Back to Journals » Journal of Pain Research » Volume 11
Duloxetine in patients with diabetic peripheral neuropathic pain in Japan: a randomized, double-blind, noninferiority comparative study with pregabalin
Authors Enomoto H , Yasuda H, Nishiyori A, Fujikoshi S, Furukawa M, Ishida M , Takahashi M, Tsuji T , Yoshikawa A, Alev L
Received 10 April 2018
Accepted for publication 24 July 2018
Published 13 September 2018 Volume 2018:11 Pages 1857—1868
DOI https://doi.org/10.2147/JPR.S170646
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Katherine Hanlon
Hiroyuki Enomoto,1 Hitoshi Yasuda,2 Atsushi Nishiyori,3 Shinji Fujikoshi,4 Masashi Furukawa,5 Mitsuhiro Ishida,6 Masashi Takahashi,1 Toshinaga Tsuji,7 Aki Yoshikawa,8 Levent Alev9
1Bio-Medicine, Medicines Development Unit, Eli Lilly Japan K.K, Tokyo, Japan; 2Foundation of Shiga Health Research Center, Shiga, Japan; 3Project Management, Global Development Division, Shionogi & Co. Ltd., Osaka, Japan; 4Statistical Science, Medicines Development Unit, Eli Lilly Japan K.K., Kobe, Japan; 5Biostatistics, Biostatistics Department, Shionogi & Co. Ltd., Osaka, Japan; 6Clinical Research Development, Shionogi & Co. Ltd., Osaka, Japan; 7Medical Affairs Department, Shionogi & Co., Ltd., Osaka, Japan; 8Scientific Communications, Medicines Development Unit, Eli Lilly Japan K.K, Kobe, Japan; 9Eli Lilly, Medical Department, Lilly Turkey, Istanbul, Turkey
Purpose: Duloxetine and pregabalin are recommended as first-line treatments for diabetic peripheral neuropathic pain (DPNP). However, studies have not reported a direct comparison between duloxetine and pregabalin. We conducted a postmarketing, randomized, double-blind study to assess the noninferiority of duloxetine compared with pregabalin after 12 weeks of treatment in adult patients with DPNP in Japan (NCT02417935).
Patients and methods: Patients (N = 303) with distal symmetrical DPNP were randomized to and were administered duloxetine (40–60 mg/day) or pregabalin (300–600 mg/day). The primary endpoint was the change from baseline in weekly mean of the 24-hour average pain score (numeric rating scale [NRS]). Noninferiority of duloxetine compared with pregabalin was assessed with the primary endpoint at week 12. Secondary measures, including night pain and worst pain, Brief Pain Inventory-Severity and Interference rating short form (BPI-SF), Clinical Global Impression of Improvement (CGI-I), Patient Global Impression of Improvement (PGI-I), and Neuropathic Pain Symptom Inventory (NPSI), health outcome measures (EuroQol 5-Dimension index and VAS), and safety were also assessed.
Results: For the 24-hour NRS average pain score, the difference between the duloxetine and pregabalin groups was 0.072 (95% CI: – 0.295, 0.439), and the upper bound of the 95% CI (0.439) did not exceed the predefined noninferiority margin (0.51), at the end of the study period. For secondary outcome measures (night pain, worst pain, BPI-SF, CGI-I, PGI-I, NPSI) and health outcome measures, both the duloxetine and pregabalin treatment groups showed an improvement from baseline with no significant between-group difference. Duloxetine and pregabalin were well tolerated and the safety profiles were consistent with previously reported results.
Conclusion: This study demonstrated the noninferior efficacy of duloxetine compared with pregabalin in the treatment of adult patients with DPNP. The safety analyses showed an acceptable tolerability based on safety profiles of duloxetine and pregabalin.
