Back to Journals » Breast Cancer: Targets and Therapy » Volume 13
Drug-Resistant Breast Cancer: Dwelling the Hippo Pathway to Manage the Treatment
Authors Kaur S, Najm MZ, Khan MA, Akhter N , Shingatgeri VM, Sikenis M , Sadaf , Aloliqi AA
Received 14 October 2021
Accepted for publication 29 November 2021
Published 14 December 2021 Volume 2021:13 Pages 691—700
DOI https://doi.org/10.2147/BCTT.S343329
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pranela Rameshwar
Simran Kaur,1 Mohammad Zeeshan Najm,1 Mohammad Aasif Khan,2 Naseem Akhter,3 Vyas M Shingatgeri,1 Mudra Sikenis,1 Sadaf,4 Abdulaziz A Aloliqi5
1School of Bioscience, Apeejay Stya University, Gurugram, Haryana, India; 2Department of Biosciences, Jamia Millia Islamia University, New Delhi, India; 3Department of Laboratory Medicine, Faculty of Applied Medical Sciences, Al Baha University, Al Baha, Saudi Arabia; 4QuickisCool, Aitele Research LLP, Sitamarhi, Bihar, India; 5Department of Medical Biotechnology, College of Applied Medical Sciences, Qassim University, Buraydah, Saudi Arabia
Correspondence: Sadaf; Abdulaziz A Aloliqi Email [email protected]; [email protected]
Abstract: Breast cancer can be categorized as a commonly occurring cancer among women with a high mortality rate. Due to the repetitive treatment cycles, it has been noted that the patients develop resistance towards the chemotherapeutic drugs and remain unresponsive towards them. Therefore, many researchers are studying various signaling pathways involved in drug resistance for cancer treatment to overcome the obstacle. Hippo signaling is a widely studied pathway involved in tumor progression and controlling cell proliferation. Hence, understanding the aspects of the gene involved Hippo pathway would provide an insight into the mechanism behind the resistance and result in the development of new treatments. Here, we review the Hippo signaling pathway in humans and how the expression of different components leads to the regulation of resistance against some of the common chemo-drugs used in breast cancer treatment. The article will also discuss the chemotherapeutics that became ineffective due to the resistance and the mechanism following the process.
Keywords: drug resistance, breast cancer, chemo drugs, Hippo signaling, YAP, LATS, TAZ
Introduction
Breast cancer can be described as a fatal disease as it contributes to increasing death rates in women worldwide and is also considered the second most common type of cancer next to lung cancer. If untreated or late-diagnosed, breast cancer can easily infilter to adjoining lymph nodes and metastasize to distant significant organs such as the liver, lung, and brain.1 According to the WHO statistics, in 2020, around 2.3 million women were diagnosed with breast cancer, besides 6,85,000 deaths globally.2 Cancer is life-threatening and remains incurable mainly because the symptoms are asymptomatic, leading to the early onset but the late prognosis. By the time cancerous cells were identified, the disease had already progressed in the patient’s body. Lagging prognosis leads to the development of local and distant metastasis depending on the nature of the tumorous growth.
Chemotherapy is one of the foremost and influential ways to treat breast cancer in most countries. However, it is essential to emphasize that while applying chemotherapy to the patient, the main hurdle that prevents the diminishing of cancerous growth is drug resistance. In this case, after a few treatment cycles, the patient starts to develop resistance against the supplied drug, thus limiting the patient’s benefits.3
Through the various studies conducted to modulate the process of drug resistance in cancer therapy, the Hippo pathway came to light. Hippo signaling proves to be an essential aspect in imparting drug resistance, leading to ineffectiveness in treatment. The hippo pathway is a signaling mechanism commonly witnessed in mammals and is involved in cellular processes at the genomic level leading to alteration in gene expression. Thus, the potential of genes in Hippo Signaling can be explored in significant mechanisms leading to malignancy and drug resistance in cancer therapy. Several recent data on various solid tumors have pointed out the alteration in the Hippo pathway, suggesting a possible association in pathogenesis and drug resistance.3–6 For example, in breast cancer, the co-transcriptional factors YAP and TAZ of the hippo pathway are deregulated with increased TAZ and YAP1. The raised expression of YAP1 and TAZ has been linked to Her2+, Luminal A, and Luminal B and triple-negative breast cancer (TNBC).7 Furthermore, the expression of YAP1 was also noticed to be linked with a low survival rate in HER2+ positive breast cancer patients. These potential pieces of evidence suggest significant crosstalk between the hippo pathway and the molecular biology of breast cancer.8,9 So, it is crucial to understand the fundamental mechanism to develop effective chemotherapeutic treatments.10
Hippo Signaling Pathway
The hippo signaling pathway includes the cascade mechanism responsible for controlling the size of the organs by maintaining cell growth and apoptosis in animals and humans.11 This pathway was first identified as a significant governor to regulate the organ’s size and was first discovered in Drosophila melanogaster.12 This led to the discovery of the 4 tumor suppressors which are; Warts (Wts),12,13 Salvador (Sav),3,14 Hippo (Hpo)15–19 and Mob-as-tumor-suppressor (Mats).20 These tumor suppressors work by forming the core kinase cascade, which controls cell proliferation and apoptosis.
