Back to Journals » Journal of Pain Research » Volume 10
Dynamic abnormalities of spontaneous brain activity in women with primary dysmenorrhea
Authors Jin L, Yang X, Liu P, Sun J, Chen F, Xu Z, Qin W, Tian J
Received 1 September 2016
Accepted for publication 13 January 2017
Published 24 March 2017 Volume 2017:10 Pages 699—707
DOI https://doi.org/10.2147/JPR.S121286
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael Schatman
Lingmin Jin,1 Xuejuan Yang,1 Peng Liu,1 Jinbo Sun,1 Fei Chen,1 Ziliang Xu,1 Wei Qin,1 Jie Tian2
1Sleep and Neuroimage Group, School of Life Science and Technology, Xidian University, Xi’an, Shaanxi, 2Institute of Automation, Chinese Academy of Sciences, Beijing, People’s Republic of China
Purpose: This study aimed to investigate the regional spontaneous brain activity changes in primary dysmenorrhea (PD) patients in different phases of the menstrual cycle by regional homogeneity (ReHo) analysis.
Patients and methods: Thirty-three PD patients and 32 healthy controls (HCs) separately received resting-state functional magnetic resonance imaging during menstrual phase and follicular phase (non-menstrual phase). Cox retrospective symptom scale (RSS), Self-Rating Anxiety Scale (SAS) and Self-Rating Depression Scale (SDS) were applied to assess related symptoms and emotions.
Results: There was no significant difference between the two groups in demographic data. The PD patients obtained higher RSS score, SAS score and SDS score than HCs. Compared with HCs, the ReHo values of the PD patients were increased in left midbrain and hippocampus, right posterior cingulate cortex (PCC), insula and middle temporal cortex (MTC) and decreased in left dorsolateral prefrontal cortex and right medial prefrontal cortex (mPFC) in menstrual phase. In non-menstrual phase, enhanced ReHo values were found in bilateral S1 and precuneus, left S2 and MTC, and reduced ReHo values were observed in left mPFC and orbital frontal cortex. RSS score positively correlated with ReHo values of midbrain and negatively correlated with mPFC and PCC.
Conclusion: Our results suggested that PD is accompanied by dynamic regional spontaneous activity changes across the menstrual cycle, and the altered regions were involved in descending pain modulation, default mode network and sensory modulation. These abnormal activations might contribute to maintain the menstrual pain.
Keywords: primary dysmenorrhea, resting-state functional magnetic resonance imaging, regional homogeneity, spontaneous brain activity, descending pain modulation
Introduction
Primary dysmenorrhea (PD), which refers to lower abdominal pain or cramps occurring just before and/or during the menstrual period in the absence of evident pathology, is regarded as one of the most frequent gynecological complaints mainly experienced by adolescents.1 A recent review reported that dysmenorrhea prevalence varied between 45% and 95% among women of reproductive age, and 10% and 25% of the women suffered severe symptoms.2 The cramps are often accompanied by low back pain, nausea, vomiting and diarrhea, resulting in a negative impact on quality of life and even short-term absence from school or work in very severe cases.2 It has been demonstrated that increased secretion of peripheral vasoactive prostanoid was one of the key factors of pathogenesis in PD, which directly gave rise to enhanced frequency of uterine contraction, decreased myometrial blood flow and further uterine hypoxia and dysmenorrhea.2 In recent years, the central disorder has been investigated and abnormal variations in brain structure and function were found in moderate-to-severe PD patients.3–10
According to the studies, the microstructure of white matter, gray matter (GM) volumes and cortical thickness were all altered in PD patients compared with healthy controls (HCs), and the disorders were mainly related to pain transmission and modulation, sensory processing and affect regulation.4,7,10 Functional neuroimaging studies reported that hypermetabolism was observed in the orbitofrontal cortex (OFC), medial prefrontal cortex (mPFC) and ventral posterior thalamus in PD patients, which suggested that the disinhibition of thalamo-orbitofrontal-prefrontal networks might partly underlie the cramps and hyperalgesia of PD.3,5 The patients also revealed abnormal pain processing when suffering experimental stimuli.5 The resting-state studies found that the functional connectivity of periaqueductal gray (PAG) and default mode network (DMN) was maladaptive/adaptive changed in PD patients.8,9 However, few studies focused on the spontaneous brain activity of the patients. On the basis of our previous studies and other findings, we will define all the aberrant brain areas as regions of interest (ROIs) and then detect the corresponding spontaneous activities in PD patients.