Keywords: serotonin and norepinephrine reuptake inhibitor, diabetic peripheral neuropathic pain, duloxetine, Japan, noninferiority, pregabalin
Introduction
The global prevalence of diabetes in adults has been estimated at 8.5%, with as many as 422 million adults living with diabetes in 2014.1 Diabetic peripheral neuropathy, which occurs in up to 50% of patients with diabetes,2 is a cause of increased morbidity and mortality and reduced quality of life.3 Manifestations of diabetic peripheral neuropathy include pain, numbness, paresthesia, and insensitivity,2,4 leading to an increased risk for burns and other foot injuries and an impact on quality of life in areas that include sleep, enjoyment of life, and daily activities.2 Neuropathic pain is one of the most frequent complications of diabetes,5,6 and up to 22% of Japanese diabetic patients have painful diabetic neuropathy.7 A recent study reported that the prevalence of neuropathy, nephropathy, or retinopathy was ~30%, with 6.4% of patients having all three microvascular complications.8
Currently, pregabalin, duloxetine and tricyclic antidepressants are the recommended first-line drugs for moderate to severe painful neuropathy by the Japanese guidelines for neuropathic pain management.9 Furthermore, duloxetine and pregabalin are listed as the first-line of treatment in the international treatment guidelines for diabetic peripheral neuropathic pain (DPNP).10,11
Duloxetine (40–60 mg daily) is approved for the treatment of DPNP in Japan. It is a serotonin and norepinephrine reuptake inhibitor and, although the exact mechanisms of the central pain inhibitory actions of duloxetine are unknown, its mechanism of action is believed to be related to its potentiation of serotonergic and noradrenergic activity in the CNS such as descending inhibitory pain pathways.12 In Japan, duloxetine is indicated for the treatment of depression and depressive state and for pain associated with diabetic neuropathy, fibromyalgia, chronic lower back pain, and osteoarthritis. In the USA, the indications for duloxetine are major depressive disorder (MDD), general anxiety disorder (GAD), DPNP, fibromyalgia, and chronic musculoskeletal pain. In the European Union, the indications for duloxetine are MDD, GAD, DPNP, and stress urinary incontinence.
Pregabalin (300–600 mg daily) is approved for the treatment of neuropathic pain and pain associated with fibromyalgia in Japan. It is thought to reduce pain by binding to alpha-2-delta subunits of presynaptic neuronal calcium channels and reducing the release of excitatory neurotransmitters involved in pain perception.13,14 In the USA and European Union, pregabalin is indicated for the management of neuropathic pain, including DPNP, postherpetic neuralgia, fibromyalgia, and generalized anxiety disorder, and as an adjunct therapy for epilepsy.
A previous “combination vs monotherapy of pregabalin and duloxetine in diabetic neuropathy” (COMBO-DN) study combined both duloxetine (60–120 mg/day) and pregabalin (300–600 mg/day) in the treatment of patients with DPNP who were not responding to standard doses of duloxetine or pregabalin.15 They examined if a combination of both medications was superior to increasing each drug to its maximum recommended dose. The COMBO-DN study included duloxetine and pregabalin in monotherapy phase, and its primary objective was to compare efficacy between high-dose monotherapy and combination therapy. Although the COMBO-DN study compared duloxetine and pregabalin directly as a secondary endpoint in the first period of the study, the primary objective was not a direct comparison between duloxetine and pregabalin nor were there any Japanese patients enrolled in the study.15 The sensitivity to pain in Japanese patients is suggested to be different from that in Western patients.16 However, there is no evidence showing a direct comparison between duloxetine and pregabalin in Japanese patients. It is useful to compare the efficacy and safety of these treatments for daily medical practice. The effectiveness of various pharmacological interventions for DPNP has been evaluated; however, the implications of the findings are limited due to indirect comparisons of clinical trials.17 Treatments for DPNP in terms of effectiveness have rarely been compared directly against each other, and network meta-analysis and direct (head-to-head) comparisons can help determine the proportional advantages of given drugs.18 There is no head-to-head study comparing duloxetine and pregabalin in the treatment of Japanese patients with DPNP.
Our objective was to assess the noninferiority of duloxetine compared to pregabalin after 12 weeks of treatment in adult patients with DPNP in Japan. Both have been evaluated as first-line treatments for patients with neuropathic pain6,9 despite different modes of action. We conducted a postmarketing, Phase IV, multicenter, randomized, double-blind, parallel-group, flexible-dose, comparative study in patients who had never taken either drug and were primarily indicated for first-line treatment of DPNP. The primary objective of the study was to assess the noninferiority of duloxetine (40–60 mg/day, approved treatment doses in Japan) compared with pregabalin (300–600 mg/day) after 12 weeks of treatment in adult patients with DPNP in Japan. The study presented here is the first of its kind to directly compare the efficacy of duloxetine with pregabalin and address whether duloxetine is not inferior to pregabalin in terms of efficacy in treating DPNP after 12 weeks of treatment in adult patients.