Initially, activated Hpo phosphorylates and activates the Wts-Mats complex.17,21 At the same time, Sav, which is also activated when phosphorylated by Hpo, activates the Wts-Mats complex by acting as a protein scaffold.17,18
The downstream target of the activated Wts is Yorkie (Yki) which plays the role of a transcriptional co-activator.22 In the case of Yki, the DNA binding domains are mainly absent. Despite the absence of the DNA binding domains, Yki binds to the Scalloped (Sd), a DNA binding partner, to regulate the transcription of genes.23 Wts is capable of phosphorylating Yki in multiple sites, which can also be viewed as an effective regulatory mechanism to downregulate the expression of Yki. Phosphorylation at multiple sites leads to restricting the nuclear localization and cytoplasmic sequestration of Yki that transcriptionally inactivates the target gene.24–26
Meanwhile, in the absence of the signaling, Yki moves towards the nucleus and interacts with Sd, another transcriptional factor. This results in activating the expression of diverse genes, including cyclin E and diap1 (Figure 1A). The whole process results in increased expression, leading to cell proliferation and a decrease in cell apoptosis expression.19
 |
Figure 1 Hippo pathway gene regulation in (A) Drosophila melanogaster (B) mammals. |
In the case of human beings, the core elements that are involved in the mammalian Hippo pathway includes Serine/threonine kinases: Examples includes Mammalian sterile 20 like kinase1/2 (MST1/2), Large tumor suppressor1/2 (LATS1/2), Salvador Homolog 1 (SAV1), and MOB kinase activator 1A/B (MOB 1A/B).
It is important to note that MOB 1A/B acts as an adapter protein for MST1/2 and LATS1/2.2
In humans, the LATS1/2-MOB1A/B complex is phosphorylated and activated by MST1/2 – SAV1 complex, which results in the phosphorylation and inactivation of the Yes-associated protein (YAP) and co-activator TAZ, which are classified as the orthologs of the Yki in mammals.27 LATS1/2 works by phosphorylating YAP/TAZ at multiple sites and promotes its binding to 14-3-3 protein, leading to cytoplasmic retention. Phosphorylation also results in ubiquitination that directs the process of proteasomal degradation.27–29
The potential of hippo signaling is that the pathway is majorly responsible for controlling cell proliferation, apoptosis, and differentiation.30 If this pathway is altered due to the inactivation of tumor suppressor genes, the YAP-TAZ will move towards the nucleus, and once they reach the nucleus, they bind to the transcriptional factors of the TEAD family (Figure 1B) and activate the expression of genes that are responsible for promoting the cell proliferation and results in disrupted tumor growth and carcinogenesis.31
In conclusion, due to the genetic changes (gene silencing, transcriptional regulations, and mutations), the activity of the tumor suppressors is subdued, which then proceed to hyper activate the YAP/TAZ and develop different types of cancer.