Regional homogeneity (ReHo), based on the hypothesis that neighboring voxels within a functional brain region showed a temporal similarity that could be modulated in different states, is proved to be an effective approach to evaluating synchronization of resting-state blood-oxygenation level dependent (BOLD) signals according to Kendall’s coefficient concordance (KCC).11,12 ReHo analysis has been applied to explore the brain functional changes in many neurological and psychiatric disorders, gastrointestinal diseases and chronic pain and gives a better understanding of the corresponding brain mechanisms.13–17 Here, we employed ReHo approach to identify regional coherence of spontaneous brain activity in PD patients.
Considering the state alterations of GM volumes and the functional connectivity in PD patients,6,8 the spontaneous activity might also be varied with the menstrual cycle. Therefore, we hypothesized that the ReHo values might dynamically change with the phases of menstrual cycle in women with PD compared with HCs, and the aberrant regions might be included in the regions involved in the descending pain modulation pathways, DMN and sensory regulation.
Materials and methods
Subjects
Thirty-four right-handed PD patients (21.5±1.2 years) and 34 age-matched right-handed HC females (22.2±1.7 years) were enrolled. All the subjects were recruited from the college students of Chengdu University of Traditional Chinese Medicine (TCM) and Sichuan University. The inclusion criteria of PD were primary dysmenorrhea (diagnosed by the details of menstrual questionnaires and pelvic ultrasound); regular menstrual cycle (28±3 days) and normal duration of menstruation (3–7 days); moderate or severe abdominal cramp pain (>4 points on a visual analog scale in which 0 means no pain and 10 means the worst pain); suffered an attack at least 4 months in the past 6 months; nulliparous and nonsmoker. The inclusion criteria for HC were same to the PD patients except menstrual pain. The exclusion criteria for all the subjects were secondary dysmenorrhea induced by endometriosis or pelvic inflammatory disease (excluded by pelvic ultrasound); recurrent pelvic or lower abdominal pain; gastrointestinal diseases including recurrent gastritis, gastric or duodenal ulcer; chronic pain; pregnant or intending to become pregnant during the course of the trial; history or evidence of serious diseases, neurological or psychiatric diseases; receiving nonsteroidal anti-inflammatory drugs (NSAIDs) (including aspirin) or analgesics or alcohol within 24 h leading up to magnetic resonance imaging (MRI) scanning; receiving oral contraceptives within 6 months prior to the study and any contraindication of MRI scanning. This project was approved by the West China Hospital Research Ethics Committee, and written informed consent was obtained from each subject.
Psychological assessment
All subjects underwent two functional MRI (fMRI) scanning. One scan was performed on the 1st–3rd day of the menstrual cycle (menstrual phase), while the other scan was performed on the 10th–12th day of the menstrual cycle (follicular phase). The scan order was randomized. Short-form McGill pain questionnaire (SF-MPQ) and Cox retrospective symptom scale (RSS)18 were used to evaluate the menstrual pain and associated symptoms experienced by each PD patient retrospectively in the past 3 months. SF-MPQ was also completed only before menstrual phase scan, and the questionnaire was replaced with an oral question by supposing there was no pain before follicular phase scan. Self-Rating Anxiety Scale (SAS) and Self-Rating Depression Scale (SDS) were applied to assess the subject’s anxiety and depression level prior to each scan.
Image acquisition
A standard birdcage head coil of a 3 T Siemens scanner (Allegra, Siemens Medical System) was used to collect imaging data at the Huaxi MR Research Center, West China Hospital of Sichuan University, Chengdu, China. Restraining foam pads were accompanied to minimize head motion and diminish the scanner noise. During the scan, subjects were requested to close the eyes and keep still in the supine position. In follicular phase, functional images were scanned using a gradient-echo echo planar imaging sequence (repetition time [TR] = 2000 ms; echo time [TE] = 30 ms; flip angle = 90°; slices = 30; field of view [FOV] = 240 mm × 240 mm; in-plane matrix resolution = 64 × 64; in-plane resolution = 3.75 mm × 3.75 mm). After that, high-resolution T1-weighted images were acquired (TR = 1900 ms; TE = 2.26 ms; flip angle = 9°; slices = 176; slices thickness = 1 mm, FOV = 256 mm × 256 mm; data matrix = 256 × 256; in-plane resolution = 1 mm × 1 mm). In menstrual phase, only functional images were obtained.
