Clin Transl Gastroenterol. 2015;6(1):e69. doi:10.1038/ctg.2014.18
63. Lim SS, Kan H, Pobiner BF, et al. Patient perceptions and preferences of biologic therapies in SLE. Lupus Sci Med. 2019;6(1):e000322. doi:10.1136/lupus-2019-000322
64. Mantovani LG, Monzini MS, Mannucci PM, et al. Differences between patients’, physicians’ and pharmacists’ preferences for treatment products in haemophilia: a discrete choice experiment. Haemophilia. 2005;11(6):589–597. doi:10.1111/j.1365-2516.2005.01159.x
65. Nolla JM, Rodríguez M, Martin-Mola E, et al. Patients’ and rheumatologists’ preferences for the attributes of biological agents used in the treatment of rheumatic diseases in Spain. Patient Prefer Adherence. 2016;10:1101–1113. doi:10.2147/PPA.S106311
66. Ratcliffe J, Buxton M, McGarry T, et al. Patients’ preferences for characteristics associated with treatments for osteoarthritis. Rheumatology. 2004;43(3):337–345. doi:10.1093/rheumatology/keh038
67. Bröckelmann PJ, McMullen S, Wilson JB, et al. Patient and physician preferences for first-line treatment of classical Hodgkin lymphoma in Germany, France and the United Kingdom. Br J Haematol. 2019;184(2):202–214. doi:10.1111/bjh.15566
68. Gajra A, McCall L, Muss HB, et al. The preference to receive chemotherapy and cancer-related outcomes in older adults with breast cancer CALGB 49907 (Alliance). J Geriatr Oncol. 2018;9(3):221–227. doi:10.1016/j.jgo.2018.02.003
69. Havrilesky LJ, Alvarez Secord A, Ehrisman JA, et al. Patient preferences in advanced or recurrent ovarian cancer. Cancer. 2014;120(23):3651–3659. doi:10.1002/cncr.28940
70. Hendriks A, Wijnen B, van Engelen R, et al. A best-worst scaling in Colombian patients to rank the characteristics of HIV/AIDS treatment. J Med Econ. 2018;21(5):468–473. doi:10.1080/13696998.2018.1440401
71. Jarmolowicz DP, Bruce AS, Glusman M, et al. On how patients with multiple sclerosis weigh side effect severity and treatment efficacy when making treatment decisions. Exp Clin Psychopharmacol. 2017;25(6):479–484. doi:10.1037/pha0000152
72. Berry D, Bradlow A, Bersellini E. Perceptions of the risks and benefits of medicines in patients with rheumatoid arthritis and other painful musculoskeletal conditions. Rheumatology. 2004;43(7):901–905. doi:10.1093/rheumatology/keh196
73. Beusterien KM, Dziekan K, Schrader S, et al. Patient preferences among third agent HIV medications: a US and German perspective. AIDS Care. 2007;19(8):982–988. doi:10.1080/09540120701294278
74. Blinman P, Mileshkin L, Khaw P, et al. Patients’ and clinicians’ preferences for adjuvant chemotherapy in endometrial cancer: an ANZGOG substudy of the PORTEC-3 intergroup randomised trial. Br J Cancer. 2016;115(10):1179–1185. doi:10.1038/bjc.2016.323
75. Casciano R, Malangone E, Ramachandran A, et al. A quantitative assessment of patient barriers to insulin. Int J Clin Pract. 2011;65(4):408–414. doi:10.1111/j.1742-1241.2010.02590.x
76. Cefalu WT, Mathieu C, Davidson J, et al. Patients’ perceptions of subcutaneous insulin in the OPTIMIZE study: a multicenter follow-up study. Diabetes Technol Ther. 2008;10(1):25–38. doi:10.1089/dia.2008.0249
77. Desplats M, Pascart T, Jelin G, et al. Are Abatacept and tocilizumab intravenous users willing to switch for the subcutaneous route of administration? A questionnaire-based study. Clin Rheumatol. 2017;36(6):1395–1400. doi:10.1007/s10067-017-3587-8
78. Dowson A, Bundy M, Salt R, et al. Patient preference for triptan formulations: a prospective study with zolmitriptan. Headache. 2007;47(8):1144–1151. doi:10.1111/j.1526-4610.2007.00805.x
79. Duarte JW, Bolge SC, Sen SS. An evaluation of patients’ preferences for osteoporosis medications and their attributes: the PREFER-International study. Clin Ther. 2007;29(3):488–503. doi:10.1016/S0149-2918(07)80087-7
80. Eliasson L, Bewley AP, Mughal F, et al. Evaluation of psoriasis patients’ attitudes toward benefit–risk and therapeutic trade-offs in their choice of treatments. Patient Prefer Adherence. 2017;11:353–362. doi:10.2147/PPA.S121838