Cancer Progression Due to Hippo Pathway
Hippo Pathway is primarily responsible for mediating cell proliferation, apoptosis, differentiation, and migration (Figure 2).32 In cancer, due to the inactivation of tumor suppressors such as LATS1/2, the pathway is not activated, it results in the hyperactivation of the YAP/TAZ, resulting in the increased expression of CRY61 and BCL2 family, which ultimately suppresses the apoptosis process as they are the factors responsible for promoting cell survival.26,33–35
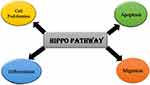 |
Figure 2 Cellular roles of Hippo pathway. |
In breast cancer, the upregulation of the YAP and TAZ (transcriptional co-activators) are mainly responsible for cancer initiation, growth, and metastasis. TAZ plays a significant part in cancer stem cells’ tumor initiation and self-renewal capacity.32 TAZ, in a way, enriches cancer originating stem cells which result in the formation of tumors.36 The luminal cells even start showing the characteristics of the basal cells due to the overexpression and activity of the TAZ oncoprotein, therefore resulting in the progression of basal-like breast cancer.32,36 YAP/TAZ mediates the activity of the other oncogenic components such as LIF and GPER. Increased expression of YAP results in the downregulation of the Leukemia Inhibitory Factor Receptor, which ultimately results in increased metastatic and invasive potential of the cells.37 The hyperactivation of YAP/TAZ results in the upregulation of the G-protein coupled estrogen receptor, which stimulates the tumor growth and movement of the cancerous cells to the surrounding tissues.32 When the Hippo pathway is not activated, the YAP/TAZ complex moves towards the nucleus. It binds to the TEAD transcriptional factors, resulting in the increased expression of many oncogenic factors such as CYR61 and CTGF, leading to progression in breast cancer.34,35,38 The hyperactivation of YAP results in elevated levels of KLF5 proteins, thus promoting cell proliferation and cell survival (Figure 3).39
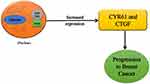 |
Figure 3 Hippo pathway genes in breast cancer progression. |
Treatment Strategies Used in Breast Cancer
Breast cancer belongs to the class of heterogeneous disease(s) and is basically divided into three subtypes depending on how the hormone receptors are expressed, which include estrogen and progesterone, Human epidermal growth factor receptor 2 (HER2), Triple-negative breast cancer.40
In breast cancer, the medicinal therapies involved are mainly chemotherapy, hormonal therapy, targeted therapy, and immunotherapy.
Chemotherapy destroys the cancer cells and shrinks the tumor growth before or after surgery. Drugs mainly used in chemotherapy for breast cancer treatment are listed (Table 1). Further, these drugs can be used in combination depending upon the medical oncologist.
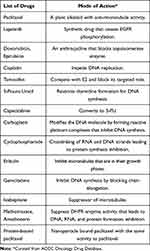 |
Table 1 Commonly Used Chemotherapeutic Drugs for Breast Cancer Treatment |
It is important to note that each tumor grows in a specified environment to help its growth and transmission. The targeted therapy works by targeting the specific components promoting the growth and survival of the cancerous cells, such as the specific gene, protein, or hormone. The identified targets are then treated, which results in the blockage of the growth of tumor cells as their niche is destroyed.
For example, the targeted therapies used to treat HER2-positive breast cancer are Trastuzumab, Pertuzumab, Neratinib, and Ado-trastuzumab emtansine. In addition, the therapies mentioned above are used in conjugation to chemotherapeutics, including taxanes and anthracyclines, to treat triple-negative breast cancer.41
Drug Resistance Mediated by Hippo Signaling
Although cancer largely remains incurable due to various aspects, it is essential to emphasize that chemotherapy is one of the most effective treatments for cancer patients. However, the hurdle that occurs with antibiotics is that drug resistance is also seen in cancer therapy due to genetic and epigenetic mutations and the metabolic mechanism involved in drug inactivation and efflux hampering the patients from the treatment.3 Various studies emphasized that Hippo signaling can be vital in imparting drug resistance and ineffective treatment. Bortezomib, an inhibitor that interacts with the hippo pathway, is being analyzed in the clinical trial Phase III to treat different cancers, including breast cancer.42 In an earlier study, it had been noticed that breast cancer stem-like cells (BCSCs), a specific group of self-renewal cells, are thought to be accountable for drug resistance and cancer spread. Interestingly, when activated, the TAZ and YAP assist in maintaining breast cancer cells with stem cell-like properties.8
Drug resistance is mainly observed due to the upregulation or the downregulation of the components that play an integral part in the hippo signaling pathway (Table 2).
