Back to Journals » OncoTargets and Therapy » Volume 8
Dynamic monitoring of plasma amino acids and carnitine during chemotherapy of patients with alimentary canal malignancies and its clinical value
Authors Wang X, Wang J, Wang Z, Wang Q, Li H
Received 14 April 2015
Accepted for publication 8 June 2015
Published 7 August 2015 Volume 2015:8 Pages 1989—1996
DOI https://doi.org/10.2147/OTT.S86562
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Daniele Santini
Video abstract presented by Xiaoyu Wang
Views: 232
Xiaoyu Wang,1 Jiaqi Wang,2 Zhenghua Wang,1 Qingjun Wang,1 Hua Li1
1Second Ward of Oncology Department, 2Traditional Chinese Medicine Department, The First Affiliated Hospital of Liaoning Medical University, Jinzhou, People’s Republic of China
Objective: The aim of this study was to observe the plasma amino acid and carnitine characteristics in patients with metastatic gastrointestinal malignancies during chemotherapy and to identify markers for the early diagnosis and evaluation of adverse reactions and prognosis of the digestive tract malignant tumor patients.
Methods: Blood samples of 30 patients with metastatic gastrointestinal malignancies were collected at four time points: before chemotherapy, the first day after chemotherapy (+1 day), bone marrow depression period (+14 days), and hematopoietic recovery period (+21 days). The plasma amino acids and carnitine from those 30 patients were determined by high-performance liquid chromatography–tandem mass spectrometry method. Simultaneously, the levels of 21 amino acids were detected in 30 healthy individuals, who were considered as control. Biochemical indexes were also detected at four time points, adverse reactions were recorded during the chemotherapy process, and patients were followed up for 1 year to observe time to progression (TTP) and progression-free survival (PFS).
Results: Compared to healthy people in the control group, patients with malignancies showed significantly increased levels of plasma amino acids such as Arg, Asp, Cit, Gly, Orn, Tyr, Val, and carnitine (such as C2). The levels of compounds such as C3, Asn, Leu, Lys, Pip, Pro, C0, C5:1 decreased significantly before chemotherapy. The levels of Cit, Cys, Lys, Pro, Tyr, Val, C0, and C2 decreased significantly on the second day of chemotherapy (+1 day), whereas the level of C3 increased significantly. During myelosuppression (+14 days), the levels of Asp, Cit, Met, and Orn were observed to still decrease significantly, whereas the level of Val appeared to increase significantly. The levels of Asp, Glu, and Met were clearly different among patients with gastric carcinoma, rectal cancer, and colon cancer. Compared to the control group, aspartate amino transferase and alanine aminotransferase were found to be higher in eight patients with hypocarnitinemia, yet TTP, PFS, and RR (response rate) were lower. No significant difference was observed for adverse reactions. The indexes in 12 patients with citrullinemia showed no difference compared with control group. All the results showed statistically significant differences (P<0.05).
Conclusion: Real-time monitoring of plasma amino acids and carnitine in patients with metastatic gastrointestinal malignancies can directly reflect the body’s metabolism and nutritional status. The results provide a reference for nutrition therapy or support for patients with alimentary canal malignancies. Hypocarnitinemia is a risk factor for gastrointestinal cancer patients and affects TTP, PFS, and RR by liver function. This study shows that tandem mass spectrometry can be used to detect blood amino acids and carnitine spectrum may be used for an early diagnosis and evaluation of adverse reactions and prognosis of the digestive tract malignant tumor patients.
Keywords: plasma amino acid, carnitine and acylcarnitines, gastrointestinal cancer, high-performance liquid chromatography–tandem mass spectrometry, chemotherapy
Introduction
Plasma amino acids and carnitine levels have a close relationship with protein metabolism and body nutrition, which can reflect the level of protein metabolism. They also act as indicators to evaluate the nutritional status. Plasma amino acid and carnitine concentrations have to be maintained at a certain level under normal conditions. Any fluctuation in the normal level indicates diseased states or some special physiological condition. This study used liquid chromatography–tandem mass spectrometry (LC–MS/MS) and isotope labeling methods to determine the levels of carnitine and plasma amino acids in malignant tumor patients undergoing chemotherapy and in healthy people (control). The study was aimed at observing the change in the levels of plasma amino acids and carnitine in advanced malignant tumor patients. This study also provides a scientific basis for clinical nutrition monitoring and reasonable nutrition support for patients with malignant tumor. The study also aims to observe the influence of hypocarnitinemia and citrullinemia on chemotherapy and provide a scientific method to improve the efficiency of chemotherapy in diseased people.