81. Emkey R, Koltun W, Beusterien K, et al. Patient preference for once-monthly ibandronate versus once-weekly alendronate in a randomized, open-label, cross-over trial: the Boniva Alendronate Trial in Osteoporosis (BALTO). Curr Med Res Opin. 2005;21(12):1895–1903. doi:10.1185/030079905X74862
82. Engelhard EA, Smit C, Vervoort SC, et al. Patients’ willingness to take multiple-tablet antiretroviral therapy regimens for treatment of HIV. Drugs Real World Outcomes. 2016;3(2):223–230. doi:10.1007/s40801-016-0070-9
83. Flood EM, Bell KF, de la Cruz MC, et al. Patient preferences for diabetes treatment attributes and drug classes. Curr Med Res Opin. 2017;33(2):261–268. doi:10.1080/03007995.2016.1253553
84. Grisanti L, Kwiatkowski A, Dyrda P, et al. Patient perspectives on intravenous biologics for rheumatologic disease. Arthritis Care Res. 2019;71(9):1234–1242. doi:10.1002/acr.23758
85. Ho KA, Acar M, Puig A, et al. What do Australian patients with inflammatory arthritis value in treatment? A discrete choice experiment. Clin Rheumatol. 2020;39(4):1077–1089. doi:10.1007/s10067-019-04843-4
86. Huynh TK, Ostergaard A, Egsmose C, et al. Preferences of patients and health professionals for route and frequency of administration of biologic agents in the treatment of rheumatoid arthritis. Patient Prefer Adherence. 2014;8:93–99. doi:10.2147/PPA.S55156
87. Johansson G, Ställberg B, Tornling G, et al. Asthma treatment preference study: a conjoint analysis of preferred drug treatments. Chest. 2004;125(3):916–923. doi:10.1378/chest.125.3.916
88. Kowacs PA, Piovesan EJ, Tepper SJ. Rejection and acceptance of possible side effects of migraine prophylactic drugs. Headache. 2009;49(7):1022–1027. doi:10.1111/j.1526-4610.2009.01431.x
89. Lim SG, Aung MO, Chung SW, et al. Patient preferences for hepatitis B therapy. Antivir Ther. 2013;18(5):663–670. doi:10.3851/IMP2482
90. Lloyd A, Nafees B, Barnett AH, et al. Willingness to pay for improvements in chronic long-acting insulin therapy in individuals with type 1 or type 2 diabetes mellitus. Clin Ther. 2011;33(9):1258–1267. doi:10.1016/j.clinthera.2011.07.017
91. Mansfield C, Sikirica MV, Pugh A, et al. Patient preferences for attributes of type 2 diabetes mellitus medications in Germany and Spain: an online discrete-choice experiment survey. Diabetes Ther. 2017;8(6):1365–1378. doi:10.1007/s13300-017-0326-8
92. Marchesini G, Pasqualetti P, Anichini R, et al. Patient preferences for treatment in type 2 diabetes: the Italian discrete-choice experiment analysis. Acta Diabetol. 2019;56(3):289–299. doi:10.1007/s00592-018-1236-6
93. McTaggart-Cowan HM, Shi P, Fitzgerald JM, et al. An evaluation of patients’ willingness to trade symptom-free days for asthma-related treatment risks: a discrete choice experiment. J Asthma. 2008;45(8):630–638. doi:10.1080/02770900802126990
94. Peyrot M, Rubin RR. Perceived medication benefits and their association with interest in using inhaled insulin in type 2 diabetes: a model of patients’ cognitive framework. Patient Prefer Adherence. 2011;5:255–265. doi:10.2147/PPA.S18799
95. van Heuckelum M, Mathijssen EGE, Vervloet M, et al. Preferences of patients with rheumatoid arthritis regarding disease-modifying antirheumatic drugs: a discrete choice experiment. Patient Prefer Adherence. 2019;13:1199–1211. doi:10.2147/PPA.S204111
96. Verhoef LM, Selten EMH, Vriezekolk JE, et al. The patient perspective on biologic DMARD dose reduction in rheumatoid arthritis: a mixed methods study. Rheumatology. 2018;57(11):1947–1955. doi:10.1093/rheumatology/key205
97. Vigneau C, Choukroun G, Isnard-Bagnis C, et al. “Doctor, can I have less frequent injection with highly efficient treatment?” A patient centered study using an electronic choice-based conjoint analysis (ePRO) to assess real world preferences regarding erythropoiesis stimulating agent to treat anaemia in chronic kidney disease (PERCEPOLIS study). Nephrol Ther. 2019;15(3):152–161. doi:10.1016/j.nephro.2018.11.009
98. Weilandt J, Diehl K, Schaarschmidt ML, et al. Patient preferences in adjuvant and palliative treatment of advanced melanoma: a discrete choice experiment. Acta Derm Venereol. 2020;100(6):1–9. doi:10.2340/00015555-3422