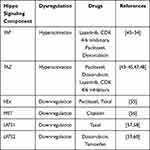 |
Table 2 Hippo Signaling Components and Their Associated Role in Drug-Resistant Breast Cancer |
Paclitaxel
Paclitaxel is the drug administered to treat different types of cancer and is classified as the widely used drug in this domain. The drug is plant-derived and is extracted from the Taxus brecifolia. Paclitaxel works by binding to the β-tubulin, thus maintaining the spindle formation at the time of mitosis.61,62 Resistance to paclitaxel can ascribe to the hyperactivation of YAP/TAZ or the downregulation of hEx. YAP can be described as a transcriptional co-activator capable of functioning as an oncoprotein by interacting and activating other transcriptional factors.35,63 For example, in the cancerous growth hyperactivation of YAP-S127A due to the abbreviated phosphorylation site, the YAP reaches the nucleus, accumulates there, and produces resistance to paclitaxel.52
TAZ can be understood as an analog to YAP. Both YAP and TAZ are termed oncogenes, resulting in cell proliferation and cancerous growth.64,65 Overexpression of TAZ results in the development of resistance against paclitaxel as TAZ’s overexpression results in the increased levels of Multidrug resistance proteins (MDR). When activated, these proteins, the cell membrane transporters, reduce the drug concentration, resulting in anti-drug resistance.3
hEx is also responsible for controlling cell proliferation as it binds to the Yki.66 So, the downregulation of hEx results in the increased activity of Yki, resulting in the development of paclitaxel resistance (Figure 4).55 Further, Cyr61 and CTGF, the target for TAZ/TEAD, provide resistance to paclitaxel-mediated breast cancer treatment. Therefore, the activity of paclitaxel was reversed to its original form by inhibiting Cyr61 and CTGF with the short hairpin RNA method.45
 |
Figure 4 Hippo signaling in regulating chemo-drugs resistance. |
Lapatinib
Lapatinib is a therapeutic drug and works as a reversible inhibitor for tyrosine kinase regions of HER2. Epidermal growth factor receptor holds significance in treating advanced or metastatic breast cancer.
It works by competing with ATP to reversibly inhibit the ATP-binding pocket by forming weak interactions. As it binds to the pocket instead of ATP, this results in the downregulation and blocking of the targeted enzymes such as Mitogen-Activated Protein Kinase and Phosphatidylinositol 3-kinase, Akt, and also results in inhibition of the mammalian target of rapamycin dependent transduction pathways, and this blocking results in the arresting the cell growth and finally results in the apoptosis of tumorous cells.67,68
It is a HER2 targeted kinase inhibitor, and it is observed that in vitro, after subsequent administration, the HER2 breast cancer cells develop resistance against the lapatinib.3 Furthermore, the association of this resistance development and hyperactivation of YAP and TAZ has been observed in previous studies. Therefore, it is believed that the outcome of decreased/minimal expression of YAP/TAZ can be positively correlated with increased sensitivity of lapatinib (Figure 4).31
CDK 4/6 Inhibitors
Cyclin-dependent kinase, also known as CDKs, helps in regulating the cell cycle. CDK 4 and 6 act as a network, manage the cell cycle, and controls cell proliferation. CDK 4 and CDK 6 works as a complex with D-type cyclins, and in the case of mitogenic stimulation, the response is produced as they work together to drive cell progressions from the G phase to the S phase.69,70
CDK 4/6 inhibitors are administered to treat oncogenic growth and are administered along with hormonal therapy, including the aromatase inhibitor or fulvestrant. These inhibitors are vital in treating hormone receptors, HER2, and advanced or metastatic breast cancer.3
However, their effectiveness is restricted due to the drug resistance imparted by the hyperactivation of YAP and TAZ.31 In ER-positive cases, the loss of FAT1 steered the hyperactivation of YAP/TAZ as the Hippo pathway is suppressed. As the suppression of pathway directs the increased expression of the YAP/TAZ, they cluster around the CDK6 promoter and facilitate the CDK 6 transcription. Additionally, increased expression of CDK6 imparts resistance to the CDK 4/6 inhibitors in the case of the breast cancer cells and the inactivation of FAT1 protein (Figure 4).3
Tamoxifen
It is an estrogen receptor modulator, and it is utilized to medicate breast cancer.44 People diagnosed with breast cancer express the ERα protein and respond to ERα antagonists.75,76
However, the resistance to hormone therapy is observed in patients and tamoxifen resistance is developed due to the downregulation of LATS2.69 The downregulation of LATS2 results in its co-localization with the ERα in the nucleus. As LATS2 can activate the ERα transcription, it promotes the increased expression of YAP, which in turn increases the transcription of estrogen receptor alpha, causing inhibition of the action of tamoxifen drug.57,60 Consequently, regulating the expression of LATS2 protein can overcome this resistance towards tamoxifen.