Imaging data preprocessing and analysis
Preprocessing of the resting-state fMRI data was performed using SPM8 software package19 and composed of the following steps. Abandoned the first five images of each functional time series for the longitudinal magnetization to reach equilibrium; slice-timing adjusted and realigned to the first volume; motion-corrected by estimating six parameters capturing translation and angular rotation relative to the first volume (2 mm or 2 degrees rotation was discarded); normalized to Montreal Neurologic Institute (MNI) space using the normalization parameters estimated by T1 structural image unified segmentation and re-sampled to 3 mm voxels; removed estimated motion parameters, linear drift and average BOLD signals in ventricular and white matter regions through linear regression and reduced the effect of low-frequency drifts and high-frequency noise by temporal band pass filtering (0.01–0.08 Hz).
Individual ReHo maps were generated by calculating KCC, used to measure the correlation of the time series of a given voxel with the time series of its 26 nearest neighbors, within a prepared mask including the following regions: superior frontal cortex, middle frontal cortex (MFC), medial frontal cortex, inferior frontal cortex (IFC), OFC, precentral, primary somatosensory area (S1), secondary somatosensory area (S2), inferior parietal cortex, cuneus, precuneus (Pcu), middle temporal cortex (MTC), inferior temporal cortex (ITC), insula, anterior and posterior cingulate cortex (PCC), thalamus, basal ganglia, hippocampus and brainstem. When the center cube was on the edge of the mask, we only calculated ReHo for a vessel if all the remaining nearest boxes were within the mask. For each participant, the KCC map was normalized by dividing KCC in each voxel by the mean KCC of the mask. At last, smooth was applied with a three-dimensional (3D) Gaussian kernel (full width at half maximum [FWHM] = 6 mm) to reduce noise and residual differences in gyral anatomy. All of these procedures were performed using DPARSF software.20
Statistical analysis
Two independent samples t-test were used to examine the consistency of age, body mass index, the onset of menses, length of menstrual cycle and menstrual phase and the differences of psychological scores (SF-MPQ, RSS, SAS and SDS) between PD patients and HC by using SPSS 20.0. All p-values were two-sided, and p<0.05 was considered statistically significant.
In the group analysis, age was regressed out as covariate. Statistical maps were evaluated to determine the differences in ReHo values with a significance voxel level of p<0.05 (false discovery rate [FDR] corrected) by using permutation test (SnPM13)21 in the following contrasts: 1) group differences between PD and HC in both menstrual phase [PD(mens) − HC(mens)] and follicular phase (non-menstrual phase) [PD(non) − HC(non)] were examined separately with two-sample t-test. 2) The interaction between phase and group [PD(mens-non) − HC(mens-non)] was examined by repeating (mens-non) measures within and independent measures between groups. Correlation analysis was applied using REST software22 to detect the relationship of the ReHo values in the above significant areas to duration, RSS, SAS and SDS scores of PD patients. The significance level was set at p<0.05 (FDR corrected). Considering that half of the PD patients experienced no pain during the scanning in menstrual phase, here we chose RSS as the symptom score instead of SF-MPQ.
Results
Demographic data and psychological information
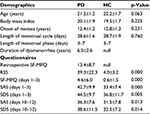
Thirty-three PD patients and 32 HC volunteers completed all the scans very well. The other three subjects were excluded because of significant head motion. There was no significant difference between the two groups in age, body mass index, the onset of menses, length of menstrual cycle and menstrual phase. Retrospective SF-MPQ, RSS scores and SF-MPQ scores of patients in menstrual phase were remarkably higher than HC group. The patients also obtained higher SAS and SDS scores both in menstrual phase and in non-menstrual phase than HCs (Table 1).
Regional spontaneous activity changes
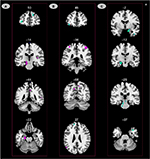
In menstrual phase, the ReHo values of the PD patients significantly increased in left midbrain and hippocampus, right PCC, MTC and insula and decreased in left dorsolateral prefrontal cortex (dlPFC) and right mPFC (Table 2; Figure 1A). Among these areas, midbrain and hippocampus survived the phase and group interaction differences (Table 2; Figure 1C). It was worth noting that PAG became remarkable during the interaction analysis. The other regions did not survive, indicating that these regions in the group differences were not statistically significant across phases. In non-menstrual phase, enhanced ReHo values were found in bilateral S1 and Pcu, left S2 and MTC. Reduced ReHo values were observed in left OFC and mPFC (Table 2; Figure 1B). Furthermore, the results of the interaction displayed three more areas that did not exist in either phase, including left pons, ITC and right temporal pole (TP) (Table 1; Figure 1C).