Materials and methods
Study design
This was a postmarketing, Phase IV, multicenter, randomized, double-blind, parallel-group, flexible-dose, comparative 12-week study in adult outpatients with DPNP in Japan. The study was conducted in 78 study centers, and the trial has been registered at ClinicalTrials.gov (NCT02417935). The study was approved by applicable ethical review boards and Pharmaceuticals and Medical Devices Agency prior to the study initiation, and was conducted following applicable laws and regulations, Good Clinical Practice (according to the International Conference on Harmonization), and the Declaration of Helsinki. Patients provided written consent after the study was explained and before study procedures were initiated.
Study population
The study included male or female outpatients ≥20 and <80 years of age who presented with pain due to distal symmetrical polyneuropathy caused by type 1 or type 2 diabetes mellitus and who had never been treated with duloxetine or pregabalin. We used the Japanese abbreviated diagnostic criteria to define diabetic polyneuropathy.19 Patients had to have a score of ≥4 on the mean 24-hour average pain score measured using the 11-point numeric rating scale (NRS) in the daily patient diary (calculated from records 7 days immediately before randomization), and HbA1c ≤9.4% (National Glycohemoglobin Standardization Program). Patients had to complete daily diary entries ≥80% of the time from screening to randomization.
Key exclusion criteria included patients who had poor glycemic control within 70 days immediately prior to visit 1 (week –2 to week –1), a history or current diagnosis of psychiatric diseases including MDD, complications of diseases that were considered to affect the assessment of DPNP, and neuropathic pain suspected to be caused by alcohol.
Treatment protocol
The study consisted of four study periods, which included a 1- to 2-week screening period, a 12-week treatment period, a 1-week tapering period, and a 1-week follow-up period (Figure 1).
  | Figure 1 Study design. |
After screening, eligible patients were randomized and the treatment period began at week 0 (baseline, visit 2) and ended at week 12 (visit 6). At week 0 (visit 2), patients were assigned to duloxetine or pregabalin in a 1:1 ratio via a computer-generated random sequence using an interactive web response system. Randomization was stratified by the baseline weekly mean of the 24-hour average pain score (NRS) (<6, ≥6) and by the duration of DPNP (<2 years, ≥2 years). Duloxetine was administered at 20 mg/day for a week followed by 40 mg/day for 3 weeks. Pregabalin was administered at 150 mg/day for a week, followed by 300 mg/day for 3 weeks. To maintain blinding, patients received both their assigned active study drug and an indistinguishable placebo (“double-dummy” design). Investigators and other study personnel were also blinded to treatment allocation. At week 4 (visit 4) and week 8 (visit 5), duloxetine and pregabalin doses could be increased in patients who did not achieve clinically significant improvement (≥30% pain improvement in the Japanese validated Brief Pain Inventory-Severity and Interference rating short form [BPI-SF]20 average pain score compared with baseline [week 0] score). At either week, duloxetine could be increased to 60 mg/day and pregabalin could be increased to 450 mg/day. If pregabalin was increased to 450 mg/day at week 4, it could be further increased to 600 mg/day at week 8. Once increased, patient doses could not be decreased again during the study.
During the tapering period (to minimize discontinuation-emergent adverse events), study drug doses were tapered down, and during the follow-up period, no study drug was administered.
Outcome measures
The primary efficacy measure was the 11-point NRS in the daily patient diary, ranging from 0 (no pain) to 10 (pain as bad as you can imagine). The mean scores of the daily NRS recorded in the diaries were calculated by week, and they were used for the analyses. The NRS measured the severity of pain over the previous 24 hours (ie, “24-hour NRS average pain score”). Change in the weekly mean of the 24-hour NRS average pain score (ie, “average pain”) was the primary endpoint, and noninferiority of duloxetine compared with pregabalin was assessed with the change in average pain using the NRS from baseline to week 12.
Secondary measures derived from the pain diaries were worst pain and night pain ratings. Other secondary measures included the BPI-SF that measures the severity of pain (worst pain, least pain, average pain, and pain right now) and the interference of pain on function (general activity, mood, walking ability, normal work, relations with other people, sleep, and enjoyment of life), and were recorded at baseline and weeks 2, 4, 8, and 12. Neuropathic Pain Symptom Inventory (NPSI)21 that assessed five different dimensions of neuropathic pain (burning spontaneous pain, pressing spontaneous pain, paroxysmal pain, evoked pain, and paresthesia/dysesthesia) was recorded at baseline and week 12. The EuroQol 5-Dimension (EQ-5D)22 index score that assessed the patient’s health utility (mobility, self-care, usual activities, pain and discomfort, and depression/anxiety) was recorded and a quality of life VAS score was also measured at baseline and week 12. The Clinical Global Impression of Improvement (CGI-I)23 and Patient Global Impression of Improvement (PGI-I)23 were recorded at weeks 2, 4, 8 and 12, and reflected the change from baseline. The Beck Depression Inventory-II,24which indicates the presence and degree of depressive symptoms, was recorded at baseline and weeks 4, 8, and 12. Response to treatment was defined by a 30% and 50% reduction in average pain measured using the NRS at endpoint. Overall, the outcome measures used in this study were similar to those used in previous Phase III clinical trials of duloxetine and pregabalin.25,26
Safety measures included frequencies of treatment-emergent adverse drug reactions (TEADRs), serious adverse events (SAEs), and laboratory measurements. The TEADRs were determined by study investigators and SAEs were defined by the protocol.