Doxorubicin
Doxorubicin falls under the category of anthracycline drugs and works by binding to the enzyme topoisomerase II and blocking its action. Since this is the enzyme that the cancerous cells require to grow and divide, the binding of doxorubicin to the topoisomerase enzyme results in cell apoptosis. Therefore, this drug is commonly administered to treat various kinds of cancer.71
Doxorubicin resistance is developed due to the hyperactivation of YAP/TAZ and the decreased expression of the LATS2. In the case of YAP, its overexpression leads to the partial actuation of the MAPK (Mitogen-Activated Protein Kinase) pathway, which promotes cell proliferation.3 It is also noted that YAP and p53 are activated when the cells accompanied by doxorubicin are subjected to treatment. Hence the activation and overexpression of wildtype YAP promote the development of resistance.72 Also, p53, in a way, controls the expression of the YAP where YAP binds to the promoter of p53 to maintain the apoptosis, whereas p53 by binding to YAP’s promoter increases its expression, thus regulating its function.72
In the case of TAZ, its hyperactivation leads to the activation of the interleukin-8 (IL-8), and the increased levels of MDR proteins in Ras-transformed MCF10A-T1K cells leads to the development of resistance.3,35
LATS2 is responsible for maintaining the expression levels of YAP/TAZ in the Hippo Pathway by phosphorylating the YAP/TAZ component to lead to cytoplasmic retention, ubiquitination, and eventually proteasomal degradation. In the case of breast cancer, the LATS2 levels are downregulated, and due to this, it is unable to phosphorylate the YAP component, which results in hyperactivation due to overexpression of the YAP component and further leads to drug resistance in cells.54
Cisplatin
Cisplatin falls under the category of chemotherapeutic drug and holds the utility to treat diverse classes of cancer. It works by forming bonds with DNA, causing damage which results in apoptosis.45 Therefore, cisplatin drug is always administered with paclitaxel as the first-line treatment.
It is found that in cisplatin resistance breast cancer cells, downregulation of the MST protein is observed. MST is an integral part of the Hippo signaling pathway, and MST 1/2 is responsible for phosphorylating YAP and TAZ and downregulating them. Through autophosphorylation, MST1/2 gets activated, which in turn phosphorylates downstream LATS kinase that helps make LATS further phosphorylate the YAP and TAZ, consequently controlling cell proliferation.73,74 Overall, the hyperactivation results in the impartment of the cisplatin resistance on the patients.55
Taxol
Taxol is a therapeutic drug and is commonly used to treat breast cancer patients. The primary role of taxol is the induction of apoptosis to destroy cancerous growth.
Again, the TAZ protein is found to be overexpressed in breast cancer cells showing resistance towards chemo-drug Taxol. Highly expressed TAZ molecules move towards the nucleus and interact with the TEAD proteins. TEAD factors are responsible for promoting the various TAZ functions.32,33
Since TAZ is a transcriptional co-activator, its increased levels mark the activation of various downstream targets, resulting in the activation and increased expression of many oncogenic factors such as CYR61 and CTGF. The TEAD response elements bind to the promoters of CYR61 and CTGF and support their elevated expression. This ultimately suppresses the apoptosis process as they are the factors that are responsible for promoting cell survival and proliferation and interrupt the cancer treatment (Figure 4).27,35–37,45
Conclusion
The development of drug resistance proves to be a significant setback for chemotherapy as it is the most commonly used procedure to treat cancer patients. The molecules involved in the hippo signaling pathway play a vital role in developing this resistance. The deregulation of the hippo pathway, be it the inactivation of tumor suppressor genes LATS1/2 or the increased expression of oncogenes YAP/TAZ, results in the disrupted expressions of downstream targets causing the cancer cells to develop resistance against the anti-cancer drugs. A recent study identified the predictive utility of YAP where the choice of drug can be used according to the YAP expression pattern. In addition, the protuberant role of YAP makes it an attractive target for the synthesis of anti-cancer drugs.77
Regulating the expression of these genes can be a better approach towards modulating cancer treatment by augmenting the effects of various chemo drugs involved in the whole process. To apply the practice in clinical treatment, the hippo pathway should be extensively studied and can be an accomplishment in targeted therapies for breast cancer treatment.
Acknowledgment
The researchers would like to thank the Deanship of Scientific Research, Qassim University, for funding the publication of this project.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sun Y-S, Zhao Z, Yang Z-N, et al. Risk factors and preventions of breast cancer. Int J Biol Sci. 2017;13(11):1387. doi:10.7150/ijbs.21635
