Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 12
Dulaglutide as an Add-on to Insulin in Type 2 Diabetes; Clinical Efficacy and Parameters Affecting the Response in Real-World Practice
Authors Lee J, Cho YK , Kim HS, Jung CH, Park JY, Lee WJ
Received 16 September 2019
Accepted for publication 11 December 2019
Published 27 December 2019 Volume 2019:12 Pages 2745—2753
DOI https://doi.org/10.2147/DMSO.S231272
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Antonio Brunetti
Jiwoo Lee, Yun Kyung Cho, Hwi Seung Kim, Chang Hee Jung, Joong-Yeol Park, Woo Je Lee
Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea
Correspondence: Woo Je Lee
Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, 88, Olympic-Ro 43-Gil, Songpa-Gu, Seoul 05505, Republic of Korea
Tel +82-2-3010-5882
Fax +82-2-2045-4034
Email [email protected]
Purpose: For patients with type 2 diabetes (T2DM) who remain above their glycemic target on insulin therapy, a combination of insulin and a glucagon-like peptide 1 receptor agonist has been recommended. However, few studies have been conducted to determine the clinical efficacy and parameters affecting the response to this combination in a real-world setting. This study aimed to investigate the clinical efficacy and parameters affecting the glycemic response to dulaglutide as an add-on to insulin therapy for T2DM in a real-world clinical setting.
Patients and methods: A retrospective study was performed in 120 patients with T2DM who had initiated dulaglutide as an add-on to insulin therapy between January 2017 and December 2018. After 6 months of treatment, the change in glycated hemoglobin (HbA1c) was evaluated. Multiple linear regression analysis was used to determine the parameters affecting the therapeutic response to dulaglutide.
Results: The mean age of the patients was 55.1 years and 57.5% were male. The mean baseline HbA1c, body mass index, and duration of diabetes were 9.1%, 27.5 kg/m2, and 17.2 years, respectively. The change in HbA1c between baseline and 6 months was −0.97% (95% confidence interval [CI]: −1.28 to −0.66%, P<0.001), the change in body weight was −2.05 kg (95% CI: −2.93 to −1.17 kg, P<0.001), and the change in total daily insulin dose was −11.67 IU (95% CI: −14.55 to −8.78 IU, P<0.001). In multiple linear regression analysis, higher baseline HbA1c was associated with a greater reduction in HbA1c. The most frequent adverse events were gastrointestinal symptoms, but these were well-tolerated.
Conclusion: Dulaglutide treatment in combination with insulin resulted in a significant improvement in HbA1c and body weight over a 6-month period in a real-world clinical setting. Higher baseline HbA1c was associated with a good clinical response.
Keywords: dulaglutide, glucagon-like peptide-1 receptor agonist, insulin therapy, glycemic control, diabetes mellitus
Introduction
Patients with type 2 diabetes (T2DM) who do not achieve their glycemic goal by taking an oral anti-diabetic drug (OAD) alone eventually require additional therapy with insulin or a glucagon-like peptide-1 receptor agonist (GLP-1 RA). The addition of insulin to OADs has been the conventional strategy for the initiation of injectable therapy. In the first instance, basal insulin administration is usually commenced and then, if the patient’s HbA1c concentration remains above their target, short-acting insulin or an alternative premixed insulin administration regimen is instituted.1 However, many patients that commence insulin or intensify their insulin treatment regimen do not reach their glycemic target, and are more likely to experience hypoglycemic events and weight gain.2
GLP-1 RAs are appropriate agents for the management of T2DM, given their potent effects to reduce blood glucose and body weight, and the low risk of hypoglycemia associated with their use.3 Therefore, there has been interest in using GLP-1 RAs as add-ons to insulin therapy because of their complementary effects on glycemic control, body weight, and hypoglycemic risk.4,5
Dulaglutide is a GLP-1 RA that is approved for once-weekly administration for the treatment of T2DM in many countries.6 The efficacy and safety of the addition of dulaglutide to insulin therapy have been assessed in several component studies of the Assessment of Weekly Administration of LY2189265 in Diabetes (AWARD) trial.7–9 However, few studies have investigated the efficacy of dulaglutide combined with insulin in a real-world setting. Moreover, studies that have aimed to determine the clinical factors associated with the efficacy of the combination of dulaglutide and insulin therapy have been few in number. Therefore, we aimed to determine the efficacy and clinical parameters affecting the glucose-lowering effect of dulaglutide as an add-on to insulin therapy in a real-world clinical setting.