Correlation between ReHo values and questionnaire data
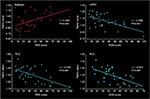
The correlations between questionnaire scores and the regions that showed significant ReHo value changes during group comparisons in both phases were analyzed. During menstrual phase, RSS score was positively correlated with the ReHo values of midbrain and negatively related to mPFC and PCC. In addition, there was a negative correlation between the ReHo value of PCC and SAS scores (Figure 2). No obvious correlation between questionnaire scores and the regions with abnormal ReHo values was detected in non-menstrual phase.
Discussion
In this study, we used ReHo analytical method to explore the resting-state spontaneous brain activity of PD patients in different phases. The present results demonstrated that the altered ReHo value regions were varied with the phases of menstrual cycle in PD patients, which was corresponding with the previous structural and functional neuroimaging findings of PD.6,8 The abnormal ReHo value regions were mostly involved in the pain modulation network and DMN in menstrual phase and sensorimotor processing and DMN in non-menstrual phase. Furthermore, midbrain, mPFC and PCC were correlated with the clinical symptom scores in PD patients, and PCC also exhibited correlation with anxiety score.
Abnormal spontaneous activities in descending pain modulation pathway
Accumulated evidences indicate that dysregulation in descending pain modulation makes a significant contribution to the development of chronic pain.23 In this study, increased ReHo values were observed in midbrain, which is considered as a key role in the endogenous descending pain inhibitory system.24 Both animal and human studies have proved that the PAG is rich in opioid receptors and manifests a notable analgesic effect.24,25 Various chronic pain such as migraine, chronic low back pain and fibromyalgia presented GM alterations or abnormal functional connectivity in PAG, and the dysfunction might be a potential pathogenic mechanism of chronic pain.26–29 An acupuncture research further supported this point based on the results that the functional connectivity between PAG and rostral ACC in migraine patients restoratively increased after treatment and the recovery was significantly associated with clinical symptom improvement.30 Previous studies of PD patients have also showed that the regional GM volume in midbrain was increased compared with HCs, and the functional connectivity of PAG with dlPFC and DMN appeared maladaptive decreased.4,8 Similarly, the correlation of midbrain and RSS score in our results inferred that the symptoms of PD patients were closely related to the dysfunction of the midbrain. The survival of PAG during interaction analysis revealed its abnormal spontaneous activity altered with the menstrual phase.
It had been suggested that dlPFC is another important region in descending pain modulation pathway and might act as a pain inhibitor during pain processing.31 Insula, which showed disordered functional connectivity to PFC in chronic pain and intrinsic connectivity with PAG,32,33 was also involved in the descending pain modulation.34 A heat allodynia study implied that the effect of pain control in dlPFC might derive from suppressing the activity of insula, midbrain and medial thalamic.35 When painful stimulation was controllable, the negative connectivity between dlPFC and insula was increased and that could be the downregulation effect of dlPFC to pain-evoked activation.36 In chronic pain patients, the abnormal GM structure was found in dlPFC and insula,37,38 and the abnormal changes restored in accordance with the reduction of pain and physical disability after treatment.39 The PD studies have been reported that the decreased metabolism was observed in dlPFC and insula3 and the cortical thickness of insula was significantly increased;7 both the regional GM volumes of dlPFC and insula covaried negatively with the menstrual pain experience.4 This study presented the consistent findings that ReHo values decreased in dlPFC and insula in menstrual phase of PD patients. Therefore, we suggested that the spontaneous activities of PD patients are abnormal in descending pain modulation during menstrual phase. DlPFC, insula and midbrain might all contribute to the dysregulation.