Materials and methods
Materials
Thirty gastrointestinal cancer patients (18 men and 12 women) undergoing chemotherapy in our hospital were selected. The study was approved by the Institutional Review Board (CWO) of The First Affiliated Hospital of Liaoning Medical University. All patients provided written informed consent for the use of their data. Among the patients included, seven were suffering with gastric carcinoma, ten with rectal cancer, and 13 with colon cancer. The median age was 55.27 years. All the patients had stage IV gastrointestinal malignant tumors before chemotherapy, which was confirmed by pathology and imaging.1 They were given XELOX chemotherapy. Control group included 30 healthy people (15 men and 15 women) who came to our hospital for physical examination, and the median age was 53.91 years. The median weight and height of the patients were 51 kg and 158 cm, respectively. The median weight and height of the healthy people (control group) were 60 kg and 160 cm, respectively. There were no differences between the two groups regarding age, weight, and height (P>0.05).
Methods
Chemotherapy methods
Chemotherapy regimen used for treatment was as follows: XELOX scheme chemotherapy (L-OHP [oxaliplatin] 130 mg/m2·dL + Xeloda [capecitabine] 1,800 mg/m2 po (orally), days 1–14, q21d [every 21 days]).2
Specimens
Patient’s specimens were collected at four time points: before chemotherapy, 1 day after chemotherapy, 14 days after chemotherapy (myelosuppression period), and 21 days after chemotherapy (post-chemotherapy recovery period). Four milliliters of fasting peripheral blood of each patient was collected, anticoagulated by heparin, and separated from plasma within 30 minutes by centrifugation (10,000 rpm for 10 minutes). After centrifugation, the plasma was transferred to clean epoxy resin (EP) tubes, refrigerated at 20°C, and tested in batches. Plasma amino acids and carnitine were analyzed at the four time points. Blood biochemical indexes including serum hemoglobin (Hb), serum albumin (ALB), aspartate transaminase (AST), and alanine transaminase (ALT) were also detected (Table 1).
Specimen processing
About 10 μL of sample was taken in 1.5 ml EP tube and 100 μL of daily working liquid was added. Then, it was centrifuged at 13,200 rpm for 2 minutes. Centrifugal supernatant was directly transferred to another 1.5 mL EP tube, which was dried at 50°C under nitrogen protection. After adding 60 μL of derivatization reagent, the tube was sealed and vibrated on a vortex mixer for 30 seconds. Then, the solution was immediately centrifuged and incubated at 65°C for 15 minutes, for derivatization to occur. The derived solution was centrifuged again and dried at 50°C under nitrogen protection. Then, it was centrifuged again and incubated at 65°C for 15 minutes, for derivatization to occur. The derived solution was centrifuged again, and was dried at 50°C under nitrogen protection. Then, 100 μL of reconstitution fluid was added, vibrated on a vortex mixer for 30 seconds, and centrifuged at 13,200 rpm for 2 minutes. About 5 μL of the sample was analyzed by LC–MS/MS.
Experimental apparatus
Experimental apparatus consisted of 3200QTRAP liquid chromatography–tandem mass spectrometer equipped with electrospray ionization source and Analyst 1.5 data processing software (Applied Biosystem Company, Foster City, CA, USA), UltiMate3000 standard liquid chromatography system, including double ternary gradient pump, automatic sampler, column temperature box, switch valve(America Diane Company), and nitrogen enrichment apparatus(Tokyo Physical and Chemical Equipment Co., Tokyo, Japan).
Chromatographic conditions
Chromataographic conditions were as follows: TC: C18 column, 150 mm ×4.6 mm ID (Agilent Technologies, Santa Clara, CA, USA); mobile phase: acetonitrile–water, gradient elution; column temperature: 50°C; sample quantity: 5 μL.
The adverse reactions that occurred during chemotherapy were recorded. RR was assessed by imaging examination. One-year follow-up was done for time to progression (TTP) and progression-free survival (PFS).