Statistical analyses
Sample size was calculated to have enough power to confirm the noninferiority of duloxetine to pregabalin, based on the primary endpoint. Assuming that the treatment difference was 0.1 (ie, duloxetine was superior to pregabalin by 0.1) and the common SD was 1.82 with a noninferiority margin of 0.51, 141 patients per group would have had a statistical power of 80% to confirm noninferiority with a 1-sided significance level of 0.025.
The noninferiority margin was estimated based on a meta-analysis of randomized placebo-controlled trials of pregabalin in Japan and overseas. The noninferiority margin was set using half of the estimated mean difference, 0.51.
All randomized patients receiving at least 1 dose of the study drug were included in the analysis. For each analysis, the patients with no available data of the item were excluded. Analyses based on the per-protocol set were also performed to examine the robustness of the primary efficacy analysis. For this analysis, patients who had major protocol deviations, such as noncompliance of study drug or use of prohibited concomitant medication, were excluded.
A mixed model repeated measures (MMRM) analysis was used for the assessment of the primary endpoint. The MMRM model included the random effect of patient and fixed categorical effects of treatment, duration of DPNP (<2 years, ≥2 years), week, and treatment-by-week interaction, as well as the continuous fixed covariates of baseline value. A 95% confidence interval (CI) for the treatment difference (duloxetine–pregabalin) at week 12 was estimated, and if the upper bound of the 95% CI did not exceed the predefined noninferiority margin (0.51), it could be concluded that duloxetine was not inferior to pregabalin.
For the secondary repeated efficacy endpoints, a similar MMRM model was used, but without the baseline value in the model for CGI-I and PGI-I, with the week being replaced by visit except for items from the diary. For the change from baseline to endpoint in NPSI, EQ-5D, and laboratory measurements, analysis of covariance was used. The model included treatment, duration of DPNP and baseline value for efficacy endpoints, and treatment and baseline value for laboratory measurements.
All statistical analyses were conducted using SAS 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
Patient disposition and baseline characteristics
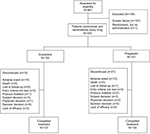
In this study that was conducted from April 2015 to May 2017, a total of 303 patients were randomly assigned to duloxetine and pregabalin treatment groups (Figure 2). Of the 303 patients who received the treatment, 152 patients received at least one dose of duloxetine (40–60 mg/day) and 151 patients received at least one dose of pregabalin (300–600 mg/day). During the treatment period, 15 of 152 patients (9.9%) in the duloxetine group and 21 of 151 patients (13.9%) in the pregabalin group discontinued the study. The reasons for discontinuation were balanced between groups (Figure 2), with the most common reason being discontinuation due to adverse events.
  | Figure 2 Patient disposition. |
Patient demographics and baseline characteristics were generally well balanced, with no major differences between treatment groups (Table 1). Of the 303 patients, 59 (19.5%) had received a previous medication, most commonly epalrestat (9.9%). In the pregabalin group, 2.0% (3/151) of patients had previously taken an investigational drug, compared with 7.9% (12/152) of patients in the duloxetine group (P=0.031). No statistically significant differences between treatment groups were observed for any other previous medication or for any of the baseline pain severity measures.
Extent of exposure
The number of patients receiving each dose in each treatment group for week 4 and week 8, and the maximum individual dose received are shown in Table 2. In the duloxetine group, at week 4, 83 patients (58.5%) were receiving the 40 mg/day dose and 59 patients (41.5%) had increased the dose to 60 mg/day. At week 8, the proportion of patients receiving each dose remained similar, with 76 patients (54.3%) receiving the 40 mg/day dose and 64 patients (45.7%) receiving the 60 mg/day dose.
  | Table 2 Dose volume |
In the pregabalin group, at week 4, 90 patients (62.5%) were receiving the 300 mg/day dose and 54 patients (37.5%) had increased the dose to 450 mg/day. At week 8, 78 patients (57.4%) were receiving the 300 mg/day dose, 37 patients (27.2%) were receiving the 450 mg/day dose and 21 patients (15.4%) were receiving the 600 mg/day dose.