99. Weiss TW, Gold DT, Silverman SL, et al. An evaluation of patient preferences for osteoporosis medication attributes: results from the PREFER-US study. Curr Med Res Opin. 2006;22(5):949–960. doi:10.1185/030079906X104740
100. Wong XY, Lim AQJ, Shen Q, et al. Patient preferences and predicted relative uptake for targeted therapies in metastatic colorectal cancer: a discrete choice experiment. Curr Med Res Opin. 2020;36(10):1677–1686. doi:10.1080/03007995.2020.1790348
101. Blinman PL, Davis ID, Martin A, et al. Patients’ preferences for adjuvant sorafenib after resection of renal cell carcinoma in the SORCE trial: what makes it worthwhile? Ann Oncol. 2018;29(2):370–376. doi:10.1093/annonc/mdx715
102. Brotherston DC, Poon I, Le T, et al. Patient preferences for oropharyngeal cancer treatment de-escalation. Head Neck. 2013;35(2):151–159. doi:10.1002/hed.22930
103. Hardtstock F, Sbarigia U, Kocaata Z, et al. Preferences of patients with chronic hepatitis B– a discrete choice experiment on the acceptability of functional cure. Patient Prefer Adherence. 2020;14:613–624. doi:10.2147/PPA.S238833
104. Hehir MK, Punga AR, Ciafaloni E. Myasthenia gravis patient and physician opinions about immunosuppressant reduction. Muscle Nerve. 2020;61(6):767–772. doi:10.1002/mus.26850
105. Islam KM, Anggondowati T, Deviany PE, et al. Patient preferences of chemotherapy treatment options and tolerance of chemotherapy side effects in advanced stage lung cancer. BMC Cancer. 2019;19(1):835. doi:10.1186/s12885-019-6054-x
106. Locadia M, van Grieken RA, Prins JM, et al. Patients’ preferences regarding the timing of highly active antiretroviral therapy initiation for chronic asymptomatic HIV-1 infection. Antivir Ther. 2006;11(3):335–341. doi:10.1177/135965350601100309
107. Postmus D, Richard S, Bere N, et al. Individual trade-offs between possible benefits and risks of cancer treatments: results from a stated preference study with patients with multiple myeloma. Oncologist. 2018;23(1):44–51. doi:10.1634/theoncologist.2017-0257
108. Poulos C, Soliman AM, Renz CL, et al. Patient preferences for endometriosis pain treatments in the United States. Value Health. 2019;22(6):728–738. doi:10.1016/j.jval.2018.12.010
109. MacLean S, Mulla S, Akl EA, et al. Patient values and preferences in decision making for antithrombotic therapy: a systematic review: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2Suppl):e1S–e23S. doi:10.1378/chest.11-2290
110. Harrison M, Rigby D, Vass C, et al. Risk as an attribute in discrete choice experiments: a systematic review of the literature. Patient. 2014;7(2):151–170. doi:10.1007/s40271-014-0048-1
111. El Masri H, Hollingworth SA, van Driel M, et al. Real-world questions and concerns about disease-modifying antirheumatic drugs (DMARDs): a retrospective analysis of questions to a medicine call center. BMC Rheumatol. 2020;4:27. doi:10.1186/s41927-020-00126-7
112. Abu Hassan H, Tohid H, Mohd Amin R, et al. Factors influencing insulin acceptance among type 2 diabetes mellitus patients in a primary care clinic: a qualitative exploration. BMC Fam Pract. 2013;14:164. doi:10.1186/1471-2296-14-164
113. Ridyard CH, Dawoud DM, Tuersley LV, et al. A systematic review of patients’ perspectives on the subcutaneous route of medication administration. Patient. 2016;9(4):281–292. doi:10.1007/s40271-015-0160-x
114. Harvey JM, Sibelli A, Chalder T, et al. Desperately seeking a cure: treatment seeking and appraisal in irritable bowel syndrome. Br J Health Psychol. 2018;23(3):561–579. doi:10.1111/bjhp.12304
115. Soekhai V, Whichello C, Levitan B, et al. Methods for exploring and eliciting patient preferences in the medical product lifecycle: a literature review. Drug Discov Today. 2019;24(7):1324–1331. doi:10.1016/j.drudis.2019.05.001
116. Whichello C, Levitan B, Juhaeri J, et al. Appraising patient preference methods for decision-making in the medical product lifecycle: an empirical comparison. BMC Med Inform Decis Mak. 2020;20(1):114.