Statistical analysis
The data were analyzed using SPSS 19.0 statistical software. Non-normal distribution measurement data used “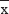 ± S
± S 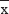 ” and showed that according to the three digestive tract malignant tumors, multiple sets were compared by the independent sample Kruskal–Wallis H test (P>0.05 indicated no statistically significant difference). Normal distribution measurement data used ‘
” and showed that according to the three digestive tract malignant tumors, multiple sets were compared by the independent sample Kruskal–Wallis H test (P>0.05 indicated no statistically significant difference). Normal distribution measurement data used ‘ 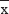 ± S’ and showed that, according to the chemotherapy time points, multiple sets were compared and analyzed by variance analysis and Bonferroni test. T-test was used to analyze AST, ALT, TTP, PFS, hypocarnitinemia, and citrullinemia among tumor patients and control group. Chi-square analysis was used to analyze RR and adverse reactions of chemotherapy. P<0.05 indicated statistically significant difference.
± S’ and showed that, according to the chemotherapy time points, multiple sets were compared and analyzed by variance analysis and Bonferroni test. T-test was used to analyze AST, ALT, TTP, PFS, hypocarnitinemia, and citrullinemia among tumor patients and control group. Chi-square analysis was used to analyze RR and adverse reactions of chemotherapy. P<0.05 indicated statistically significant difference.
Ethics statement
The study was approved by the institutional review board (CWO) of The First Affiliated Hospital of Liaoning Medical University. All patients provided written informed consent for the use of their data.
Results
Biochemical changes in each period of intensive chemotherapy in patients with alimentary canal malignancies
This study showed that HB and ALB levels of the digestive tract malignant tumor patients dropped significantly compared to control group during chemotherapy (P<0.05) (Table 1). HB and ALB reached a minimum level 14 days after chemotherapy compared to pre-chemotherapy (P<0.05). The values of HB and ALB at 21 days after chemotherapy (hematopoietic recovery) were close to those present before chemotherapy (P<0.05). AST and ALT levels had significantly increased during chemotherapy compared with control group (P<0.05). AST and ALT levels were the highest at 14 days after chemotherapy compared with pre-chemotherapy values (P<0.05). AST and ALT levels at 21 days after chemotherapy (hematopoietic recovery) dropped down and were close to those at pre-chemotherapy (P<0.05).
Plasma amino acids before chemotherapy in patients with metastatic alimentary canal malignancies
This study showed that the plasma amino acids and carnitine spectrum of gastric cancer, rectal cancer, and colon cancer before chemotherapy were clearly different, including asparaginate (Asn), leucine (Leu), piperamide (Pip), and free carnitine (C0) (P<0.05). The other amino acids and carnitine spectrum showed no significant difference (P>0.05) (Table 2).
Plasma amino acids in each period of intensive chemotherapy in patients with alimentary canal malignancies
A significant increase in the levels of a few plasma amino acids and carnitine, which included arginine (Arg), asparagine (Asp), citrulline (Cit), glycine (Gly), ornithine (Orn), tyrosine (Tyr), valine (Val), and acetyl-l-carnitine (C2), was observed in the digestive tract malignant tumor patients before chemotherapy compared to control group (P<0.05). But the levels of some plasma amino acids and carnitine, which included propionyl-carnitine (C3), Asn, Leu, lysine (Lys), Pip, proline (Pro), C0, and pentene acyl carnitine (C5:1), decreased significantly (P<0.05). The levels of the remaining amino acids and carnitine showed no statistical significance compared to the control group.
The levels of some plasma amino acids and carnitine, including Cit, cysteine (Cys), Lys, Pro, Tyr, Val, C0, and C2, of the digestive tract malignant tumor patients 1 day after chemotherapy were shown to decrease significantly, but the C3 level showed a significant rise (P< 0.05) compared to healthy control group. The levels of some plasma amino acids, such as Asp, Cit, methionine (Met), and Orn, and carnitine of the digestive tract malignant tumor patients decreased significantly (P<0.05) 14 days after chemotherapy, whereas the level of Val presented an increasing trend (P< 0.05) (Table 3).