Materials and Methods
Study Population
We retrospectively reviewed the data associated with patients who initiated dulaglutide treatment as an add-on to insulin between January 2017 and December 2018 at Asan Medical Center (Seoul, Republic of Korea). Of these, patients who continued combined dulaglutide and insulin therapy for at least 6 months and self-reported high levels of compliance were included. Initially, 166 patients were identified. Of these, patients who were lost to follow-up (n=13), stopped taking dulaglutide due to adverse events within the first month (n=14), used short-acting GLP-1 RAs prior to dulaglutide (n=9), self-reported low levels of compliance with therapy (n=6), were hospitalized for example, for diabetic foot infection treatment, kidney transplantation, or pancreaticoduodenectomy) (n=3), or stopped administering insulin (n=1) were excluded. Therefore, 120 patients who recorded regular dulaglutide and insulin use for at least 6 months were included in the analysis.
The present study was conducted in accordance with the principles of the Declaration of Helsinki and Korean Good Clinical Practice, and was approved by the Institutional Review Board of Asan Medical Center (IRB No. 2018-1050).
Assessment of Treatment Efficacy
Baseline data, including age, sex, body weight, body mass index (BMI), duration of diabetes, total daily insulin dose, presence of hypertension or dyslipidemia, and use of medication, were collected through a review of electronic medical records. Laboratory measurements were also performed at baseline: HbA1c, fasting plasma glucose (FPG), postprandial 2 hr plasma glucose (PP2), fasting C-peptide, total cholesterol (TC), low-density lipoprotein-cholesterol (LDL-C), high-density lipoprotein-cholesterol (HDL-C), triglycerides (TG), creatinine, glomerular filtration rate (GFR), and liver enzymes. After 3 months (± 4 weeks) and 6 months (± 4 weeks) of dulaglutide treatment, all these measurements were repeated.
The primary objective of the study was to evaluate the efficacy of once-weekly dulaglutide as an add-on to insulin over 6 months by assessing the change in HbA1c from baseline. The secondary objectives of the study were to evaluate the changes in FPG, body weight, and total daily insulin dose between baseline and 6 months. These parameters were also evaluated after 3 months of treatment.
Assessment of Clinical Parameters and Adverse Events
Parameters affecting the glycemic response to dulaglutide treatment were assessed using multiple linear regression analysis. For the subgroup analysis, the changes in HbA1c in groups of patients were analyzed according to age, baseline HbA1c, BMI, and duration of diabetes.
We also reviewed the adverse events recorded in the medical records of each patient who underwent dulaglutide and insulin treatment.
Statistical Analysis
Continuous variables are expressed as the mean ± standard deviation (SD) and categorical variables are expressed as percentages. Student’s t-tests were used to compare the means of continuous variables, and the χ2 test was used for categorical variables. The paired t-test was used to compare the changes in HbA1c, FPG, body weight, and total daily insulin dose between baseline and 6 months. A linear mixed model was used to analyze repeated measurements. Linear regression analysis was conducted to assess the relationships between clinical parameters and the therapeutic efficacy of dulaglutide. All statistical analyses were performed using SPSS software version 21.0 for Windows (IBM, Inc., Armonk, NY). P<0.05 was considered to represent statistical significance.
