Back to Journals » International Journal of General Medicine » Volume 13
Dynamic Changes of Blood Lipids in Breast Cancer Patients After (Neo)adjuvant Chemotherapy: A Retrospective Observational Study
Authors Xu L, Dong Q, Long Y, Tang X, Zhang N, Lu K
Received 20 July 2020
Accepted for publication 11 September 2020
Published 14 October 2020 Volume 2020:13 Pages 817—823
DOI https://doi.org/10.2147/IJGM.S273056
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Liuyue Xu,1 Qian Dong,2 Yaoying Long,1 Xiaoqiong Tang,1 Nan Zhang,2 Kai Lu2
1Department of Hematology, The First Affiliated Hospital of Chongqing Medical University, Chongqing 400016, People’s Republic of China; 2Department of Cardiology, The First Affiliated Hospital of Chongqing Medical University, Chongqing 400016, People’s Republic of China
Correspondence: Kai Lu
Department of Cardiology, The First Affiliated Hospital of Chongqing Medical University, No. 1, Yixueyuan Road, Chongqing 400016, People’s Republic of China
Tel/Fax +86 23 89011562
Email [email protected]; [email protected]
Background: Previous studies indicated that the (neo)adjuvant chemotherapy for breast cancer can cause significant dyslipidemia in patients, but how long this abnormality can persist is unclear so far. The purpose of this study is to investigate whether (neo)adjuvant chemotherapy has a long-term effect on blood lipids in breast cancer patients.
Methods: A total of 159 newly diagnosed female breast cancer patients receiving the (neo)adjuvant chemotherapy subsequently and 159 female healthy controls were enrolled into the observational study. All participants’ blood lipid profiles which included TC, TG, HDL-C, and LDL-C before and at the end of the 1st and 12th month after chemotherapy were retrieved from the electronic medical record system. The blood lipid profiles and the percentage of dyslipidemia before and after chemotherapy in breast cancer patients and controls were compared.
Results: Compared with the baseline before chemotherapy, TC, LDL-C, and TG increased significantly at the end of the 1st month after chemotherapy, but only the abnormal increase in TG (2.98± 0.71 mmol/L vs 2.82± 0.63 mmol/L, P< 0.05) and LDL-C (1.82± 0.42 mmol/L vs 1.59± 0.42 mmol/L, P< 0.05) continued until the 12th month after chemotherapy. Levels of HDL-C in breast cancer patients and all the blood lipid parameters in controls remained stable during the observation period. The percentage of dyslipidemia in breast cancer patients rose from 41.5% at baseline to 54.1% at the 12th month after chemotherapy. Subgroup analysis demonstrated that the increase in dyslipidemia percentage was more pronounced in patients with low body mass index and aged over 50 years.
Conclusion: The (neo)adjuvant chemotherapy used for treating breast cancers can cause significant abnormalities in blood lipid profiles, and the abnormal increase in LDL-C and TG can last at least 12 months after chemotherapy, which indicates long-term management of blood lipid is necessary for those patients.
Keywords: breast cancer, dyslipidemia, adjuvant chemotherapy
Introduction
Breast cancer has become the most frequently diagnosed malignant tumor and the second leading cause of cancer-related death among women worldwide.1–3 Because of the huge advances in breast cancer treatment including the (neo)adjuvant chemotherapy over the past few decades, the breast cancer survival rate has improved significantly.3,4 However, due to the toxic and side effects of (neo)adjuvant chemotherapy drugs on cardiovascular system, and that many risk factors are shared by breast cancer and cardiovascular disease, cardiovascular disease has become the leading cause of death for breast cancer survivors.5,6 Well management of cardiovascular risk factors for a long time may be an important measure to improve the prognosis of breast cancer survivors. Extensive literature have reported that those commonly used drugs for (neo)adjuvant chemotherapy, such as anthracycline and taxane, can cause significant dyslipidemia in breast cancer patients after chemotherapy,7–9 which is the most well known risk factor for cardiovascular disease. At the same time, there is growing evidence showing that dyslipidemia is unfavorable for the prognosis of breast cancer.10,11 However, most of those studies only focus on change in blood lipid profiles before and shortly after chemotherapy and few researchers have been able to draw on any structured research into long-term evaluation of blood lipid levels after chemotherapy. Based on the above, the present study intended to observe the change of blood lipid profiles in patients with breast cancer within 12 months after completing all cycles of chemotherapy, so as to add new evidence to the importance of strengthening long-term lipid management after (neo)adjuvant chemotherapy in breast cancer patients.