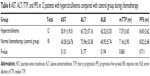
AST, ALT, TTP, PFS, RR, and adverse reactions in eight patients with hypocarnitinemia compared with control group during chemotherapy
This study showed that AST and ALT levels of eight patients with hypocarnitinemia were significantly higher than those of the control group (P<0.05), while the mTTP (the median TTP), PFS, and RR were lower than those of the control group (P<0.05). The occurrence of adverse reactions such as diarrhea, nausea, vomiting, and myelosuppression showed no significant difference in the control group (P>0.05) (Tables 4 and 5).
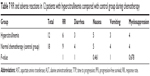
AST, ALT, TTP, PFS, RR, and adverse reactions in 12 patients with hypercitrullinemia compared with control group during chemotherapy
No obvious difference was found in the levels of AST, ALT, TTP, PFS, and RR in 12 patients with hypercitrullinemia compared to the control group. The occurrence of adverse reactions such as diarrhea, nausea, vomiting, and myelosuppression also showed no significant difference (P>0.05) (Tables 6 and 7).
Discussion
At present, there are few reports on the dynamic monitoring of the change and relationships in serum albumin, plasma amino acids, and carnitines of the gastrointestinal malignancies during chemotherapy. This research used the advanced LC–MS/MS to detect the samples. The analysis process of the LC–MS/MS included ionizing molecules to be detected, separating ions according to mass-to-charge ratio (m/z), and detecting various ions with a set of scan modes. LC-MS/MS could be used to detect dozens of metabolites present in the body both qualitatively and quantitatively.3,4 The metabolic state of the body and its variation, and amino acids, organic acids, fatty acids, free carnitine, and acyl carnitine metabolism could also be evaluated by this method.5 This study used the advanced technology that is generally used to screen genetic metabolic diseases in newborns, to analyze amino acid and carnitine metabolism changes and prognosis of tumor patients.
This study evaluated 30 patients with stage IV gastrointestinal malignant tumors before chemotherapy, who were not able to undergo operation. Significant differences in the levels of Asn, Leu, Pip, and C0 were observed in three patients with digestive tract malignant tumor before chemotherapy (P<0.05). Among the gastric cancer patients, Asn was observed to be the highest, Leu and Pip the highest among colorectal cancer patients, and C0 the highest among the colon cancer patients. The study showed that all the three types of digestive tract malignant tumors had different impact on the nutritional status of patients, and hence Leu, Pip, and C0 are considered as significant markers for the identification of digestive tract malignant tumor.
This study showed that patients’ serum albumin level before chemotherapy decreased significantly compared to normal level, fell to the lowest level at 14 days after chemotherapy, and recovered close to normal level at 21 days after chemotherapy; a similar change was also observed for plasma amino acids. In the case of malnutrition, low concentration of albumin level was not due to the descending liver ability to synthesize proteins, but because of the lack of nutrients required for amino acid synthesis in the body.6,7 Serum albumin level often acts as an index to evaluate malnutrition in clinical settings, but the change period is longer than that of plasma amino acids. Hence, monitoring plasma amino acid levels indicates patients’ nutritional status and the effect of nutritional support, in time.
Carnitine is a quaternary ammonium compound biosynthesized from amino acids. It contains a left-handed and a right-handed form, but only l-carnitine shows physiological activities.5,8 Carnitine in the human body is found in two forms: free carnitine and fatty acyl carnitine. Carnitine plays an important role in the oxidation pathway of long chain fatty acids and their transport through mitochondrial membrane.9,10 Excess acyl formed due to oxidation and carnitine can result in the formation of acyl carnitine.11 Free acyl can inhibit the cell enzyme activity, which can cause metabolic acidosis. So carnitine is an indispensable compound to energy metabolism.12,13
At present no clear reports are available that emphasize the relationship of amino acid and carnitine spectral changes with the digestive tract malignant tumors. This study shows that the levels of both free and total carnitine of the digestive tract malignant tumor patients are lower than the control group. Levels of free carnitine fall further after chemotherapy, and this indirectly affects the function of liver. This might be associated with the inhibition of mitochondrial oxidation caused by tumor cells. The abnormality in cell energy results in cell damage, whereas energy metabolism of normal cells is enhanced so that carnitines are further consumed. As a result, the carnitine levels of tumor patients are lower than the normal individuals. Levels of free carnitine fall further during chemotherapy, which may be related to the limited reserve of carnitine. Much of carnitine is lost during chemotherapy due to diarrhea, nausea, vomiting, and loss of appetite, which results in hypocarnitinemia.