Results
Baseline Characteristics
The baseline characteristics of the participants are shown in Table 1. Their mean age was 55.1 ± 11.0 years and 57.5% of them (n=69) were male. The mean baseline HbA1c, BMI, and duration of diabetes were 9.1 ± 1.7%, 27.5 ± 3.6 kg/m2, and 17.2 ± 8.3 years, respectively. The mean total daily insulin dose at baseline was 47.8 ± 25.6 international units (IU). The patients were categorized into two subgroups according to their change in HbA1c between baseline and 6 months (≥ 1% vs < 1%). Those who showed a reduction in HbA1c ≥ 1% were defined as “responders”. When the baseline characteristics of responders and non-responders were compared, the responders had significantly higher HbA1c and FPG.
 |
Table 1 Baseline Characteristics of the Study Participants, Classified According to Their Change in HbA1c |
Before initiating dulaglutide therapy, most of the patients (85.8%) had been treated with a combination of insulin and OADs. In this subgroup, 40.0% of the patients had been treated with basal insulin plus OADs (n=48), and 24.2% had been treated with both basal and prandial insulin, plus OADs (n=29). After the initiation of dulaglutide, 50.8% (n = 61) of the patients were taking insulin plus metformin, and 33.3% (n = 40) were taking insulin, metformin, and sulfonylurea (Supplementary Tables 1 and 2).
Efficacy of Dulaglutide as an Add-on to Insulin Therapy
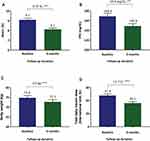
After 6 months of taking dulaglutide, HbA1c, FPG, body weight, and total daily insulin dose had all significantly decreased. At this time point, the change in HbA1c was −0.97% (95% confidence interval [CI]: −1.28 to −0.66%, P < 0.001; Figure 1A), and the change in FPG was −19.88 mg/dL (95% CI: −32.82 to −6.95 mg/dL, P = 0.003; Figure 1B). The change in body weight was −2.05 kg (95% CI: −2.93 to −1.17 kg, P < 0.001; Figure 1C), and the change in total daily insulin dose was −11.67 IU after 6 months of treatment (95% CI: −14.55 to −8.78 IU, P < 0.001; Figure 1D). Of the patients, 23.3% achieved an HbA1c < 7% during this period (n = 28).
There were also significant changes in HbA1c, body weight, and total daily insulin dose between baseline and 3 months (all P < 0.001), but not in FPG (P = 0.071) (Supplementary Figure 1).
Clinical Parameters Affecting the Efficacy of Add-on Dulaglutide
To identify the parameters that predict the glucose-lowering effect of dulaglutide, univariate and multiple linear regression analysis of the reduction in HbA1c was performed. Univariate regression analysis showed that the reduction in HbA1c between baseline and 6 months was associated with higher baseline HbA1c and FPG (Table 2). Similarly, after multiple linear regression analysis, involving adjustment for age, sex, baseline HbA1c, baseline body weight, and the changes in total daily insulin dose, higher baseline HbA1c significantly affected the reduction in HbA1c in patients treated with add-on dulaglutide for 6 months (Table 3). However, age, sex, body weight, and the changes in total daily insulin dose were not associated with the reduction in HbA1c.
 |
Table 2 Univariate Linear Regression Analysis of the Reduction in HbA1c |
 |
Table 3 Multiple Linear Regression Analysis of the Reduction in HbA1c |
Subgroup Analysis of the Efficacy of Dulaglutide as an Add-on to Insulin Therapy
Patients were categorized into the following subgroups: age < 65 years (n = 101) or ≥ 65 years (n = 19); HbA1c < 10.0% (n = 79) or ≥ 10.0% (n = 41); BMI ≤ 25 kg/m2 (n = 34) or > 25 kg/m2 (n = 86); and DM duration ≤ 15 years (n = 50) or > 15 years (n = 70). HbA1c significantly decreased in the subgroup with baseline HbA1c ≥ 10.0%, whereas little reduction in HbA1c was identified in patients with baseline HbA1c < 10.0% (2.0% vs 0.5%, P < 0.001). In the BMI subgroups of ≤ 25 kg/m2 and > 25 kg/m2, HbA1c decreased by 0.5% and 1.1%, respectively (P = 0.088). However, when the patients were categorized according to age or DM duration, the reductions in HbA1c did not differ between the subgroups (Figure 2).
