Back to Journals » OncoTargets and Therapy » Volume 13
Durable Clinical Response of Advanced Lung Adenocarcinoma Harboring EGFR-19del/T790M/BRAFV600E Mutations After Treating with Osimertinib and Dabrafenib Plus Trametinib: A Case Report
Authors Ding H, Zhuang Z, Xie J, Huang H, Tao Z, Liu Z
Received 21 December 2019
Accepted for publication 30 June 2020
Published 10 August 2020 Volume 2020:13 Pages 7933—7939
DOI https://doi.org/10.2147/OTT.S240775
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jianmin Xu
Honggang Ding,1,* Zhenjie Zhuang,1,* Jingyi Xie,1 Haifu Huang,2 Zhigang Tao,3 Zhanhua Liu4
1Guangzhou University of Chinese Medicine, Guangzhou, People’s Republic of China; 2Shenzhen Hospital of Guangzhou University of Chinese Medicine, Shenzhen, People’s Republic of China; 3Mygene Medical Technology, Guangzhou, People’s Republic of China; 4Department of Cancer Center, The First Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhanhua Liu Email [email protected]
Abstract: EGFR-T790M and BRAFV600E are the common resistance mechanisms to EGFR-tyrosine kinase inhibitors (TKIs). Standard treatment for the triple mutations of EGFR-19del, T790M, and BRAFV600E is still under debate. Herein, we present a case of therapeutic efficacy of osimertinib and dabrafenib plus trametinib on a 63-year-old man with advanced lung adenocarcinoma. This patient reached a remarkable progression-free survival of 9 months without any serious adverse reaction. At the progression of the disease, C797S mutation in cis was detected by liquid biopsy. Subsequently, brigatinib with cetuximab was administered but no curative effect was observed. Then, therapy was changed to apatinib combined with osimertinib. The subsequent CT scan showed that the lesions reached stable disease (SD), and the survival benefit has been evaluated. This case showed that the combination treatment of osimertinib and dabrafenib plus trametinib might be a great treatment option for NSCLC patients with triple mutations (EGFR-19del/T790M/BRAFV600E).
Keywords: EGFR-19del, T790M, BRAFV600E, EGFR TKI, combination therapy
Introduction
Numerous resistance mechanisms of first and third generation EGFR TKIs (epidermal growth factor receptor tyrosine kinase inhibitors) have been elucidated, with EGFR-T790M being the most commonly acquired resistance mutation.1 As a third generation EGFR-TKI, osimertinib has demonstrated significant efficacy against T790M mutation.2 However, acquired resistance to osimertinib after treatment inevitably arises, of which mechanisms include acquired C797S mutation and BRAFV600E mutation.3,4 Currently, the standard treatment for acquired C797S mutation is still under debate. As for the BRAFV600E mutation, a pre-clinical study and a case study5,6 have proven that combining BRAF or MEK inhibitor with osimertinib at the same time could exert a significant inhibitory effect on this acquired resistance. However, the progression-free survival (PFS) of treatment regarding BRAF or MEK inhibitor plus osimertinib was unclear.
Herein, we report the first clinical evidence of efficacy generated by a combination of dabrafenib plus trametinib and osimertinib to target concurrent EGFR-19del, T790M, and BRAFV600E in a lung adenocarcinoma patient. Furthermore, liquid biopsy at disease progression showed a new acquired mutation, C797S in cis, in addition to the original mutation after treatment. Subsequently, brigatinib and cetuximab were administered but without obvious response. Combination treatment of apatinib and osimertinib was applied and the therapeutic effect has been evaluated. The whole course of treatment and changes of tumor indicators during the treatment are shown in Figures 1 and 2, respectively.