This study also found that TTP, PFS, and RR of patients with hypocarnitinemia were lower compared to normal chemotherapy patients, which illustrated that patients with hypocarnitinemia were not sensitive to chemotherapy and had low treatment efficacy and poor prognosis. C2 of patients with hypocarnitinemia was higher than normal, which was because long-chain acyl carnitine was mainly oxidized in mitochondria,14 and the oxidation process could be affected by tumors. Because of a lack of carnitine acyl transferase, ester acyl exchange disorder occurred for the accumulation of acyl coenzyme A. A series of adverse complications, including liver damage, was also observed. So hypocarnitinemia is a poor prognosis factor of digestive tract malignant tumor. People can improve treatment efficacy and quality of life by taking carnitine.
This study found that the citrulline level of the digestive tract malignant tumor patients was higher compared to control group. Citrulline plays an important role in the urea cycle. Citrulline is produced from blood ammonia by the action of enzymes, and then is transported out of the mitochondria. Citrulline combines with aspartic acid to form pure ammonia acyl succinic acid in the presence of ammonia acyl succinyl synthetase.15 Presence of high citrulline level indicates urea cycle disorder and accumulation of large amounts of blood ammonia in the body, which may cause damage to the liver.16 No significant difference was noted in factors such as AST, ALT, TTP, PFS, RR, diarrhea, nausea, vomiting, and myelosuppression due to hypercitrullinemia compared with normal chemotherapy group.
Glutamine was found to be significantly lower in tumor patients compared to control group during the whole chemotherapy period (including recovery period) (Table 3) and nearly fourfold lower than pre-chemotherapy period. Reasons for the significant decrease in glutamine include loss of appetite caused by capecitabine, inadequate intake or absorption of basic amino acids, and inadequate intake of total amino acids. Glutamine can improve the body’s metabolism, increase the total number of lymphocytes, and improve the body’s immune status and oxidation resistance.17 Lack of glutamine results in decreased immune function after chemotherapy, increased intestinal mucosal permeability, secondary bacterial translocation, and systemic infection.18 This might be the cause of aggravating diarrhea after oral capecitabine. Hence patients are advised to take sufficient glutamine supplements before chemotherapy so as to maintain their nutritional status and improve their immune levels during the disease period.
In summary, as two of the nutritional assessment index, amino acid spectrum and carnitine spectrum can indicate the patients’ nutritional status much earlier and help to provide required nutritional support. Analysis of these spectrums showed abnormal levels of free carnitine, citrulline, glutamine, and essential amino acids in tumor patients compared to control group. The abnormality not only leads to systemic metabolic disorder but also affects all of the plasma amino acids, trace nutrients, and vitamins. Monitoring of amino acids and carnitine spectrum can provide some references and guidance for chemotherapy regimens and nutrition therapy. Patients with malignant tumors should be encouraged to take enteral nutrition, vitamins, and trace elements much earlier to overcome the metabolic disorder. Moreover, it reduces the nutritional risk caused by digestive tract infection, mucosal inflammation, fever, diarrhea and other complications during the chemotherapy.
This study also showed that tandem mass spectrometry can be used to detect blood amino acids and carnitine spectrum may be used for an early diagnosis and in the evaluation of adverse reactions and prognosis of the digestive tract malignant tumor patients. Long-term follow-up of all the tumor patients was not possible in this study due to limited time and funds. More clinical research is necessary to study the significance of blood amino acids and carnitine spectrum in detecting digestive tract malignant tumors in the future.
Disclosure
The authors certify that all affiliations with or financial involvement in, within the past 5 years and in the near future, any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript are completely disclosed (eg, employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received, or pending royalties). The authors report no conflicts of interest in this work.