Efficacy
Primary efficacy measure
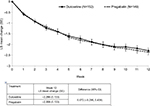
In the analysis of the 24-hour NRS average pain scores using an 11-point NRS in the daily patient diary by MMRM, the least-squares mean (standard error [SE]) change in 24-hour NRS average pain at the end of the study period (week 12) was –2.286 (0.133) in the duloxetine group and –2.358 (0.133) in the pregabalin group (Figure 3). The treatment difference was 0.072 (95% CI: –0.295, 0.439), and the upper bound of the 95% CI (0.439) did not exceed the predefined noninferiority margin (0.51), demonstrating the noninferiority of duloxetine compared with pregabalin. In the per-protocol set, the noninferiority of duloxetine compared to pregabalin was also demonstrated to show the robustness of the primary efficacy analysis (data not shown).
Secondary efficacy measures
The mean changes from baseline to week 12, for the secondary outcome measures, are shown in Table 3. For measures, such as night pain and worst pain scores, BPI-SF, CGI-I, PGI-I, and NPSI, both the duloxetine and pregabalin treatment groups showed an improvement from baseline, with no significant between-group difference. Similarly, both treatment groups showed improvement from baseline on the health outcome measures, and no significant between-group differences were observed in the EQ-5D index and VAS scores.
Proportions of patients with ≥30% or ≥50% reduction in 24-hour NRS average pain score
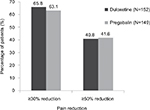
When treatment groups were compared with respect to the proportions of patients achieving a ≥30% or ≥50% reduction on the 24-hour NRS average pain score, in the duloxetine group, 65.8% (100/152) of patients achieved a ≥30% reduction compared to 63.1% (94/149) of patients in the pregabalin group (Figure 4). In addition, in the duloxetine group, 40.8% (62/152) of patients achieved a ≥50% reduction compared to 41.6% (62/149) of patients in the pregabalin group.
  | Figure 4 Percentage of patients with ≥30% or ≥50% reduction in average pain at endpoint. |
Safety
Treatment-emergent adverse drug reactions
Patients generally tolerated the treatment well, with no new safety concerns or events reported as related to duloxetine. The TEADRs reported in ≥3 patients in any treatment group are shown in Table 4. More patients in the pregabalin group (54 [35.8%]) than in the duloxetine group (45 [29.6%]) reported TEADRs. In the duloxetine group, the most common TEADRs reported in three or more patients were somnolence (18 [11.8%]), nausea (11 [7.2%]), dizziness (6 [3.9%]), vomiting (3 [2.0%]), stomatitis (3 [2.0%]), headache (3 [2.0%]), and decreased appetite (3 [2.0%]). In the pregabalin group, the most common TEADRs reported in three or more patients were somnolence (22 [14.6%]), dizziness (16 [10.6%]), nausea (5 [3.3%]), feeling abnormal (4 [2.6%]), edema peripheral (3 [2.0%]), and vertigo (3 [2.0%]).
  | Table 4 Frequency of treatment-emergent adverse drug reactions that occurred in at least three patients Abbreviation: TEADR, treatment-emergent adverse drug reaction. |
Adverse events leading to discontinuation
The incidence of adverse events leading to discontinuation was low overall (duloxetine: 10 [6.6%], pregabalin: 12 [7.9%]), and similar between the treatment groups (P=0.665). The most common adverse events leading to discontinuation were nervous system disorders (duloxetine: 1 [0.7%], pregabalin: 5 [3.3%]) and gastrointestinal system disorders (duloxetine: 1 [0.7%], pregabalin: 3 [2.0%]).
SAEs and deaths
SAEs were reported in fewer patients in the duloxetine group (1 [0.7%]) compared with the pregabalin group (6 [4.0%]). In the duloxetine group, a vascular disorder (shock, 1 [0.7%]) was reported as an SAE, and in the pregabalin group, most SAEs were classified as gastrointestinal disorders (3 [2.0%]). All SAEs were reported in one patient each and were not considered possibly related to study drug, except for congestive cardiac failure (1 [0.7%]) and myocardial infarction (1 [0.7%]) (accompanied by a clinically significant abnormal electrocardiogram) which both occurred in the same patient in the pregabalin group. Both of these events were considered possibly related to study drug, and the myocardial infarction led to discontinuation. No deaths were reported during this study.