 |
Figure 1 Flowchart of the whole course of treatment. |
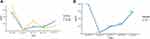 |
Figure 2 Line chart of the changes of tumor markers during the process of the treatment. (A) Changes of CA-125 and CA-199 from Apr 2016 to Jun 2019. (B) Changes of CEA from Apr 2016 to Jun 2019. |
Case Report
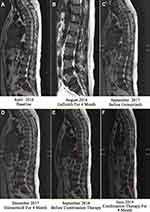
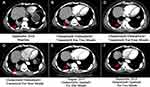
A 63-year-old man with more than 40-year history of second-hand smoking presented with recurrent pain in his left lumbar back for more than half a year, and was diagnosed with stage IV lung adenocarcinoma with metastases to the spine (Figure 3A). Owing to the detection of EGFR-19 del by tissue biopsy, gefitinib (250 mg qd) was administered in April 2016. In addition, the magnetic resonance imaging (MRI) after 4 months of gefitinib treatment showed the patient reached a stable disease (SD) (Figure 3B). But his disease progressed after a progression-free survival (PFS) of 17 months and an MRI scan revealed the deterioration of bone metastasis (Figure 3C). At disease progression, his liquid biopsy revealed an EGFR-T790M mutation. Subsequently, the patient was treated with osimertinib (80 mg qd), and achieved a stable disease (SD) after 4 months based on criteria in Response Evaluation Criteria In Solid Tumors (RECIST) 1.1 (Figure 3D). However, after 12 months of treatment with osimertinib, the patient’s left lumbar back recurred. Results from computed tomography (CT) of his lung and MRI of his lumbar showed that the disease progressed (Figures 3E and 4A). Subsequently, the patient was recommended for chemotherapy, but he refused due to a fear of a series of adverse events. In this case, we advised him to take a plasma next-generation sequencing (NGS) testing, and eventually, a BRAFV600E (AF=8.78%) mutation was identified in addition to EGFR-19 del and EGFR-T790M (AF=2.38%) mutation (Figure 5A and B).
Due to the lack of recommendation for the subsequent treatment from the National Comprehensive Cancer Network (NCCN) guideline of Non-Small Cell Lung Cancer (Version 2017) at that time, we conducted a literature review to identify the optimal treatment strategy for the patient. A Phase 2 trial showed substantial anti-tumor activity and acceptable toxicity of dabrafenib plus trametinib in patients with treatment-naïve BRAFV600E mutation in advanced NSCLC7 and a pre-clinical study6 demonstrated that combined BRAF inhibitor with osimertinib might be a feasible treatment strategy for these triple mutations.
Therefore, a combination treatment with osimertinib (80 mg qd) and dabrafenib (150 mg bid) plus trametinib (2 mg qd) was administered with the consent of the patient in September 2018. As a result, the patient’s back pain relieved significantly within two weeks. The follow-up CT reexamination also demonstrated his lung lesions decreased and reached SD (Figure 4B and C). Furthermore, he reached a remarkable PFS of 9 months, which was rarely reported for this therapeutic regimen recently. Notably, although the patient was treated with a multi-drug combination therapy, only mild adverse reactions such as intermittent low fever and paronychia occurred during the treatment.
In June 2019, he returned to the clinic due to recurring back pain. CT scan and MRI showed an enlarged pulmonary lesion together with metastases in subcarinal lymph node and the aggravation of bone metastases lesions, respectively (Figures 3F and 4D). Liquid biopsy of plasma revealed the acquired mutation of EGFR cis-C797S (AF=6.17% and 7.76%), in addition to the original mutations (19Del/T790M/BRAFV600E) (Figure 5C and D).
Fortunately, a combination of brigatinib and cetuximab was demonstrated to have significant suppression effect on NSCLC cell lines with EGFR-C797S and T790M mutations by inhibition of phosphorylation of EGFR and the decreased expression of EGFR.8 Moreover, Wang et al9 reported that a combination of brigatinib and cetuximab reached a PFS of 9 months in an advanced lung adenocarcinoma patient with triple mutations (EGFR-19del/C797S/T790M). This evidence provided us with clues for the subsequent treatment option for this patient. Thus, the combination therapy mentioned above was changed to brigatinib (90 mg qd) together with cetuximab (250 mg/m2) from June 2019 to July 2019. But it was regrettable that no obvious response was observed and his bone pain persisted in the treatment process. Oxycontin (20 mg/q12h) was applied for bone pain remission. Another therapeutic regimen for our patient was imperative.