2. World Health Organization. Available from: https://www.who.int/news-room/fact-sheets/detail/breast-cancer.
3. Zeng R, Dong J. The Hippo signaling pathway in drug resistance in cancer. Cancers. 2021;13(2):318. doi:10.3390/cancers13020318
4. Kyriazoglou A, Liontos M, Zakopoulou R, et al. The role of the hippo pathway in breast cancer carcinogenesis, prognosis, and treatment: a systematic review. Breast Care. 2021;16(1):6–15. doi:10.1159/000507538
5. Varelas X. The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development. 2014;141(8):1614–1626. doi:10.1242/dev.102376
6. Zhang K, Qi HX, Hu ZM, et al. YAP and TAZ Take Center Stage in Cancer. Biochemistry. 2015;54(43):6555–6566. doi:10.1021/acs.biochem.5b01014
7. Maugeri-Saccà M, Barba M, Pizzuti L, et al. The Hippo transducers TAZ and YAP in breast cancer: oncogenic activities and clinical implications. Expert Rev Mol Med. 2015;17:e14. doi:10.1017/erm.2015.12
8. Maugeri-Saccà M, De Maria R. Hippo pathway and breast cancer stem cells. Crit Rev Oncol Hematol. 2016;99:115–122. PMID: 26725175. doi:10.1016/j.critrevonc.2015.12.004
9. Shi P, Feng J, Chen C. Hippo pathway in mammary gland development and breast cancer. Acta Biochim Biophys Sin. 2015;47(1):53–59. PMID: 25467757. doi:10.1093/abbs/gmu114
10. Wei C, Wang Y, Xiangqi L. The role of Hippo signal pathway in breast cancer metastasis. Onco Targets Ther. 2018;11:2185. doi:10.2147/OTT.S157058
11. Zhao B, Tumaneng K, Guan KL. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol. 2011;13(8):877–883. doi:10.1038/ncb2303
12. Justice RW, Zilian O, Woods DF, et al. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 1995;9(5):534–546. doi:10.1101/gad.9.5.534
13. Xu T, Wang W, Zhang S, et al. Identifying tumor suppressors in genetic mosaics: the Drosophila LATS gene encodes a putative protein kinase. Development. 1995;121(4):1053–1063. doi:10.1242/dev.121.4.1053
14. Tapon N, Harvey KF, Bell DW, et al. Salvador Promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell. 2002;110(4):467–478. doi:10.1016/S0092-8674(02)00824-3
15. Harvey KF, Pfleger CM, Hariharan IK. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell. 2003;114(4):457–467. doi:10.1016/S0092-8674(03)00557-9
16. Jia J, Zhang W, Wang B, et al. The Drosophila Ste20 family kinase dMST functions as a tumor suppressor by restricting cell proliferation and promoting apoptosis. Genes Dev. 2003;17(20):2514–2519. doi:10.1101/gad.1134003
17. Wu S, Huang J, Dong J, et al. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell. 2003;114(4):445–456. doi:10.1016/S0092-8674(03)00549-X
18. Pantalacci S, Tapon N, Pierre L. The Salvador partner Hippo promotes apoptosis and cell-cycle exit in Drosophila. Nat Cell Biol. 2003;5(10):921–927. doi:10.1038/ncb1051
19. Udan RS, Kango-Singh M, Nolo R, et al. Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat Cell Biol. 2003;5(10):914–920.
20. Lai Z-C, Wei X, Shimizu T, et al. Control of cell proliferation and apoptosis by mob as tumor suppressor, mats. Cell. 2005;120(5):675–685. doi:10.1016/j.cell.2004.12.036
21. Wei X, Shimizu T, Lai Z-C. Mob as tumor suppressor is activated by Hippo kinase for growth inhibition in Drosophila. EMBO J. 2007;26(7):1772–1781. doi:10.1038/sj.emboj.7601630
22. Huang J, Wu S, Barrera J, et al. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122(3):421–434. doi:10.1016/j.cell.2005.06.007
23. Wu S, Liu Y, Zheng Y, et al. The TEAD/TEF family protein scalloped mediates transcriptional output of the hippo growth-regulatory pathway. Dev Cell. 2008;14(3):388–398. doi:10.1016/j.devcel.2008.01.007
24. Oh H, Irvine KD. In vivo analysis of Yorkie phosphorylation sites. Oncogene. 2009;28(17):1916–1927. doi:10.1038/onc.2009.43
25. Oh H, Irvine KD. In vivo regulation of Yorkie phosphorylation and localization. Development (Cambridge, England). 2008;135:1081–1088. doi:10.1242/dev.015255
26. Dong J, Feldmann G, Huang J, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130(6):1120–1133. doi:10.1016/j.cell.2007.07.019
27. Zheng Y, Pan D. The Hippo signaling pathway in development and disease. Dev Cell. 2019;50(3):264–282. doi:10.1016/j.devcel.2019.06.003
28. Zhao B, Li L, Tumaneng K, et al. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF β-TRCP. Genes Dev. 2010;24(1):72–85. doi:10.1101/gad.1843810
29. Liu C-Y, Zha Z-Y, Zhou X, et al. The hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCFβ-TrCP E3 ligase. J Biol Chem. 2010;285(48):37159–37169. doi:10.1074/jbc.M110.152942
30. Hoa L, Kulaberoglu Y, Gundogdu R, et al. The characterization of LATS2 kinase regulation in Hippo-YAP signaling. Cell Signal. 2016;28(5):488–497. doi:10.1016/j.cellsig.2016.02.012
31. Atkins M, Potier D, Romanelli L, et al. An ectopic network of transcription factors regulated by hippo signaling drives growth and invasion of a malignant tumor model. Curr Biol. 2016;26(16):2101–2113.