Abnormal spontaneous activities in DMN
In this study, a majority of ReHo values changed regions gathered in DMN, including mPFC, PCC, hippocampus and MTC in menstrual phase and mPFC, Pcu and MTC in non-menstrual phase. DMN is referred to brain activation of subjects when they received no task or pay no attention to the present sensory world by using resting-state functional MRI.40 It is considered as one of the most important networks for pain connectome and plays a significant role in the persistence of pain.41 In chronic pain conditions, the functional connectivity within DMN, especially chronic pain hubs PCC/Pcu and mPFC to other regions, exhibited abnormal enhancement or diminution.42–44 The aberrant functional connectivity between DMN and other regions such as insula and PAG also had been reported.42,44–47 Furthermore, following therapy, the restorative reduction of connectivity between the DMN and insula correlated positively with diminished clinical pain.46 Similarly with other chronic pain, a resting-state study of PD patients showed hypoconnectivity between ventromedial PFC and ACC and hyperconnectivity between ventromedial PFC and dorsomedial PFC during both menstrual phase and non-menstrual phase.9 Our research found that the spontaneous activation of DMN dynamically changed in PD patients across the menstrual cycle comparing with HCs, which was in keeping with the previous findings. Moreover, the results also displayed significant relations between DMN (mPFC and PCC) and RSS scores in menstrual phase, and then we suggested that DMN might contribute to maintaining the cramps in PD patients during menstrual phase.
Abnormal spontaneous activities in other sensory regions
OFC was considered as the crucial region in sensory integration, self-control and emotional expression.48,49 Patients presented the corresponding behavioral changes such as deficit in recognition of emotional expression following OFC lesions.49 The animal study had been proved that the OFC was selectively connected with other prefrontal (dlPFC and mPFC) and sensory cortices including olfactory, gustatory, somatosensory, auditory and visual processing and the amygdala.50 Furthermore, OFC was found to participate in pain modulation.51–53 The irritable bowel syndrome patients exhibited enhanced cortical thickness in OFC, and the cortical thickening was strongly correlated with greater severity of abdominal pain complaints.51 The OFC of PD patients also manifested increased cortical thickness and hypermetabolism, and the abnormal changes were separately correlations with PD durations and pain ratings.3,7 In agreement with this, we found the decreased ReHo values of OFC in PD patients in non-menstrual phase compared with HC group. Meanwhile, the ReHo values of S1 and S2 enhanced in non-menstrual phase. The aberrant spontaneous activities in OFC, S1 and S2 might implicate in hyperalgesia or allodynia in PD patients even in the absence of pain.54,55
There are some limitations in this study. It is difficult to collect image on time in PD patients when the cramp occurring, so about half of the patients did not experience noticeable pain during the menstrual phase scanning. We have not tested the hormone levels in the study. So the influence of the hormone cross group and phase cannot be excluded. We evaluated only the regional spontaneous activities in this study; brain networks of PD patients will be explored in the future.
Conclusion
The imaging data presented here implied that PD is accompanied by dynamic regional spontaneous activity changes during menstrual phase and non-menstrual phase. The abnormal activated regions were basically involved in descending pain modulation pathways, DMN and sensory processing. Among these regions, midbrains, mPFC and PCC were correlated with symptom experience during menstrual phase. In light of the results, we suggest that abnormal activations of descending pain modulation network and DMN may have close correlations with the maintenance of menstrual pain. It remains to be illuminated the causal relationship between these functional changes and the repeated regular cramps.
Acknowledgments