According to the previous report, combination therapy of TKI and angiogenic agents could help to overcome the resistance of TKI.10 Recently, a Phase III clinical trial (NEJ026 trial) showed that TKI combined with angiogenic agents significantly prolong PFS in EGFR-positive advanced NSCLC patients compared with TKI alone.11 Therefore, a combination treatment of apatinib and osimertinib was administered based on the patient’s intention and accessibility of treatment considerations in mid-July 2019. Notably, his bone pain was significantly released within a week without taking oxycontin. In August and September 2019, a CT scan showed that the pulmonary lesion had shrunk and reached SD (Figure 4E and F). In December 2019, our patient returned to our department, and imaging examination, as well as liquid biopsy, was performed for therapeutic effect evaluation.
Discussion
Here, we presented the first case of a lung adenocarcinoma patient with triple mutations reaching a remarkable PFS of 9 months and tolerant toxicity under the combination treatment of osimertinib and dabrafenib plus trametinib. In addition, an acquired cis-C797S mutation was also identified after the combination treatment.
Osimertinib and dabrafenib plus trametinib might be a great treatment option for NSCLC patients with triple mutations (19Del/T790M/BRAFV600E). According to the NCCN guideline, osimertinib and dabrafenib plus trametinib were recommended as the preferred therapy for EFGR mutations (19Del/L858R/T790M) and BRAFV600E mutation in NSCLC patients, respectively.12 However, when it came to the triple mutations (19Del/T790M/BRAFV600E), the therapeutic effect of combined molecular targeted therapy is unclear.
Recently, Solassol et al13 have reported a case regarding a 68-year-old female never-smoker with triple mutations (19Del/T790M/BRAFV600E) who experienced a comprehensive treatment including treatment of dabrafenib plus trametinib alternation with osimertinib. This patient was administered with dabrafenib plus trametinib alternation with osimertinib at the disease progression after two cycles of chemotherapy. The PFS of the patient was only 6 months. By contrast, the patient in our case was only administered with molecular targeted therapy during the whole disease course. Additionally, osimertinib and dabrafenib plus trametinib were applied together but not altered. Of note, the PFS of our patient after the combined treatment was 9 months with a benefit of 3 months longer than that of the aforementioned patient. Indeed, any survival benefit in post-line therapy of advanced NSCLC is noteworthy. The difference of PFS benefit might result from the different treatment plan through the disease course of patients. It seemed that the efficacy of combined molecular targeted therapy might be better than chemotherapy followed with targeted therapy in these triple mutations.
Subsequently, the cis-C797S mutation was found in our patient. EGFR-C797S mutation was regarded as an important common acquired mechanism leading to resistance to all covalent EGFR-TKIs and the compromised efficacy of osimertinib.14,15 Though the combination of brigatinib and cetuximab was considered as a promising therapy for concurrent mutations (EGFR-19del/C797S/T790M), it did not exert a curative effect on our patient. We speculated that the difference in treatment outcome might result from the difference in the mutation pattern, for there was another BRAFV600E mutation in our patient. Afterward, due to the lack of the subsequent treatment recommendation for our patient, a combination of apatinib and osimertinib was applied. Recently, a prospective study showed that apatinib combined with osimertinib could help to overcome the resistance of osimertinib, which reached a median PFS of 4 months.16 The further therapeutic effect and survival benefit of this treatment on our patient has been evaluated.
Indeed, the post-line treatment options for advanced NSCLC patients with multiple gene mutations are controversial. Our case showed that osimertinib and dabrafenib plus trametinib could be a great treatment option for triple mutations (19Del/T790M/BRAFV600E) in NSCLC patients. Additionally, full management of treatment for patients with multiple gene mutations is needed. Treatment adjustment should be evidence-based and take the patient’s intention, as well as the accessibility of the treatment into consideration.
Conclusion
In conclusion, our case showed a remarkable PFS of 9 months with a combination of dabrafenib plus trametinib and osimertinib in lung adenocarcinoma harboring EGFR-19del, T790M and BRAFV600E before acquiring the cis-C797S mutation. This case could be clinical evidence and sheds some light for NSCLC patients with concurrent mutations (19Del/T790M/BRAFV600E). In addition, full management strategy of treatment NSCLC patients with multiple gene mutations and more studies to find the standard treatment for the acquired cis-C797S mutation in addition to triple mutation (19Del/T790M/BRAFV600E) are still needed.