32. Lin KC, Park HW, Guan K-L. Deregulation and therapeutic potential of the hippo pathway in Cancer. Ann Rev Cancer Biol. 2018;2:59–79. doi:10.1146/annurev-cancerbio-030617-050202
33. Rosenbluh J, Nijhawan D, Cox A, et al. β-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell. 2012;151(7):1457–1473. doi:10.1016/j.cell.2012.11.026
34. Zhang H, Liu CY, Zha ZY, et al. TEAD transcription factors mediate the function of TAZ in cell growth and epithelial-mesenchymal transition. J Biol Chem. 2009;284(20):13355–13362. doi:10.1074/jbc.M900843200
35. Zhao B, Ye X, Yu J, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22(14):1962–1971.
36. Skibinski A, Breindel J, Prat A, et al. The Hippo transducer TAZ interacts with the SWI/SNF complex to regulate breast epithelial lineage commitment. Cell Rep. 2014;6(6):1059–1072. doi:10.1016/j.celrep.2014.02.038
37. Chen D, Sun Y, Wei Y, et al. LIFR is a breast cancer metastasis suppressor upstream of the Hippo-YAP pathway and a prognostic marker. Nat Med. 2012;18(10):1511–1517. doi:10.1038/nm.2940
38. Hiemer SE, Szymaniak AD, Varelas X. The transcriptional regulators TAZ and YAP direct transforming growth factor β-induced tumorigenic phenotypes in breast cancer cells*♦. J Biol Chem. 2014;289(19):
39. Zhi X, Zhao D, Zhou Z, et al. YAP promotes breast cell proliferation and survival partially through stabilizing the KLF5 transcription factor. Am J Pathol. 2012;180(6):2452–2461. doi:10.1016/j.ajpath.2012.02.025
40. Guiu S, Michiels S, André F, et al. Molecular subclasses of breast cancer: how do we define them? The IMPAKT 2012 Working Group Statement. Ann Oncol. 2012;23(12):2997–3006. doi:10.1093/annonc/mds586
41. Martin HL, Smith L, Tomlinson DC. Multidrug-resistant breast cancer: current perspectives. Breast Cancer. 2014;6:1.
42. Ye S, Eisinger-Mathason TS. Targeting the Hippo pathway: clinical implications and therapeutics. Pharmacol Res. 2016;103:270–278. doi:10.1016/j.phrs.2015.11.025
43. Lin C-H, Pelissier FA, Zhang H, et al. Microenvironment rigidity modulates responses to the HER2 receptor tyrosine kinase inhibitor lapatinib via YAP and TAZ transcription factors.”. Mol Biol Cell. 2015;26(22):3946–3953. doi:10.1091/mbc.E15-07-0456
44. Li Z, Razavi P, Li Q, et al. Loss of the FAT1 tumor suppressor promotes resistance to CDK4/6 inhibitors via the hippo pathway. Cancer Cell. 2018;34(6):893–905. doi:10.1016/j.ccell.2018.11.006
45. Lai D, Ho KC, Hao Y, et al. Taxol resistance in breast cancer cells is mediated by the hippo pathway component TAZ and its downstream transcriptional targets Cyr61 and CTGF. Cancer Res. 2011;71(7):2728–2738.
46. Zhao Y, Yang X. Regulation of sensitivity of tumor cells to antitubulin drugs by Cdk1-TAZ signaling. Oncotarget. 2015;6(26):21906. doi:10.18632/oncotarget.4259
47. Bartucci M, Dattilo R, Moriconi C, et al. TAZ is required for metastatic activity and chemoresistance of breast cancer stem cells. Oncogene. 2015;34(6):681–690.
48. Cordenonsi M, Zanconato F, Azzolin L, et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 2011;147(4):759–772. doi:10.1016/j.cell.2011.09.048
49. Jeong W, Kim SB, Sohn BH, et al. Activation of YAP1 is associated with poor prognosis and response to taxanes in ovarian cancer. Anti Cancer Res. 2014;34(2):811–817.