Abnormal laboratory values
The analyses of treatment-emergent abnormal laboratory measures demonstrated that significantly more duloxetine-treated patients experienced abnormally low uric acid compared with pregabalin-treated patients (10 [6.7%] vs 2 [1.4%], respectively; P=0.035). No other laboratory measures showed statistically significant differences between treatment groups.
Changes in HbA1c and glucose from baseline
The changes in HbA1c and glucose from baseline to endpoint are shown in Table 5. The change in HbA1c (duloxetine: 0.17%, pregabalin: 0.14%) was statistically significant in both treatment groups, as was the change in non-fasting glucose (duloxetine: 1.30 mmol/L, pregabalin: 0.85 mmol/L). The change in fasting glucose was statistically significant in the duloxetine group (0.81 mmol/L), but not in the pregabalin group (0.17 mmol/L). Despite these changes, glucose levels remained within the normal range.
Discussion
This 12-week, postmarketing, Phase IV, multicenter, randomized, double-blind, parallel-group, flexible-dose study demonstrated the noninferiority of duloxetine compared with pregabalin for pain relief in patients with DPNP, as measured by the change from baseline in the 24-hour NRS average pain score.
The noninferiority of duloxetine was supported by most secondary efficacy measures including night pain and worst pain scores, response rate based on change in 24-hour NRS average pain scores, BPI-SF, CGI-I, PGI-I, and NPSI. Similarly, for health outcome measures, both duloxetine and pregabalin showed improvement from baseline for the EQ-5D index and VAS scores, but with no significant difference between groups. The safety analyses of TEADRs and clinical laboratory tests showed acceptable tolerability and safety profiles for duloxetine and pregabalin. Generally, no new or unexpected safety observations were seen with regard to duloxetine treatment. The TEADRs reported in both treatment groups were consistent with the respective drug profiles. The incidence of adverse events leading to discontinuation was generally consistent with those most frequently reported in each treatment group and respective drug profile.
The efficacy and safety of both duloxetine and pregabalin in Japanese patients with DPNP have been confirmed previously by Phase III studies.25,26 However, the study presented here is the first to demonstrate the noninferiority of duloxetine compared to pregabalin in Japanese patients with DPNP. These results support the National Institute for Health and Care Excellence10,11 and the Japan Diabetes Society9 guidelines, which recommend duloxetine and pregabalin as first-line pharmaceutical treatments for DPNP. Differences in the safety profiles of duloxetine and pregabalin give physicians more treatment options for patients with DPNP.
Our results are similar to those of a previous Phase III study in patients with DPNP in Japan, where duloxetine showed superiority over placebo in reducing pain scores and improving health outcome measures.25 In that study, duloxetine (40–60 mg/day) demonstrated significantly greater improvement in average pain scores over 12 weeks compared with placebo.25 Similarly, a previous 14-week, randomized, placebo-controlled study with pregabalin (300–600 mg/day) also demonstrated efficacy in reducing pain in Japanese patients with DPNP.26 In a 12-week, open-label study in patients with an inadequate response to gabapentin, duloxetine was noninferior to pregabalin for the treatment of pain in patients with DPNP.27 Overall, the findings from these studies support the results from our study.
The COMBO-DN study examined whether a combination of duloxetine and pregabalin would be superior to increasing each drug to its maximum dose in patients with DPNP who were not responding to standard doses.15 Patients received duloxetine 60 mg/day or pregabalin 300 mg/day for 8 weeks, followed by 8 weeks of treatment with duloxetine 120 mg/day, pregabalin 600 mg/day, or a combination of duloxetine 60 mg/day and pregabalin 300 mg/day. This study found that there were no significant differences between combination and high-dose monotherapy for the primary outcome measure Brief Pain Inventory Modified Short Form, although most secondary endpoints favored combination therapy. Although our study did not use combination therapy, future examination of combination therapy in Japanese patients would be useful.
Unlike in our study, exploratory analyses of the COMBO-DN study found that improvement in pain relief during the first 8 weeks was greater with duloxetine 60 mg/day than with pregabalin 300 mg/day.15 In addition, in a network meta-analysis, serotonin and norepinephrine reuptake inhibitors were more effective than anticonvulsants in the treatment of DPNP.18 Differences in the doses and study designs may account for the different results between studies.