 |
Figure 2 Change in HbA1c between baseline and 6 months in each subgroup of participants. |
Adverse Events
The incidences of specific adverse events are shown in Table 4. Adverse events were reported in 36 (31.6%) and 20 patients (16.7%) after 3 and 6 months of dual treatment, respectively. The most common adverse events at both time points were gastrointestinal [30 (26.3%) vs 16 (13.3%)], in particular nausea [(24 (21.1%) vs 12 (10.0%)]. There were also other gastrointestinal adverse events, including abdominal pain, dyspepsia, and diarrhea. In addition, 2.6% (n = 3) and 0.8% (n = 1) of patients reported hypoglycemia during the first 3 and 6 months of treatment, but no severe hypoglycemia was reported. Overall, the incidence of adverse events tended to decrease with time.
 |
Table 4 Adverse Events Recorded |
Discussion
In this analysis of real-world data, the addition of once-weekly dulaglutide to insulin treatment for 6 months was effective in reducing HbA1c, as well as body weight and total daily insulin dose. Although the combination of dulaglutide and insulin was associated with a significant improvement in HbA1c, irrespective of age, baseline BMI, and duration of diabetes, higher baseline HbA1c did affect the glucose-lowering effect of add-on dulaglutide.
The changes in HbA1c, FPG, body weight, and total daily insulin dose after 6 months of dulaglutide as an add-on to insulin treatment were −0.97%, −19.88 mg/dL, −2.05 kg, and −11.67 IU, respectively. The AWARD-9 trial9 compared the effects of adding dulaglutide or placebo with insulin glargine, thereby having a similar design to the present study, because 78.3% of the participants in the present study were administering basal insulin when dulaglutide was added. In the AWARD-9 trial, the mean changes in HbA1c and body weight between baseline and 28 weeks were −1.44% and −2.41 kg, respectively, which are larger than the changes achieved in the present study. This disparity might be explained by real-world factors, such as lower compliance with follow-up and/or medication. In addition, the mean changes in glargine dose was increased in the AWARD-9 trial (13 ± 2 IU), and the decreased changes in total daily insulin dose at 6 months (−11.67 ± 1.46 IU) are also consistent with a smaller reduction in HbA1c than in the clinical trial. Moreover, the percentages of participants achieving an HbA1c < 7% were 23.3% in the present study, but 66.7% in the AWARD-9 trial. However, in the present study, the baseline HbA1c was higher (9.1%) than in the AWARD-9 trial (8.4%), which might explain the lower proportion of patients reaching this HbA1c target in the present study.
Overall, however, the results of the present study are consistent with those of previous clinical trials. The DURATION-7 study10 determined the efficacy of once-weekly exenatide or placebo added to titrated basal insulin in patients with T2DM, and showed a 1% reduction in HbA1c in the exenatide arm after 28 weeks of treatment. The HARMONY-6 trial11 analyzed the efficacy of adding once-weekly albiglutide vs thrice-daily prandial insulin to basal insulin administration. Following 26 weeks of treatment, the change in HbA1c was −0.82% in the albiglutide group. The BEGIN: Victoza Add-On study12 compared the effects of once-daily liraglutide and once-daily insulin aspart on basal insulin, finding a 0.74% reduction in HbA1c in the liraglutide arm. Finally, the SUSTAIN-5 trial13 assessed the efficacy and safety of once-weekly semaglutide in addition to basal insulin. At the end of 30 weeks of treatment, the reductions of HbA1c associated with the administration of 0.5 or 1.0 mg semaglutide were 1.4% and 1.8%, respectively.