Methods
Study Population
This was a single-centered observational study conducted from March 2016 to August 2018 in the First Affiliated Hospital of Chongqing Medical University (CQMU). Adult females who were histologically diagnosed as breast cancer in this period were invited to participate in the current study continuously and excluded from the following situations: the presence of other cancers; a known family history of dyslipidemia or having received drugs affecting lipid levels or combined with diseases that affect blood lipid metabolism or need blood lipid management, such as atherosclerotic cardiovascular and cerebrovascular diseases, diabetes, chronic liver disease, etc.; receiving combined endocrine therapy. In total, 159 women who met all the above criteria were enrolled. A control group was made up of 159 adult female volunteers of a similar age and no major diseases history who had not received any chemotherapy. In the current study, the (neo)adjuvant chemotherapy includes both adjuvant chemotherapy that is postoperative chemotherapy and neoadjuvant chemotherapy that is preoperative chemotherapy.
The contents and purposes of this study were thoroughly explained to the participants prior to the study, and written consent was obtained from all of them. The study protocols were made according to the Declaration of Helsinki and received ethical approval from the First Affiliated Hospital of CQMU.
Data Collection and Biochemical Variables Determination
All clinical data were collected from the electronic medical records by two independent investigators. Height and weight were measured to the nearest 0.1 cm and 0.1 kg, respectively, when participants stood upright and barefoot in light clothes. Body mass index (BMI) was calculated as body weight (kg) divided by the squared height (m2). Total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) levels were detected using fasting blood before and at the end of the 1st and 12th month after chemotherapy. Enzymatic methods were used to measure TC and TG levels, while homogeneous enzyme colorimetry methods were used to measure HDL-C and LDL-C levels. All the biochemical variables were tested in the central laboratory of the First Affiliated Hospital of CQMU on automatic biochemical analyzers (Roche Cobas C701, Basel, Switzerland). Dyslipidemia was defined as fasting serum TC>5.2 mmol/L or TG>1.7 mmol/L or LDL-C>3.1 mmol/L or HDL-C<1.0 mmol/L.12,13 The chemotherapy regiments the patients received in the present study included TAC (docetaxel, doxorubicin, and cyclophosphamide, cycled every 3 weeks for six cycles), AC-T (doxorubicin and cyclophosphamide, cycled every 3 weeks for four cycles, followed by docetaxel, cycled every 3 weeks for four cycles) and CEF (5-fluorouracil, epirubicin and cyclophosphamide, cycled every 3 weeks for six cycles).
Data Analysis
All data analysis was performed using the SPSS (version 19.0, IBM, Armonk, NY, USA). Continuous variables were presented as mean±standard deviation (SD) and categorical variables as frequency (proportion). In the descriptive analysis, the basic characteristics of the breast cancer and normal controls were presented and compared. Continuous variables were compared with Student’s t-test or non-parametric tests based on distributional properties. Categorical variables were compared with χ2 test. All statistical tests were two-sided, and P-value<0.05 was regarded as significant.
Results
Basic Characteristics of the Breast Cancer Patients and Controls
As shown in Table 1, the average age of breast cancer patients and controls was 48.42±9.86 years and 48.71±9.10 years, respectively. The proportion of premenopausal women was slightly higher in controls than breast cancer patients (40.2% vs 38.4%, P>0.05). The rates of smoking and alcohol drinking among breast cancer patients are very low (5.7% and 13.2%), and there is no significant difference from the controls. In addition, breast cancer patients had a higher BMI compared with the controls (24.22±4.12 vs 22.19±3.27 kg/m2; P<0.05). Most of the patients were at stage I and II (77.3%), and no stage IV patients were enrolled. The most commonly used chemotherapy regimen is TAC (67.9%), followed by ACT (28.9%). And 40.9% of the breast cancer patients underwent breast-conserving surgery in the end. The prevalence of positive HER-2 and ER in enrolled breast cancer patients is 30.8% and 61.6%, respectively.