As in the study reported here, a previous 12-week open-label study has also shown that duloxetine and pregabalin were generally safe and tolerable in the treatment of DPNP.28 However, discontinuation due to adverse events was significantly greater in the duloxetine group (19.6%) compared with the pregabalin group (10.4%),28 whereas in our study, discontinuation due to adverse events was lower and similar between treatment groups (duloxetine: 6.6%, pregabalin: 7.9%). In our study, HbA1c significantly increased from baseline in both treatment groups, with no difference between groups, similar to a previous study comparing duloxetine with pregabalin and with duloxetine plus gabapentin in patients with DPNP.28 Both duloxetine and pregabalin did not worsen diabetes, as measured by changes in glucose and HbA1c in patients with DPNP.
There are limitations in the interpretation of results from this study. Due to the inclusion and exclusion criteria that are inherent to enrolling patients in clinical trials, the patients in this study may not completely represent the general population of patients with DPNP or patients with other forms of diabetic neuropathy, such as radiculoplexus neuropathy or treatment-induced neuropathy. Results from clinical trials are not always indicative of real-world results, as patients vary in their characteristics, levels of adherence, and comorbidities that may affect their response to duloxetine. For example, most of the patients in this study were Japanese, had moderate HbA1c levels, and were relatively young. Potential differences due to gender, ethnicity or cultural considerations may be underrepresented in this sample. In addition, the study period was for a duration of 12 weeks, and consequently this study did not assess long-term effectiveness or safety.
Conclusion
Our study demonstrated the noninferiority of duloxetine compared with pregabalin, as measured by change from baseline in the 24-hour NRS average pain score in patients with DPNP. Overall, duloxetine and pregabalin were well tolerated and the safety profiles were consistent with previously reported results.
Acknowledgments
The authors thank all study participants. Medical writing assistance was provided by Deborah D’Souza, Syneos Health and editing assistance was provided by Rebecca Lew, PhD, CMPP, of ProScribe – Envision Pharma Group, funded by Eli Lilly Japan K.K. and Shionogi & Co. Ltd. The study NCT02417935 was conducted by Eli Lilly Japan K.K. and was funded by Eli Lilly Japan K.K. and Shionogi & Co. Ltd., manufacturer and licensee of Cymbalta® in Japan.
Disclosure
HE, SF, MT and AY are employees of Eli Lilly Japan K.K. AN is an employee of Shionogi & Co. Ltd., and MF, MI and TT are employees and minor stockholders of Shionogi & Co. Ltd. LA is an employee of Eli Lilly Turkey. SF and LA hold shares in Eli Lilly and Company. HY reports speaking fees from Nippon Boehringer Ingelheim Co. Ltd., Eli Lilly Japan K.K., Shionogi & Co. Ltd., Sanwa Kagaku Kenkyusyo Co. Ltd., and Sumitomo Dainippon Pharma Co. Ltd., and consulting fees from Shionogi & Co. Ltd. The authors report no other conflicts of interest in this work.
References
World Health Organization (WHO). Global Report on Diabetes. Geneva: WHO Press; 2016. Available from: http://apps.who.int/iris/bitstream/10665/204871/1/9789241565257_eng.pdf. Accessed January, 18, 2018. | ||
Tesfaye S, Selvarajah D. Advances in the epidemiology, pathogenesis and management of diabetic peripheral neuropathy. Diabetes Metab Res Rev. 2012;28(Suppl 1):8–14. | ||
Tesfaye S. Recent advances in the management of diabetic distal symmetrical polyneuropathy. J Diabetes Investig. 2011;2(1):33–42. | ||
Vileikyte L, Rubin RR, Leventhal H. Psychological aspects of diabetic neuropathic foot complications: an overview. Diabetes Metab Res Rev. 2004;20(Suppl 1):S13–S18. | ||
Abbott CA, Malik RA, van Ross ER, Kulkarni J, Boulton AJ. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes Care. 2011;34(10):2220–2224. | ||
Tesfaye S, Boulton AJ, Dickenson AH. Mechanisms and management of diabetic painful distal symmetrical polyneuropathy. Diabetes Care. 2013;36(9):2456–2465. | ||
Tsuji M, Yasuda T, Kaneto H, et al. Painful diabetic neuropathy in Japanese diabetic patients is common but underrecognized. Pain Res Treat. 2013;2013:318352. | ||