117. Kaplan RM, Crespi CM, Dahan E, et al. Comparison of rating scale, time tradeoff, and conjoint analysis methods for assessment of preferences in prostate cancer. Med Decis Making. 2019;39(7):816–826. doi:10.1177/0272989X19873667
118. Meara A, Crossnohere NL, Bridges JFP. Methods for measuring patient preferences: an update and future directions. Curr Opin Rheumatol. 2019;31(2):125–131. doi:10.1097/BOR.0000000000000587
119. Smolen JS, Landewe R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76(6):960–977. doi:10.1136/annrheumdis-2016-210715
120. Johnson FR, Zhou M. Patient preferences in regulatory benefit-risk assessments: a US perspective. Value Health. 2016;19(6):741–745. doi:10.1016/j.jval.2016.04.008
121. Reen GK, Silber E, Langdon DW. Multiple sclerosis patients’ understanding and preferences for risks and benefits of disease-modifying drugs: a systematic review. J Neurol Sci. 2017;375:107–122. doi:10.1016/j.jns.2016.12.038
122. Kaehler KC, Blome C, Forschner A, et al. Preferences of German melanoma patients for interferon (IFN) α-2b toxicities (the DeCOG “GERMELATOX survey”) versus melanoma recurrence to quantify patients’ relative values for adjuvant therapy. Medicine. 2016;95(46):e5375. doi:10.1097/MD.0000000000005375
123. Kuchuk I, Bouganim N, Beusterien K, et al. Preference weights for chemotherapy side effects from the perspective of women with breast cancer. Breast Cancer Res Treat. 2013;142(1):101–107. doi:10.1007/s10549-013-2727-3
124. Lee JY, Kim K, Lee YS, et al. Treatment preferences of advanced ovarian cancer patients for adding bevacizumab to first-line therapy. Gynecol Oncol. 2016;143(3):622–627. doi:10.1016/j.ygyno.2016.10.021
125. Lloyd A, McIntosh E, Price M. The importance of drug adverse effects compared with seizure control for people with epilepsy: a discrete choice experiment. PharmacoEconomics. 2005;23(11):1167–1181. doi:10.2165/00019053-200523110-00008
126. Merlino LA, Bagchi I, Taylor TN, et al. Preference for fractures and other glucocorticoid-associated adverse effects among rheumatoid arthritis patients. Med Decis Making. 2001;21(2):122–132. doi:10.1177/0272989X0102100205
127. Osilla KC, Wagner G, Garnett J, et al. Patient and provider characteristics associated with the decision of HIV coinfected patients to start hepatitis C treatment. AIDS Patient Care STDS. 2011;25(9):533–538. doi:10.1089/apc.2011.0048
128. Poulos C, Kinter E, Yang JC, et al. Patient preferences for injectable treatments for multiple sclerosis in the United States: a discrete-choice experiment. Patient. 2016;9(2):171–180. doi:10.1007/s40271-015-0136-x
129. Utz KS, Hoog J, Wentrup A, et al. Patient preferences for disease-modifying drugs in multiple sclerosis therapy: a choice-based conjoint analysis. Ther Adv Neurol Disord. 2014;7(6):263–275. doi:10.1177/1756285614555335
130. Pacou M, Basso F, Gore C, et al. Patient and physician preferences for the treatment of chronic hepatitis C virus infections: does the perspective matter? Eur J Gastroenterol Hepatol. 2015;27(9):1063–1068. doi:10.1097/MEG.0000000000000410
131. Chancellor J, Martin M, Liedgens H, et al. Stated preferences of physicians and chronic pain sufferers in the use of classic strong opioids. Value Health. 2012;15(1):106–117. doi:10.1016/j.jval.2011.07.002
132. daCosta DiBonaventura M, Copher R, Basurto E, et al. Patient preferences and treatment adherence among women diagnosed with metastatic breast cancer. Am Health Drug Benefits. 2014;7(7):386–396.
133. Das AK, Malik A, Haddad PM. A qualitative study of the attitudes of patients in an early intervention service towards antipsychotic long-acting injections. Ther Adv Psychopharmacol. 2014;4(5):179–185. doi:10.1177/2045125314542098
134. Fraenkel L, Nowell WB, Michel G, et al. Preference phenotypes to facilitate shared decision-making in rheumatoid arthritis. Ann Rheum Dis. 2018;77(5):678–683. doi:10.1136/annrheumdis-2017-212407
135. Husni ME, Betts KA, Griffith J, et al. Benefit-risk trade-offs for treatment decisions in moderate-to-severe rheumatoid arthritis: focus on the patient perspective. Rheumatol Int. 2017;37(9):1423–1434. doi:10.1007/s00296-017-3760-z
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