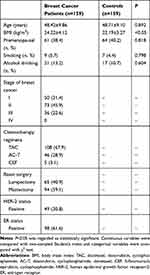 |
Table 1 Participants’ Demographics and Baseline Characteristics |
The Change of Blood Lipid Profiles Before and After the (Neo)adjuvant Chemotherapy
Table 2 shown the changes of blood lipids before and at the 1st month and 12th month after (neo)adjuvant chemotherapy. In the breast cancer patients, with the exception of HDL-C, the levels of TC, TG, and LDL-C were all significantly increased at the end of the 1st month after chemotherapy compared with baseline before chemotherapy (all P<0.05). Among them, the change in LDL-C levels was the most significant, with a 45.9% increase compared to baseline (1.59±0.42 vs 2.32±0.31, P<0.05). At the end of the12th month after chemotherapy, the levels of TC, TG, and LDL-C fell somewhat but were still above the baseline level. It should be noted that the TC level at the 12th month after chemotherapy was no longer statistically significant compared to baseline. HDL-C remained stable before and after chemotherapy. At the 12th month after chemotherapy, HDL-C was even slightly below baseline. In the control group, all the above-mentioned blood lipid indexes did not have any statistically significant change during the follow-up period.
 |
Table 2 The Change of Blood Lipid Profiles Before and After (Neo)adjuvant Chemotherapy in Breast Cancer Patients and Controls |
The Change of Dyslipidemia Percentage Before and After the (Neo)adjuvant Chemotherapy Stratified by Age, BMI, and Chemotherapy Regimens
As shown in Table 3, the total percentage of dyslipidemia increased by 23.3% from baseline of 41.5–64.8% at the end of the 1st month after chemotherapy, but fell back to 54.1% at the 12th month. Patients were grouped according to age, BMI, and chemotherapy regimen and the trends of change in the proportion of dyslipidemia in each subgroup was further analyzed.
 |
Table 3 The Change of Dyslipidemia Percentage Before and After (Neo)adjuvant Chemotherapy According to Age, BMI, and Chemotherapy Regimens |
The effect of chemotherapy on dyslipidemia seemed to be related to age. In patients aged between 20–39 years, the changes in dyslipidemia did not reach statistical significance. In patients aged between 40–49 years, the percentage of dyslipidemia increased by 21.8% at the 1st month after chemotherapy (35.9% vs 57.7%, P<0.05), but fell to 52.6% (vs baseline, P>0.05) at the 12th month. In patients aged over 50 years, the percentage of dyslipidemia increased to as high as 93.6% from 63.8% at baseline (vs baseline, P<0.05) and stayed at 83% (vs baseline, P>0.05) at the 12th month after chemotherapy.
The prevalence of dyslipidemia in patients stratified by BMI varied from 15.0% to 55.4%. Chemotherapy had a dramatic effect on the blood lipid levels of breast cancer patients with low-weight (BMI<18 kg/m2), with the dyslipidemia rate rising sharply from 15.0% before chemotherapy to 55.0% (P<0.05) at the 1st month after chemotherapy. The dyslipidemia rate was also as high as 40.0% at the 12th month after chemotherapy, which was almost three times the baseline level. Chemotherapy had similar effects on blood lipid levels in normal (18 kg/m2<BMI<24 kg/m2) and overweight (BMI>24 kg/m2) breast cancer patients. The rate of dyslipidemia increased by around 20.0% from baseline at the 1st month after chemotherapy in the two groups (38.6% vs 59.0%, P<0.05; 55.4% vs 76.8%, P<0.05), which was still about 10.0% above baseline at the 12th month but with no statistical significance (38.6% vs 49.4%, P>0.05; 55.4% vs 66.1%, P>0.05).
As to the effects of chemotherapy regimens on blood lipid levels, TAC’s seemed longer lasting than that of AC-T. In those receiving TAC, the dyslipidemia rate increased from 30.8% to 62.0% (P<0.05) at the 1st month after chemotherapy and fell to 52.8% (P<0.05) at the 12th month after the end of chemotherapy. The corresponding rate for those receiving AC-T was 54.3%, 78.3% (P<0.05), and 63.0% (P>0.05).
Discussion
Many previous studies have noted that the (neo)adjuvant chemotherapy can increase the prevalence of cardiovascular disease risk factors in breast cancer patients, and cardiovascular disease has surpassed breast cancer itself as the first cause of death for them.5 In recent years, many studies have focused on the effect of the (neo)adjuvant chemotherapy on blood lipid levels, and the immediate rise in cholesterol and triglyceride levels after chemotherapy in breast cancer patients has almost become an indisputable fact.14–16 But we need to note that most studies did not follow the change of blood lipids after chemotherapy for a long time. The long-term effect of chemotherapy on blood lipids determines whether long-term management of patients’ lipid metabolism is necessary, which has been proven to play a major role in reducing atherosclerotic cardiovascular disease by massive research. Our results showed that the effect of chemotherapy on blood lipids would gradually get weak over time, but the incidence of dyslipidemia in patients within 12 months after chemotherapy was still higher than baseline. If we consider the fact that breast cancer patients already have higher prevalence of cardiovascular risk factors, the result must be taken seriously.