This research has received funding from the National Basic Research Program of China under grant numbers 2014CB543203, 2012CB518501 and 2015CB856403; the National Natural Science Foundation of China under grant numbers 81271644, 81471811, 81471738, 61401346 and 31200837 and the Fundamental Research Funds for the Central Universities.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Dawood MY. Primary dysmenorrhea: advances in pathogenesis and management. Obstet Gynecol. 2006;108(2):428–441. | ||
Iacovides S, Avidon I, Baker FC. What we know about primary dysmenorrhea today: a critical review. Hum Reprod Update. 2015;21(6):762–778. | ||
Tu CH, Niddam DM, Chao HT, et al. Abnormal cerebral metabolism during menstrual pain in primary dysmenorrhea. Neuroimage. 2009;47(1):28–35. | ||
Tu CH, Niddam DM, Chao HT, et al. Brain morphological changes associated with cyclic menstrual pain. Pain. 2010;150(3):462–468. | ||
Vincent K, Warnaby C, Stagg CJ, Moore J, Kennedy S, Tracey I. Dysmenorrhoea is associated with central changes in otherwise healthy women. Pain. 2011;152(9):1966–1975. | ||
Tu CH, Niddam DM, Yeh TC, et al. Menstrual pain is associated with rapid structural alterations in the brain. Pain. 2013;154(9):1718–1724. | ||
Liu P, Yang J, Wang G, et al. Altered regional cortical thickness and subcortical volume in women with primary dysmenorrhoea. Eur J Pain. 2016;20(4):512–520. | ||
Wei SY, Chao HT, Tu CH, et al. Changes in functional connectivity of pain modulatory systems in women with primary dysmenorrhea. Pain. 2016;157(1):92–102. | ||
Wu TH, Tu CH, Chao HT, et al. Dynamic changes of functional pain connectome in women with primary dysmenorrhea. Sci Rep. 2016;6:24543. | ||
Liu P, Wang G, Liu Y, et al. White matter microstructure alterations in primary dysmenorrhea assessed by diffusion tensor imaging. Sci Rep. 2016;6:25836. | ||
Jiang L, Xu T, He Y, et al. Toward neurobiological characterization of functional homogeneity in the human cortex: regional variation, morphological association and functional covariance network organization. Brain Struct Funct. 2015;220(5):2485–2507. | ||
Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22(1):394–400. | ||
Zhang Z, Liu Y, Jiang T, et al. Altered spontaneous activity in Alzheimer’s disease and mild cognitive impairment revealed by regional homogeneity. Neuroimage. 2012;59(2):1429–1440. | ||
Dong M, Qin W, Zhao L, et al. Expertise modulates local regional homogeneity of spontaneous brain activity in the resting brain: an fMRI study using the model of skilled acupuncturists. Hum Brain Mapp. 2014;35(3):1074–1084. | ||
Wang T, Li S, Jiang G, et al. Regional homogeneity changes in patients with primary insomnia. Eur Radiol. 2016;26(5):1292–1300. | ||
Ke J, Qi R, Liu C, et al. Abnormal regional homogeneity in patients with irritable bowel syndrome: a resting-state functional MRI study. Neurogastroenterol Motil. 2015;27(12):1796–1803. | ||
Bao CH, Liu P, Liu HR, et al. Differences in regional homogeneity between patients with Crohn’s disease with and without abdominal pain revealed by resting-state functional magnetic resonance imaging. Pain. 2016;157(5):1037–1044. | ||
Cox DJ, Meyer RG. Behavioral treatment parameters with primary dysmenorrhea. J Behav Med. 1978;1(3):297–310. | ||
SPM: members & collaborators of the Wellcome Trust Centre for Neuroimaging. Available from: http://www.fil.ion.ucl.ac.uk/spm/software/spm8/. Accessed April 9, 2016. | ||
Chao-Gan Y, Yu-Feng Z. DPARSF: a MATLAB toolbox for “Pipeline” data analysis of resting-state fMRI. Front Syst Neurosci. 2010;4:13. | ||
Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Human Brain Mapping. 2002;15:1–25. | ||
Song XW, Dong ZY, Long XY, et al. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6(9):e25031. | ||
Ossipov MH, Morimura K, Porreca F. Descending pain modulation and chronification of pain. Curr Opin Support Palliat Care. 2014;8(2):143–151. | ||
Behbehani MM. Functional characteristics of the midbrain periaqueductal gray. Prog Neurobiol. 1995;46(6):575–605. | ||
Linnman C, Moulton EA, Barmettler G, Becerra L, Borsook D. Neuroimaging of the periaqueductal gray: state of the field. Neuroimage. 2012;60(1):505–522. | ||
Truini A, Tinelli E, Gerardi MC, et al. Abnormal resting state functional connectivity of the periaqueductal grey in patients with fibromyalgia. Clin Exp Rheumatol. 2016;34(2 suppl 96):129–133. | ||
Yu R, Gollub RL, Spaeth R, Napadow V, Wasan A, Kong J. Disrupted functional connectivity of the periaqueductal gray in chronic low back pain. Neuroimage Clin. 2014;6:100–108. | ||
