Back to Journals » International Medical Case Reports Journal » Volume 13
Dupilumab-Associated Blepharoconjunctivitis with Giant Papillae
Authors Vingopoulos F , Lazzaro DR
Received 19 May 2020
Accepted for publication 26 June 2020
Published 28 July 2020 Volume 2020:13 Pages 303—305
DOI https://doi.org/10.2147/IMCRJ.S263068
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Filippos Vingopoulos, Douglas R Lazzaro
New York University School of Medicine, Department of Ophthalmology, New York, NY 10017, USA
Correspondence: Douglas R Lazzaro
New York University School of Medicine, Department of Ophthalmology, 222 East 41st Street, 4th Floor, New York, NY 10017, USA
Tel +1 (929) 455-5051
Email [email protected]
Purpose: To describe a case of severe dupilumab-associated blepharoconjunctivitis with giant papillae treated with high potency corticosteroid eyedrops, without discontinuing or reducing dupilumab therapy.
Case Report: A 22-year-old Latin American female with a long history of severe atopic dermatitis (AD) with no ocular involvement presented 20 weeks after starting treatment with dupilumab injections with blurry vision, multiple chalazia, eyelid swelling and severe conjunctival injection in both eyes. She also reports having a hordeolum 2 months prior and severely dry eyes starting 2 weeks prior. Slit-lamp examination revealed severe conjunctivitis with macroscopically visible giant papillae in the right lower tarsal conjunctiva. The diagnosis of severe dupilumab-associated blepharoconjunctivitis was made and difluprednate 0.05% eyedrops two times a day for 7 days was initiated. Given the severity of her AD and her marked skin improvement with dupilumab, it was decided to continue dupilumab without reducing the dose. At 2-day follow-up, conjunctival injection had markedly improved, and at 2-month follow-up, her examination was unremarkable. Currently, our patient only uses dexamethasone 0.1% drops few times a week as per needed for occasional eye irritation.
Conclusion: As dupilumab injections begin to claim a rightful place in medicine, the ophthalmic community may start encountering dupilumab-associated ocular surface disease all more often and potentially play an important role in identifying, characterizing and treating the adverse ocular effects from this novel medication.
Keywords: dupilumab, blepharoconjunctivitis, conjunctivitis, atopic dermatitis, dupilumab adverse reactions
Introduction
Dupilumab is a human monoclonal antibody that blocks interleukin (IL)-4 and IL-13 signaling approved by the US Food and Drug Administration (FDA) in 2017 as the first biological systemic treatment for moderate-to-severe atopic dermatitis (AD).1
In the randomised clinical trials used for dupilumab FDA approval, up to 22% of patients with AD on dupilumab experience some kind of dupilumab-associated ocular surface disease (DOSD).2 More than 90% of DOSD cases are mild or moderate and controlled with artificial tears and/or mast cell stabilisers.3 Few severe cases have been recently reported in real-life clinical practice, including cases of follicular, proliferative and cicatricial conjunctivitis4–9 A single case of severe papillary blepharoconjunctivitis has also been described.10 Due to the novelty of this treatment, there are currently no established guidelines for treating severe DOSD cases.
We herein present a case of dupilumab-associated severe blepharoconjunctivitis with giant papillae treated with high potency corticosteroid eyedrops, without discontinuing or reducing dupilumab therapy.
Case Presentation
A 22-year-old Latin American female with a long history of severe AD with up to 90% of body surface area involvement, but no ocular involvement, was referred 20 weeks after starting treatment with dupilumab injections 300 mg biweekly, for ophthalmologic evaluation. With dupilumab, nearly complete resolution of her widespread eczematous dermatitis had been achieved, while prior treatments including topical corticosteroids and immunosuppressives failed to improve her debilitating skin disease.
The patient presented with blurry vision, green ocular discharge, multiple chalazia, eyelid swelling and severe conjunctival injection in both eyes (Figure 1). She also reports having a hordeolum 2 months prior and severely dry eyes starting 2 weeks prior.
 |
Figure 1 External photograph showing bilateral eyelid swelling and severe conjunctival injection. |
Slit-lamp examination revealed severe conjunctivitis with macroscopically visible giant papillae in the right lower tarsal conjunctiva (Figure 2). The diagnosis of severe dupilumab-associated blepharoconjunctivitis was made and difluprednate 0.05% eyedrops two times a day for 7 days was initiated. Given the severity of her AD and her marked skin improvement with dupilumab, it was decided to continue dupilumab 300 mg every 14 days without reducing the dose. At 2-day follow-up, conjunctival injection had markedly improved and at 2-month follow-up her examination was unremarkable except from a chalazion and mild dryness. Currently, our patient only uses dexamethasone 0.1% drops few times a week as per needed for occasional eye irritation.
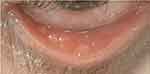 |
Figure 2 External photograph showing macroscopically visible giant papillae in the right lower tarsal conjunctiva. |
Health Insurance Portability and Accountability Act (HIPPA) compliance was maintained throughout the study as well as adherence to the tenets of the Declaration of Helsinki. An institutional review board approval was not required to publish data of a single patient. Written informed consent was obtained from the patient for chart review and publication of this case report including the images prior to study commencement.