One previous real-world study of the use of other GLP-1 RAs in 108 patients has also been conducted, which compared the addition of once-weekly exenatide or once-daily liraglutide to basal insulin.14 The addition of exenatide reduced HbA1c by 0.7% after 12 months and the change in HbA1c was –0.8% when liraglutide was added to insulin. Furthermore, the addition of exenatide and liraglutide were both associated with 2.3 kg reductions in body weight in this study. These real-world findings were also similar to those of the present study with respect to the reductions in HbA1c and body weight achieved.
We have shown here that patients with a higher baseline HbA1c achieve a larger HbA1c reduction. Consistent results have been reported following subgroup analyses of data from clinical trials and observational studies of dulaglutide administration.15–20 In previous studies of the addition of other long-acting GLP-1 RAs, including liraglutide and albiglutide, to insulin, participants with higher baseline HbA1c values also achieved larger reductions in HbA1c.11,21 In the present analysis, other factors, such as age, sex, baseline body weight, and changes in total daily insulin dose, did not affect the change in HbA1c achieved. These findings were also consistent with those of previous studies, which showed that dulaglutide treatment lowers blood glucose irrespective of age, sex, duration of diabetes, baseline BMI, and estimated GFR.8,15,16,18,22
The adverse events associated with dulaglutide use reported in this study were similar to those previous reported.23,24 The most commonly reported adverse events were gastrointestinal disorders, including nausea and dyspepsia, but these were tolerated over the period of the study. By contrast, only three hypoglycemic events were reported during the 6 months of the study, and no severe hypoglycemic events. It is important to find the optimal balance between glycemic control and the risk of hypoglycemia in patients using high doses of insulin. Accordingly, the high efficacy and low risk of hypoglycemia associated with the use of dulaglutide alongside insulin may mean that this should be the preferred option for patients requiring a high dose of insulin.
To the best of our knowledge, the present study is the first to determine the efficacy of the addition of dulaglutide to insulin therapy in a real-world setting. Only a few previous studies have demonstrated the efficacy of dulaglutide in a real-world setting,17,19,25,26 but these studies mainly compared the efficacy of the combination of dulaglutide and other OADs.
This study had several limitations. First, the 6-month duration of follow-up was relatively short; therefore, we could not investigate the long-term effects of dulaglutide treatment. However, the efficacy of dulaglutide at 26 weeks was similar to that shown at 56 weeks in a previous study.27 Consequently, this duration of follow-up may be sufficient to appropriately assess the efficacy of dulaglutide. Second, various anti-diabetic medications had been used by the participants before the initiation of dulaglutide, and continued to be used alongside dulaglutide, which may have added variation to the efficacy of the add-on dulaglutide. Third, the adverse events recorded were those described in the participants’ medical records, so there may have been some missed events. Lastly, only Korean patients were included in the present study; therefore, the results may not be generalizable to other populations.
Conclusion
Once-weekly dulaglutide treatment as an add-on to insulin therapy improved glycemic control, reduced body weight, reduced total daily insulin dose, and was associated with a low risk of adverse events in a real-world situation. Furthermore, baseline HbA1c level was shown to affect the extent of the reduction in HbA1c.
Abbreviations
AWARD, Assessment of Weekly Administration of LY2189265 in Diabetes; BMI, body mass index; CI, confidence interval; FPG, fasting plasma glucose; GFR, glomerular filtration rate; GLP-1 RA, glucagon-like peptide-1 receptor agonist; HbA1c, glycated hemoglobin; HDL-C, high-density lipoprotein-cholesterol; IU, international units; LDL-C, low-density lipoprotein-cholesterol; OAD, oral anti-diabetic drug; PP2, postprandial 2 hr plasma glucose; SD, standard deviation; T2DM, type 2 diabetes mellitus; TC, total cholesterol; TG, triglyceride.
Disclosure
The authors report no conflicts of interest in this work.