References
Brusselaers N, Vall A, Mattsson F, et al. Tumour staging of oesophageal cancer in the Swedish Cancer Registry: a nationwide validation study. Acta Oncol. 2015;54(6):903–908. | ||
Schmoll HJ, Twelves C, Sun W, et al. Effect of adjuvant capecitabine or fluorouracil, with or without oxaliplatin, on survival outcomes in stage III colon cancer and the effect of oxaliplatin on post-relapse survival: a pooled analysis of individual patient data from four randomised controlled trials. Lancet Oncol. 2014;15(13):1481–1492. | ||
Arbeláez P, Borrull F, Pocurull E, et al. Determination of high-intensity sweeteners in river water and wastewater by solid-phase extraction and liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2015;1393:106–114. | ||
Gonzalez de Vega C, Alberts D, Chawla V, et al. Use of radiofrequency power to enable glow discharge optical emission spectroscopy ultrafast elemental mapping of combinatorial libraries with nonconductive components: nitrogen-based materials. Anal Bioanal Chem. 2014; 406(29):7533–7538. | ||
Morand R, Donzelli M, Haschke M, et al. Quantification of plasma carnitine and acylcarnitines by high-performance liquid chromatography-tandem mass spectrometry using online solid-phase extraction. Anal Bioanal Chem. 2013;405(27):8829–8836. | ||
Cascales-Miñana B, Muñoz-Bertomeu J, Flores-Tornero M, et al. The phosphorylated pathway of serine biosynthesis is essential both for male gametophyte and embryo development and for root growth in Arabidopsis. Plant Cell. 2013;25(6):2084–2101. | ||
Ishikawa T, Kubota T, Horigome R, et al. Branched-chain amino acids to tyrosine ratio (BTR) predicts intrahepatic distant recurrence and survival for early hepatocellular carcinoma. Hepatogastroenterology. 2013;60(128):2055–2059. | ||
Abidin Z, Khan MZ, Khatoon A, et al. Ameliorative effects of L-carnitine and vitamin E (α-tocopherol) on haematological and serum biochemical parameters in White Leghorn cockerels given ochratoxin: a contaminated feed. Br Poult Sci. 2013;54(4):471–477. | ||
Giangregorio N, Console L, Tonazzi A, et al. Identification of amino acid residues underlying the antiport mechanism of the mitochondrial carnitine/acylcarnitine carrier by site-directed mutagenesis and chemical labeling. Biochemistry. 2014;53(44):6924–6933. | ||
Wohlfarth A, Gandhi AS, Pang S, et al. Metabolism of synthetic cannabinoids PB-22 and its 5-fluoro analog, 5F-PB-22, by human hepatocyte incubation and high-resolution mass spectrometry. Anal Bioanal Chem. 2014;406(6):1763–1780. | ||
Pormsila W, Morand R, Krähenbühl S, et al. Capillary electrophoresis with contactless conductivity detection for the determination of carnitine and acylcarnitines in clinical samples. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879(13–14):921–926. | ||
Van Hove JL, Saenz MS, Thomas JA, et al. Succinyl-CoA ligase deficiency: a mitochondrial hepatoencephalomyopathy. Pediatr Res. 2010;68(2):159–164. | ||
Gucciardi A1, Pirillo P, Di Gangi IM, et al. A rapid UPLC-MS/MS method for simultaneous separation of 48 acylcarnitines in dried blood spots and plasma useful as a second-tier test for expanded newborn screening. Anal Bioanal Chem. 2012;404(3):741–751. | ||
Ortori CA1, Dubern JF, Chhabra SR, et al. Simultaneous quantitative profiling of N-acyl-L-homoserine lactone and 2-alkyl-4(1H)-quinolone families of quorum-sensing signaling molecules using LC-MS/MS. Anal Bioanal Chem. 2011;399(2):839–850. | ||
Kleijer WJ, Garritsen VH, van der Sterre ML, et al. Prenatal diagnosis of citrullinemia and argininosuccinic aciduria: evidence for a transmission ratio distortion in citrullinemia. Prenat Diagn. 2006;26(3):242–247. | ||
Saudubray JM, Touati G, Delonlay P, et al. Liver transplantation in urea cycle disorders. Eur J Pediatr. 1999;158(Suppl 2):S55–S59. | ||
Hu K, Zhang JX, Feng L, et al. Effect of dietary glutamine on growth performance, non-specific immunity, expression of cytokine genes, phosphorylation of target of rapamycin (TOR), and anti-oxidative system in spleen and head kidney of Jian carp (Cyprinus carpio var. Jian). Fish Physiol Biochem. 2015;41(3):635–649. | ||
Pujos-Guillot E, Pickering G, Lyan B, et al. Therapeutic paracetamol treatment in older persons induces dietary and metabolic modifications related to sulfur amino acids. Age (Dordr). 2012;34(1):181–193. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




 ± S)
± S)