Mainero C, Boshyan J, Hadjikhani N. Altered functional magnetic resonance imaging resting-state connectivity in periaqueductal gray networks in migraine. Ann Neurol. 2011;70(5):838–845. | ||
Rocca MA, Ceccarelli A, Falini A, et al. Brain gray matter changes in migraine patients with T2-visible lesions: a 3-T MRI study. Stroke. 2006;37(7):1765–1770. | ||
Li Z, Liu M, Lan L, et al. Altered periaqueductal gray resting state functional connectivity in migraine and the modulation effect of treatment. Sci Rep. 2016;6:20298. | ||
Tracey I. Neuroimaging of pain mechanisms. Curr Opin Support Palliat Care. 2007;1(2):109–116. | ||
Coulombe MA, Erpelding N, Kucyi A, Davis KD. Intrinsic functional connectivity of periaqueductal gray subregions in humans. Hum Brain Mapp. 2016;37(4):1514–1530. | ||
Fomberstein K, Qadri S, Ramani R. Functional MRI and pain. Curr Opin Anaesthesiol. 2013;26(5):588–593. | ||
Benarroch EE. Descending monoaminergic pain modulation: bidirectional control and clinical relevance. Neurology. 2008;71(3):217–221. | ||
Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain. 2003;126(pt 5):1079–1091. | ||
Brascher AK, Becker S, Hoeppli ME, Schweinhardt P. Different brain circuitries mediating controllable and uncontrollable pain. J Neurosci. 2016;36(18):5013–5025. | ||
Obermann M, Rodriguez-Raecke R, Naegel S, et al. Gray matter volume reduction reflects chronic pain in trigeminal neuralgia. Neuroimage. 2013;74:352–358. | ||
Apkarian AV, Sosa Y, Sonty S, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004;24(46):10410–10415. | ||
Seminowicz DA, Wideman TH, Naso L, et al. Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J Neurosci. 2011;31(20):7540–7550. | ||
Andrews-Hanna JR, Smallwood J, Spreng RN. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci. 2014;1316:29–52. | ||
Kucyi A, Davis KD. The dynamic pain connectome. Trends Neurosci. 2015;38(2):86–95. | ||
Baliki MN, Mansour AR, Baria AT, Apkarian AV. Functional reorganization of the default mode network across chronic pain conditions. PLoS One. 2014;9(9):e106133. | ||
Hemington KS, Wu Q, Kucyi A, Inman RD, Davis KD. Abnormal cross-network functional connectivity in chronic pain and its association with clinical symptoms. Brain Struct Funct. 2016;221(8):4203–4219. | ||
Kucyi A, Moayedi M, Weissman-Fogel I, et al. Enhanced medial prefrontal-default mode network functional connectivity in chronic pain and its association with pain rumination. J Neurosci. 2014;34(11):3969–3975. | ||
Xue T, Yuan K, Zhao L, et al. Intrinsic brain network abnormalities in migraines without aura revealed in resting-state fMRI. PLoS One. 2012;7(12):e52927. | ||
Napadow V, Kim J, Clauw DJ, Harris RE. Decreased intrinsic brain connectivity is associated with reduced clinical pain in fibromyalgia. Arthritis Rheum. 2012;64(7):2398–2403. | ||
As-Sanie S, Kim J, Schmidt-Wilcke T, et al. Functional connectivity is associated with altered brain chemistry in women with endometriosis-associated chronic pelvic pain. J Pain. 2016;17(1):1–13. | ||
Rudebeck PH, Murray EA. The orbitofrontal oracle: cortical mechanisms for the prediction and evaluation of specific behavioral outcomes. Neuron. 2014;84(6):1143–1156. | ||
Jonker FA, Jonker C, Scheltens P, Scherder EJ. The role of the orbitofrontal cortex in cognition and behavior. Rev Neurosci. 2015;26(1):1–11. | ||
Cavada C, Company T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suarez F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb Cortex. 2000;10(3):220–242. | ||
Hubbard CS, Becerra L, Heinz N, et al. Abdominal pain, the adolescent and altered brain structure and function. PLoS One. 2016;11(5):e156545. | ||
Smith JK, Marciani L, Humes DJ, Francis ST, Gowland P, Spiller RC. Anticipation of thermal pain in diverticular disease. Neurogastroenterol Motil. 2016;28(6):900–913. | ||
Lim M, Kim JS, Kim DJ, Chung CK. Increased low- and high-frequency oscillatory activity in the prefrontal cortex of fibromyalgia patients. Front Hum Neurosci. 2016;10:111. | ||
Brinkert W, Dimcevski G, Arendt-Nielsen L, Drewes AM, Wilder-Smith OH. Dysmenorrhoea is associated with hypersensitivity in the sigmoid colon and rectum. Pain. 2007;132(suppl 1):S46–S51. | ||
Granot M, Yarnitsky D, Itskovitz-Eldor J, Granovsky Y, Peer E, Zimmer EZ. Pain perception in women with dysmenorrhea. Obstet Gynecol. 2001;98(3):407–411. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.