References
1. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S90–S102. doi:10.2337/dc19-S009
2. Yki-Jarvinen H, Kauppinen-Makelin R, Tiikkainen M, et al. Insulin glargine or NPH combined with metformin in type 2 diabetes: the LANMET study. Diabetologia. 2006;49(3):442–451. doi:10.1007/s00125-005-0132-0
3. Eng C, Kramer CK, Zinman B, Retnakaran R. Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta-analysis. Lancet. 2014;384(9961):2228–2234. doi:10.1016/s0140-6736(14)61335-0
4. Maiorino MI, Chiodini P, Bellastella G, Capuano A, Esposito K, Giugliano D. Insulin and glucagon-like peptide 1 receptor agonist combination therapy in type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Care. 2017;40(4):614–624. doi:10.2337/dc16-1957
5. Diamant M, Nauck MA, Shaginian R, et al. Glucagon-like peptide 1 receptor agonist or bolus insulin with optimized basal insulin in type 2 diabetes. Diabetes Care. 2014;37(10):2763–2773. doi:10.2337/dc14-0876
6. Jendle J, Grunberger G, Blevins T, Giorgino F, Hietpas RT, Botros FT. Efficacy and safety of dulaglutide in the treatment of type 2 diabetes: a comprehensive review of the dulaglutide clinical data focusing on the AWARD Phase 3 clinical trial program. Diabetes Metab Res Rev. 2016;32(8):776–790. doi:10.1002/dmrr.2810
7. Blonde L, Jendle J, Gross J, et al. Once-weekly dulaglutide versus bedtime insulin glargine, both in combination with prandial insulin lispro, in patients with type 2 diabetes (AWARD-4): a randomised, open-label, phase 3, non-inferiority study. Lancet. 2015;385(9982):2057–2066. doi:10.1016/s0140-6736(15)60936-9
8. Tuttle KR, Lakshmanan MC, Rayner B, et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 2018;6(8):605–617. doi:10.1016/s2213-8587(18)30104-9
9. Pozzilli P, Norwood P, Jodar E, et al. Placebo-controlled, randomized trial of the addition of once-weekly glucagon-like peptide-1 receptor agonist dulaglutide to titrated daily insulin glargine in patients with type 2 diabetes (AWARD-9). Diabetes Obes Metab. 2017;19(7):1024–1031. doi:10.1111/dom.12937
10. Guja C, Frias JP, Somogyi A, et al. Effect of exenatide QW or placebo, both added to titrated insulin glargine, in uncontrolled type 2 diabetes: the DURATION-7 randomized study. Diabetes Obes Metab. 2018;20(7):1602–1614. doi:10.1111/dom.13266
11. Rosenstock J, Fonseca VA, Gross JL, et al. Advancing basal insulin replacement in type 2 diabetes inadequately controlled with insulin glargine plus oral agents: a comparison of adding albiglutide, a weekly GLP-1 receptor agonist, versus thrice-daily prandial insulin lispro. Diabetes Care. 2014;37(8):2317–2325. doi:10.2337/dc14-0001
12. Mathieu C, Rodbard HW, Cariou B, et al. A comparison of adding liraglutide versus a single daily dose of insulin aspart to insulin degludec in subjects with type 2 diabetes (BEGIN: VICTOZA ADD-ON). Diabetes Obes Metab. 2014;16(7):636–644. doi:10.1111/dom.12262
13. Rodbard HW, Lingvay I, Reed J, et al. Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. J Clin Endocrinol Metab. 2018;103(6):2291–2301. doi:10.1210/jc.2018-00070
14. Stryker MD, Kane MP, Busch RS. A real-world, observational study of weekly exenatide added to basal insulin in patients with type 2 diabetes mellitus (NCT02895672). Endocrinol Diabetes Metab. 2018;1(1):e00004. doi:10.1002/edm2.4