We must also emphasize that whether the effect of chemotherapy on blood lipids is long-lasting is still controversial in the literature. Arpino et al14 observed the change of blood lipid levels in breast cancer patients for 2 years after adjuvant chemotherapy. In their study, the plasma level of LDL-C cholesterol increased by 5.4 mg/dL per year and that of triglycerides increased by 10.73 mg/dL per year, which was consistent with ours. In this study, the vast majority of patients used endocrine therapy alone or combined with chemotherapy, less than 20% of patients used chemotherapy alone, and the chemotherapy regimen used was not described in detail. In another study with a similar design, with the exception of TG levels, the effect of chemotherapy on other blood lipids largely disappeared within 6 months after chemotherapy in patients receiving anthracycline plus taxane chemotherapy.17 Our study showed the presence of dyslipidemia would last longer in patients treated with the TAC regimen compared to those with the AC-T regimen, which indicated patients exposed to anthracyclines for less time might have a lower chance for developing dyslipidemia after chemotherapy.
The results of the present study indicated that the impact of the (neo)adjuvant chemotherapy on lipid levels of breast cancers varied with age. This was consolidated by one retrospective study conducted in Chinese to determine the elements associated with lipid profiles after chemotherapy, which showed age of over 50 years was a strong and independent risk factor for the elevation of LDL-C and TC.7 But, beyond this, no more evidence was found to support our result after extensive literature searching. This study also found that obese/overweight could significantly contribute to the increase of LDL-C and TC after chemotherapy, which was inconsistent with ours that suggested chemotherapy had a greater effect on lipid profiles in patients with light body weight (BMI<18 kg/m2).7 However, considering the sample size of the low-weight population was small in the current study, we need to treat this result with caution.
In breast cancer patients, weight gain and dyslipidemia after chemotherapy have attracted much attention.14–20 and some certain potential mechanisms under this have been revealed. Studies have shown that doxorubicin, which was widely used for the (neo)adjuvant chemotherapy for breast cancer, could regulate a series of genes involved in lipoprotein metabolism in liver cells such as ATP binding cassette transporter A1 (ABCA1) and apoA1.8 Meanwhile, it was found that doxorubicin and paclitaxel could increase apoB protein levels and paclitaxel could decrease low density lipoprotein receptor (LDLR) protein level.8 Another study found that short-time infusion of paclitaxel could dysregulate the cholesterol pathway by gene ontology analysis in breast cancer patients.21,22 In addition, some previous studies in rats have shown that cyclophosphamide could depress cytochrome P450 activity, which in turn may cause reduction in the activity of fat-splitting enzymes, such as lecithin cholesterol acyl transferase (LCAT) and lipoprotein lipase (LPL).23,24 But until now, there have been no well-designed studies to see how long these changes can last, and whether these changes are parallel to those in blood lipid profiles. More research is needed to reveal the mechanism of dyslipidemia caused by chemotherapy.
There is growing evidence that long-term lipid management is essential for patients with breast cancer to improve their prognosis.25 Dyslipidemia, especially elevated LDL-C level, is the most important independent risk factor for atherosclerotic cardiovascular disease (ASCVD). Our study adds new evidence that dyslipidemia caused by chemotherapy does not diminish rapidly after chemotherapy. Persistent dyslipidemia, along with other risk factors which have high prevalence among breast cancer survivors, could significantly increase their risk for ASCVD. To make matters worse, it has been shown that even short-term chemotherapy could quickly cause the release of inflammatory factors in vascular walls and endothelial dysfunction, which in turn lead to lipid deposition and thickening of carotid intima, indicating that chemotherapy could significantly accelerate the formation of atherosclerosis.26,27 In addition, it was reported that hypercholesterolemia was associated with a decreased response of tumors to endocrine therapies. Cholesterol can be converted to 27-hydroxycholesterol which could increase estrogen receptor-dependent growth and liver X receptor-dependent metastasis in a mouse models of breast cancer.28 Thus, lowering circulating cholesterol levels may be a useful strategy to prevent significant cardiac morbidity and mortality and improve the long-term prognosis for breast cancer survivors.