Ethics
The patient provided written informed consent for the use of his information for research purposes, including publishing the case details and accompanying images. Institutional approval was not required to publish the case details.
Acknowledgment
We owe thanks to the patient and his family.
Author Contributions
Honggang Ding and Zhenjie Zhuang are co-first authors. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Disclosure
Z Tao is an employee of Mygene Medical Technology. The authors report no other possible conflicts of interest in this work.
References
1. Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3(75):75ra26.
2. Mok T, Wu Y, Ahn M, et al. Osimertinib-Therapie bei EGFR-T790M-Punktmutation beim NSCLC. Atemwegs-und Lungenkrankheiten. 2017;376:629–640.
3. Thress KS, Paweletz CP, Felip E, et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non–small cell lung cancer harboring EGFR T790M. Nat Med. 2015;21(6):560.
4. Jamal-Hanjani M, Huebner A, McGranahan N, Swanton CJ. LBA11 defining the lethal subclone in metastatic lung cancer. Ann Oncol. 2018;29(suppl_8):mdy424. doi:10.1093/annonc/mdy424
5. Ho -C-C, Liao W-Y, Lin C-A, Shih J-Y, Yu C-J, Yang JC. Acquired BRAF V600E mutation as resistant mechanism after treatment with osimertinib. J Thorac Oncol. 2017;12(3):567–572.
6. Vojnic M, Kubota D, Kurzatkowski C, et al. Acquired BRAF rearrangements induce secondary resistance to EGFR therapy in EGFR-mutated lung cancers. J Thorac Oncol. 2019;14(5):802–815.
7. Planchard D, Smit EF, Groen HJ, et al. Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol. 2017;18(10):1307–1316.
8. Uchibori K, Inase N, Araki M, et al. Brigatinib combined with anti-EGFR antibody overcomes osimertinib resistance in EGFR-mutated non-small-cell lung cancer. Nat Commun. 2017;8:14768.
9. Wang X, Zhou L, Yin JC, Wu X, Shao YW, Gao BJ. Lung adenocarcinoma harboring EGFR 19del/C797S/T790M triple mutations responds to brigatinib and anti-EGFR antibody combination therapy. J Thorac Oncol. 2019;14(5):e85–e88.
10. Zhao J, Zou M, Lv J, et al. Effective treatment of pulmonary adenocarcinoma harboring triple EGFR mutations of L858R, T790M, and cis-C797S by osimertinib, bevacizumab, and brigatinib combination therapy: a case report. Onco Targets Ther. 2018;11:5545.
11. Saito H, Fukuhara T, Furuya N, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, Phase 3 trial. Lancet Oncol. 2019;20(5):625–635. doi:10.1016/S1470-2045(19)30035-X
12. Ettinger DS, Wood DE, Aggarwal C, et al. NCCN guidelines insights: non–small cell lung cancer, version 1.2020: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2019;17(12):1464–1472.
13. Solassol J, Vendrell J, Senal R, Audran P, Leenhardt F, Quantin X. Challenging BRAF/EGFR co-inhibition in NSCLC using sequential liquid biopsies. Lung Cancer. 2019;133:45–47.
14. Niederst MJ, Hu H, Mulvey HE, et al. The allelic context of the C797S mutation acquired upon treatment with third-generation EGFR inhibitors impacts sensitivity to subsequent treatment strategies. Clin Cancer Res. 2015;21(17):3924–3933.
15. Ercan D, Choi HG, Yun C-H, et al. EGFR mutations and resistance to irreversible pyrimidine-based EGFR inhibitors. Clin Cancer Res. 2015;21(17):3913–3923.
16. Yang X, Zhao J, Liang L, Xu L. P1.14-16 resolving resistance to osimertinib by combining apatinib and osimertinib in EGFR-mutant NSCLC patients. J Thorac Oncol. 2019;14(10):S559. doi:10.1016/j.jtho.2019.08.1167
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.