15. Pantalone KM, Patel H, Yu M, Fernandez Lando L. Dulaglutide 1.5 mg as an add-on option for patients uncontrolled on insulin: subgroup analysis by age, duration of diabetes and baseline glycated haemoglobin concentration. Diabetes Obes Metab. 2018;20(6):1461–1469. doi:10.1111/dom.13252
16. Gallwitz B, Dagogo-Jack S, Thieu V, et al. Effect of once-weekly dulaglutide on glycated haemoglobin (HbA1c) and fasting blood glucose in patient subpopulations by gender, duration of diabetes and baseline HbA1c. Diabetes Obes Metab. 2018;20(2):409–418. doi:10.1111/dom.13086
17. Unni S, Wittbrodt E, Ma J, et al. Comparative effectiveness of once-weekly glucagon-like peptide-1 receptor agonists with regard to 6-month glycaemic control and weight outcomes in patients with type 2 diabetes. Diabetes Obes Metab. 2018;20(2):468–473. doi:10.1111/dom.13107
18. Kaneko S, Oura T, Matsui A, Shingaki T, Takeuchi M. Efficacy and safety of subgroup analysis stratified by baseline HbA1c in a Japanese phase 3 study of dulaglutide 0.75 mg compared with insulin glargine in patients with type 2 diabetes. Endocr J. 2017;64(12):1165–1172. doi:10.1507/endocrj.EJ17-0189
19. Yoo JH, Cho YK, Lee J, et al. Clinical efficacy and parameters affecting the response to dulaglutide treatment in patients with type 2 diabetes: a retrospective, real-world data study. Diabetes Ther. 2019;10(4):1453–1463. doi:10.1007/s13300-019-0658-7
20. Wysham C, Guerci B, D’Alessio D, Jia N, Botros FT. Baseline factors associated with glycaemic response to treatment with once-weekly dulaglutide in patients with type 2 diabetes. Diabetes Obes Metab. 2016;18(11):1138–1142. doi:10.1111/dom.12702
21. Ahmann A, Rodbard HW, Rosenstock J, et al. Efficacy and safety of liraglutide versus placebo added to basal insulin analogues (with or without metformin) in patients with type 2 diabetes: a randomized, placebo-controlled trial. Diabetes Obes Metab. 2015;17(11):1056–1064. doi:10.1111/dom.12539
22. Boustani MA, Pittman I, Yu M, Thieu VT, Varnado OJ, Juneja R. Similar efficacy and safety of once-weekly dulaglutide in patients with type 2 diabetes aged ≥65 and <65 years. Diabetes Obes Metab. 2016;18(8):820–828. doi:10.1111/dom.12687
23. Zhang L, Zhang M, Zhang Y, Tong N. Efficacy and safety of dulaglutide in patients with type 2 diabetes: a meta-analysis and systematic review. Sci Rep. 2016;6:18904. doi:10.1038/srep18904
24. Htike ZZ, Zaccardi F, Papamargaritis D, Webb DR, Khunti K, Davies MJ. Efficacy and safety of glucagon-like peptide-1 receptor agonists in type 2 diabetes: a systematic review and mixed-treatment comparison analysis. Diabetes Obes Metab. 2017;19(4):524–536. doi:10.1111/dom.12849
25. Mody R, Huang Q, Yu M, et al. Adherence, persistence, glycaemic control and costs among patients with type 2 diabetes initiating dulaglutide compared with liraglutide or exenatide once weekly at 12-month follow-up in a real-world setting in the United States. Diabetes Obes Metab. 2018. doi:10.1111/dom.13603
26. Mody R, Grabner M, Yu M, et al. Real-world effectiveness, adherence and persistence among patients with type 2 diabetes mellitus initiating dulaglutide treatment. Curr Med Res Opin. 2018;34(6):995–1003. doi:10.1080/03007995.2017.1421146
27. Wysham C, Blevins T, Arakaki R, et al. Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD-1). Diabetes Care. 2014;37(8):2159–2167. doi:10.2337/dc13-2760
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.