Yokoyama H, Oishi M, Takamura H, et al. Large-scale survey of rates of achieving targets for blood glucose, blood pressure, and lipids and prevalence of complications in type 2 diabetes (JDDM 40). BMJ Open Diabetes Res Care. 2016;4(1):e000294. | ||
The Japan Diabetes Society (JDS). 9. Treatment of Diabetic Neuropathy. Evidence-based practice guideline for the treatment for diabetes in Japan. 2013. Available from: http://www.jds.or.jp/modules/en/index.php?content_id=44. Accessed January 18, 2018. | ||
NICE National Institute for Health and Care Excellence. Neuropathic pain – pharmacological management. 2013. Available from: https://www.nice.org.uk/guidance/cg173/evidence/full-guideline-pdf-4840898221. Accessed January 18, 2018. | ||
Pop-Busui R, Boulton AJ, Feldman EL, et al. Diabetic Neuropathy: A Position Statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136–154. | ||
Kinoshita J, Takahashi Y, Watabe AM, Utsunomiya K, Kato F. Impaired noradrenaline homeostasis in rats with painful diabetic neuropathy as a target of duloxetine analgesia. Mol Pain. 2013;9:59. | ||
Verma V, Singh N, Singh Jaggi A, Jaggi AS. Pregabalin in neuropathic pain: evidences and possible mechanisms. Curr Neuropharmacol. 2014;12(1):44–56. | ||
Stahl SM, Porreca F, Taylor CP, Cheung R, Thorpe AJ, Clair A. The diverse therapeutic actions of pregabalin: is a single mechanism responsible for several pharmacological activities? Trends Pharmacol Sci. 2013;34(6):332–339. | ||
Tesfaye S, Wilhelm S, Lledo A, et al. Duloxetine and pregabalin: high-dose monotherapy or their combination? The “COMBO-DN study” – a multinational, randomized, double-blind, parallel-group study in patients with diabetic peripheral neuropathic pain. Pain. 2013;154(12):2616–2625. | ||
Robertson O, Robinson SJ, Stephens R. Swearing as a response to pain: a cross-cultural comparison of British and Japanese participants. Scand J Pain. 2017;17:267–272. | ||
Griebeler ML, Morey-Vargas OL, Brito JP, et al. Pharmacologic interventions for painful diabetic neuropathy: an umbrella systematic review and comparative effectiveness network meta-analysis. Ann Intern Med. 2014;161(9):639–649. | ||
Yasuda H. Pharmacological interventions for painful diabetic neuropathy: comparative analysis using network meta-analysis. J Diabetes Investig. 2015;6(6):620–622. | ||
Yasuda H, Sanada M, Kitada K, et al. Rationale and usefulness of newly devised abbreviated diagnostic criteria and staging for diabetic polyneuropathy. Diabetes Res Clin Pract. 2007;77(Suppl 1):S178–S183. | ||
Uki J, Mendoza T, Cleeland CS, Nakamura Y, Takeda F. A brief cancer pain assessment tool in Japanese: the utility of the Japanese Brief Pain Inventory – BPI-J. J Pain Symptom Manage. 1998;16(6):364–373. | ||
Bouhassira D, Attal N, Fermanian J, et al. Development and validation of the Neuropathic Pain Symptom Inventory. Pain. 2004;108(3):248–257. | ||
EuroQol Group. EuroQol – a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. | ||
Guy W. ECDEU Assessment Manual for Psychopharmacology, Revised. US Department of Health, Education, and Welfare publication (ADM). Rockville, MD: National Institute of Mental Health; 1976:76–338. | ||
Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. | ||
Yasuda H, Hotta N, Nakao K, Kasuga M, Kashiwagi A, Kawamori R. Superiority of duloxetine to placebo in improving diabetic neuropathic pain: results of a randomized controlled trial in Japan. J Diabetes Investig. 2011;2(2):132–139. | ||
Satoh J, Yagihashi S, Baba M, et al. Efficacy and safety of pregabalin for treating neuropathic pain associated with diabetic peripheral neuropathy: a 14 week, randomized, double-blind, placebo-controlled trial. Diabet Med. 2011;28(1):109–116. | ||
Tanenberg RJ, Irving GA, Risser RC, et al. Duloxetine, pregabalin, and duloxetine plus gabapentin for diabetic peripheral neuropathic pain management in patients with inadequate pain response to gabapentin: an open-label, randomized, noninferiority comparison. Mayo Clin Proc. 2011;86(7):615–626. | ||
Irving G, Tanenberg RJ, Raskin J, Risser RC, Malcolm S. Comparative safety and tolerability of duloxetine vs. pregabalin vs. duloxetine plus gabapentin in patients with diabetic peripheral neuropathic pain. Int J Clin Pract. 2014;68(9):1130–1140. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.