Our study has several limitations. First of all, the current study is single-centered and the sample is relatively small, which will inevitably undermine the persuasiveness of the results. We are cautious to extend the results to other populations. Secondly, endocrine therapy such as tamoxifen, toremifene and aromatase inhibitors is essential for breast cancer patients,29–31 which can also lead to dyslipidemia. We did not include patients using these drugs due to the limited sample size, which makes it impossible to carefully distinguish their effect on blood lipids from that of (neo)adjuvant chemotherapy. Thirdly, it is well known that the blood lipid profiles can be affected by various elements such as diet pattern, physical exercise, comorbidities, and so on. We did not adjust for the effects of diet and physical exercise because of the unavailability of proper and easy tools. We ruled out those with comorbidities which can affect lipids metabolism such as diabetes and chronic liver diseases when enrolling participants to minimize the confounding effects of these factors.
Conclusions
The (neo)adjuvant chemotherapy used for the treatment of female breast cancers can cause significant abnormalities in blood lipid profiles. The abnormal increase in LDL-C and TC caused by chemotherapy can last at least 12 months. In addition, the (neo)adjuvant chemotherapy can also lead to an obvious increase of the percentage of dyslipidemia which is more profound in patients with older age and lower BMI. The above results suggest that long-term management of blood lipid profiles is necessary for breast cancer patients who have received (neo)adjuvant chemotherapy.
Funding
This study was funded by grants from the Cultivation Fund of the First Affiliated Hospital of Chongqing Medical University (Grant No. PYJJ2017-28; PYJJ2018-16) and the Hehuang Fund of Chinese Association of Integrative Medicine (Grant No. HMP2003001P).
Disclosure
All authors declared no conflicting interests for this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34.
2. DeSantis CE, Fedewa SA, Goding Sauer A, et al. Breast cancer statistics, 2015: convergence of incidence rates between black and white women. CA Cancer J Clin. 2016;66(1):31–42. doi:10.3322/caac.21320
3. Li T, Mello-Thoms C, Brennan PC. Descriptive epidemiology of breast cancer in China: incidence, mortality, survival and prevalence. Breast Cancer Res Treat. 2016;159(3):395–406. doi:10.1007/s10549-016-3947-0
4. Hendrick RE, Baker JA, Helvie MA. Breast cancer deaths averted over 3 decades. Cancer. 2019;125(9):1482–1488. doi:10.1002/cncr.31954
5. Patnaik JL, Byers T, DiGuiseppi C, et al. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res. 2011;13(3):R64. doi:10.1186/bcr2901
6. Abdel-Qadir H, et al. A Population-Based Study of Cardiovascular Mortality Following Early-Stage Breast Cancer. JAMA Cardiol. 2017;2(1):88–93.
7. Yeo W, Mo FKF, Pang E, et al. Profiles of lipids, blood pressure and weight changes among premenopausal Chinese breast cancer patients after adjuvant chemotherapy. BMC Womens Health. 2017;17(1):55. doi:10.1186/s12905-017-0409-8
8. Sharma M, Tuaine J, McLaren B, et al. Chemotherapy Agents Alter Plasma Lipids in Breast Cancer Patients and Show Differential Effects on Lipid Metabolism Genes in Liver Cells. PLoS One. 2016;11(1):e0148049. doi:10.1371/journal.pone.0148049
9. Rzymowska J. Effect of cytotoxic chemotherapy on serum lipid levels in breast cancer patients. Pathobiology. 1999;67(3):129–132. doi:10.1159/000028062
10. Guan X, Liu Z, Zhao Z, et al. Emerging roles of low-density lipoprotein in the development and treatment of breast cancer. Lipids Health Dis. 2019;18(1):137. doi:10.1186/s12944-019-1075-7
11. Fichtali K, Bititi A, Elghanmi A, et al. Serum Lipidomic Profiling in Breast Cancer to Identify Screening, Diagnostic, and Prognostic Biomarkers. Biores Open Access. 2020;9(1):1–6. doi:10.1089/biores.2018.0022
12. Jacobson TA, Ito MK, Maki KC, et al. National Lipid Association recommendations for patient-centered management of dyslipidemia: part 1 - executive summary. J Clin Lipidol. 2014;8(5):473–488. doi:10.1016/j.jacl.2014.07.007
13. Grundy SM, Cleeman JI, Merz CNB, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227–239. doi:10.1161/01.CIR.0000133317.49796.0E
14. Arpino G, De Angelis C, Buono G, et al. Metabolic and anthropometric changes in early breast cancer patients receiving adjuvant therapy. Breast Cancer Res Treat. 2015;154(1):127–132. doi:10.1007/s10549-015-3586-x
15. Dieli-Conwright CM, et al. An observational study to examine changes in metabolic syndrome components in patients with breast cancer receiving neoadjuvant or adjuvant chemotherapy. Cancer. 2016;122(17):2646–2653.