Discussion
Atopic dermatitis (AD) is the most common chronic inflammatory skin disease, with a prevalence between 2–10% in adults and 15–30% in children.11 Current treatment options are limited and symptoms for moderate-to-severe atopic dermatitis are infrequently adequately controlled with topical corticosteroids. Dupilumab is the first FDA-approved biological systemic treatment for moderate-to-severe atopic dermatitis.1 In addition to improving symptoms of atopic dermatitis, clinical trials have reported dupilumab to reduce patient-reported symptoms of depression, anxiety and improve overall quality of life.1
DOSD is a common adverse event of dupilumab and if left untreated may lead to cicatricial conjunctivitis and subsequent visual impairment;4,6 high index of suspicion and multidisciplinary awareness for early recognition and appropriate treatment of this adverse event is warranted.
Both medium and high potency topical corticosteroids and calcineurin-inhibitor eyedrops have been described in real-life clinical practice as effective treatment options for severe DOSD with continued dupilumab treatment.2,6 As AD patients are prone to herpetic keratitis, topical steroids should be used with caution and the use of periocular tacrolimus ointment may also be considered.12 The two cases of cicatricial blepharoconjunctivitis reported in the literature either reduced5 or stopped4 dupilumab infusions along with topical or oral corticosteroids, respectively.4,5 The single case of papillary blepharoconjunctivitis reported combined antibiotic/dexamethasone eyedrops along with lid hygiene measures.10
In our case of severe blepharoconjunctivitis with giant papillae, remission of ocular disease was achieved with high potency corticosteroid eyedrops without reducing the dupilumab dose frequency. The recent consensus recommendation by an international panel of eczema experts concluded that dupilumab treatment should be continued when possible for patients with AD and ocular complications, with appropriate referral to an ophthalmologist.13
As dupilumab injections begin to claim a rightful place in medicine, the ophthalmic community may start encountering dupilumab-associated ocular surface disease all more often and potentially play an important role in identifying, characterizing and treating the adverse ocular effects from this novel medication.
Disclosure
The authors have no conflicting interests.
References
1. Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375(24):2335–2348. doi:10.1056/NEJMoa1610020
2. Akinlade B, Guttman-Yassky E, de Bruin-weller M, et al. Conjunctivitis in dupilumab clinical trials. Br J Dermatol. 2019;181(3):459–473. doi:10.1111/bjd.17869
3. Wollenberg A, Ariens L, Thurau S, van Luijk C, Seegräber M, de Bruin-weller M. Conjunctivitis occurring in atopic dermatitis patients treated with dupilumab–clinical characteristics and treatment. J Allergy Clin Immunol Pract. 2018;6(5):1778–80.e1. doi:10.1016/j.jaip.2018.01.034
4. Barnes AC, Blandford AD, Perry JD. Cicatricial ectropion in a patient treated with dupilumab. Am J Ophthalmol Case Rep. 2017;7:120–122. doi:10.1016/j.ajoc.2017.06.017
5. Levine RM, Tattersall IW, Gaudio PA, King BA. Cicatrizing blepharoconjunctivitis occurring during dupilumab treatment and a proposed algorithm for its management. JAMA Dermatol. 2018;154(12):1485–1486. doi:10.1001/jamadermatol.2018.3427
6. Agnihotri G, Shi K, Lio PA. A clinician’s guide to the recognition and management of dupilumab-associated conjunctivitis. Drugs R D. 2019;19(4):311–318. doi:10.1007/s40268-019-00288-x
7. Fukuda K, Ishida W, Kishimoto T, Fukushima A. Development of conjunctivitis with a conjunctival proliferative lesion in a patient treated with dupilumab for atopic dermatitis. Allergol Int. 2019;68(3):383–384. doi:10.1016/j.alit.2018.12.012
8. Maudinet A, Law-Koune S, Duretz C, Lasek A, Modiano P, Tran THC. Ocular surface diseases induced by dupilumab in severe atopic dermatitis. Ophthalmol Ther. 2019;8(3):485–490. doi:10.1007/s40123-019-0191-9
9. Shen E, Xie K, Jwo K, Smith J, Mosaed S. Dupilumab-induced follicular conjunctivitis. Ocul Immunol Inflamm. 2019;27(8):1339–1341. doi:10.1080/09273948.2018.1533567
10. Paulose SA, Sherman SW, Dagi Glass LR, Suh LH. Dupilumab-associated blepharoconjunctivitis. Am J Ophthalmol Case Rep. 2019;16:100550. doi:10.1016/j.ajoc.2019.100550
11. Malik K, Heitmiller K, Czarnowicki T. An update on the pathophysiology of atopic dermatitis. Dermatol Clin. 2017;35(3):317–326. doi:10.1016/j.det.2017.02.006
12. Nahum Y, Mimouni M, Livny E, Bahar I, Hodak E, Leshem YA. Dupilumab-induced ocular surface disease (DIOSD) in patients with atopic dermatitis: clinical presentation, risk factors for development and outcomes of treatment with tacrolimus ointment. Br J Ophthalmol. 2020;104(6):776–779. doi:10.1136/bjophthalmol-2019-315010
13. Thyssen JP, de Bruin-weller MS, Paller AS, et al. Conjunctivitis in atopic dermatitis patients with and without dupilumab therapy - international eczema council survey and opinion. J Eur Acad Dermatol Venereol. 2019;33(7):1224–1231.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