16. Li X, Liu Z-L, Wu Y-T, et al. Status of lipid and lipoprotein in female breast cancer patients at initial diagnosis and during chemotherapy. Lipids Health Dis. 2018;17(1):91. doi:10.1186/s12944-018-0745-1
17. Tian W, Yao Y, Fan G, et al. Changes in lipid profiles during and after (neo)adjuvant chemotherapy in women with early-stage breast cancer: A retrospective study. PLoS One. 2019;14(8):e0221866. doi:10.1371/journal.pone.0221866
18. Bjorge T, Lukanova A, Jonsson H, et al. Metabolic syndrome and breast cancer in the me-can (metabolic syndrome and cancer) project. Cancer Epidemiol Biomarkers Prev. 2010;19(7):1737–1745. doi:10.1158/1055-9965.EPI-10-0230
19. Gu K, Chen X, Zheng Y, et al. Weight change patterns among breast cancer survivors: results from the Shanghai breast cancer survival study. Cancer Causes Control. 2010;21(4):621–629. doi:10.1007/s10552-009-9491-z
20. Saquib N, et al. Weight gain and recovery of pre-cancer weight after breast cancer treatments: evidence from the women’s healthy eating and living (WHEL) study. Breast Cancer Res Treat. 2007;105(2):177–186.
21. Panis C, Binato R, Correa S, et al. Short infusion of paclitaxel imbalances plasmatic lipid metabolism and correlates with cardiac markers of acute damage in patients with breast cancer. Cancer Chemother Pharmacol. 2017;80(3):469–478. doi:10.1007/s00280-017-3384-8
22. Rajan VP, Menon KM. Involvement of microtubules in lipoprotein degradation and utilization for steroidogenesis in cultured rat luteal cells. Endocrinology. 1985;117(6):2408–2416. doi:10.1210/endo-117-6-2408
23. McClure MT, Stupans I. Investigation of the mechanism by which cyclophosphamide alters cytochrome P450 in male rats. Biochem Pharmacol. 1992;43(12):2655–2658. doi:10.1016/0006-2952(92)90155-C
24. Sudharsan PT, Mythili Y, Sudhahar V, et al. Role of lupeol and its ester on cyclophosphamide-induced hyperlipidaemic cardiomyopathy in rats. J Pharm Pharmacol. 2005;57(11):1437–1444. doi:10.1211/jpp.57.11.0009
25. Manthravadi S, Shrestha A, Madhusudhana S. Impact of statin use on cancer recurrence and mortality in breast cancer: A systematic review and meta-analysis. Int J Cancer. 2016;139(6):1281–1288. doi:10.1002/ijc.30185
26. Kalabova H, Melichar B, Ungermann L, et al. Intima-media thickness, myocardial perfusion and laboratory risk factors of atherosclerosis in patients with breast cancer treated with anthracycline-based chemotherapy. Med Oncol. 2011;28(4):1281–1287. doi:10.1007/s12032-010-9593-1
27. Militaru A, Avram A, Cimpean AM, et al. The Assessment of Left Ventricle Function and Subclinical Atherosclerosis in Patients with Acute Myeloid Leukemia. Vivo. 2018;32(6):1599–1607. doi:10.21873/invivo.11420
28. Kim Y, Park SK, Han W, et al. Serum high-density lipoprotein cholesterol and breast cancer risk by menopausal status, body mass index, and hormonal receptor in Korea. Cancer Epidemiol Biomarkers Prev. 2009;18(2):508–515. doi:10.1158/1055-9965.EPI-08-0133
29. Sahebkar A, Serban M-C, Penson P, et al. The Effects of Tamoxifen on Plasma Lipoprotein(a) Concentrations: systematic Review and Meta-Analysis. Drugs. 2017;77(11):1187–1197. doi:10.1007/s40265-017-0767-4
30. Chi F. Effects of toremifene versus tamoxifen on breast cancer patients: a meta-analysis. Breast Cancer. 2013;20(2):111–122.
31. Amir E, Seruga B, Niraula S, et al. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systematic review and meta-analysis. J Natl Cancer Inst. 2011;103(17):1299–1309. doi:10.1093/jnci/djr242
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