50. Xia Y, Chang T, Wang Y, et al. YAP promotes ovarian cancer cell tumorigenesis and is indicative of a poor prognosis for ovarian cancer patients. PLoS One. 2014;9(3):e91770. doi:10.1371/journal.pone.0091770
51. Zhang X, George J, Deb S, et al. The Hippo pathway transcriptional co-activator, YAP, is an ovarian cancer oncogene. Oncogene. 2011;30(25):2810–2822. doi:10.1038/onc.2011.8
52. Huo X, Zhang QI, Liu AM, et al. Overexpression of Yes-associated protein confers doxorubicin resistance in hepatocellullar carcinoma. Oncol Rep. 2013;29(2):840–846. doi:10.3892/or.2012.2176
53. Jiang N, Hjorth-Jensen K, Hekmat O, et al. In vivo quantitative phosphoproteomic profiling identifies novel regulators of castration-resistant prostate cancer growth. Oncogene. 2015;34(21):2764–2776. doi:10.1038/onc.2014.206
54. Huang J-M, Nagatomo I, Suzuki E, et al. YAP modifies cancer cell sensitivity to EGFR and survivin inhibitors and is negatively regulated by the non-receptor type protein tyrosine phosphatase 14. Oncogene. 2013;32(17):2220–2229. doi:10.1038/onc.2012.231
55. Visser-Grieve S, Hao Y, Yang X. Human homolog of Drosophila expanded, hEx, functions as a putative tumor suppressor in human cancer cell lines independently of the Hippo pathway. Oncogene. 2012;31(9):1189–1195. doi:10.1038/onc.2011.318
56. Ren A, Yan G, You B, et al. Down-regulation of mammalian sterile 20–Like Kinase 1 by heat shock protein 70 mediates cisplatin resistance in prostate cancer cells. Cancer Res. 2008;68(7):2266–2274. doi:10.1158/0008-5472.CAN-07-6248
57. Visser S, Yang X. LATS tumor suppressor: a new governor of cellular homeostasis. Cell Cycle. 2010;9(19):3892–3903. doi:10.4161/cc.9.19.13386
58. Ji D, Deeds SL, Weinstein EJ. A screen of shRNAs targeting tumor suppressor genes to identify factors involved in A549 paclitaxel sensitivity. Oncol Rep. 2007;18(6):1499–1505.
59. Kawahara M, Hori T, Chonabayashi K, et al. Kpm/LATS2 is linked to chemosensitivity of leukemic cells through the stabilization of p73. Blood. 2008;112(9):3856–3866.
60. Lit LC, Scott S, Zhang H, et al. LATS2 is a modulator of estrogen receptor alpha. Anti Cancer Res. 2013;33(1):53–63.
61. Horwitz SB. “Taxol (paclitaxel): mechanisms of action.” Annals of oncology: official. J Eur Soc Med Oncol. 1994;5:S3–S6.
62. Risinger AL, Giles FJ, Mooberry SL. Microtubule dynamics as a target in oncology. Cancer Treat Rev. 2009;35(3):255–261. doi:10.1016/j.ctrv.2008.11.001
63. Lamar JM, Stern P, Liu H, et al. The Hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain. Proc Natl Acad Sci. 2012;109(37):E2441–E2450. doi:10.1073/pnas.1212021109
64. Lei Q-Y, Zhang H, Zhao B, et al. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol Cell Biol. 2008;28(7):2426–2436. doi:10.1128/MCB.01874-07
65. Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–378. doi:10.1016/j.ejphar.2014.07.025
66. Zhao Y, Yang X. WWTR1 (WW domain containing transcription regulator 1). Atlas Genet Cytogenet Oncol Haematol. 2014;18(11):849. doi:10.4267/2042/54169
67. Xia W, Mullin RJ, Keith BR, et al. Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene. 2002;21(41):6255–6263. doi:10.1038/sj.onc.1205794
68. Ahn ER, Vogel CL. Dual HER2-targeted approaches in HER2-positive breast cancer. Breast Cancer Res Treat. 2012;131(2):371–383. doi:10.1007/s10549-011-1781-y
69. Otto T, Sicinski P. Cell cycle proteins as promising targets in cancer therapy. Nat Rev Cancer. 2017;17(2):93–115. doi:10.1038/nrc.2016.138
70. Sherr CJ, Roberts JM. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 2004;18(22):2699–2711. doi:10.1101/gad.1256504
71. Available from: https://www.cancerresearchuk.org/about-cancer/cancer-in-general/treatment/cancer-drugs/drugs/doxorubicin#:~:text=Doxorubicin%20is%20a%20type%20of,combination%20with%20other%20chemotherapy%20drugs.
72. Bai N, Zhang C, Liang N, et al. Yes-associated protein (YAP) increases chemosensitivity of hepatocellular carcinoma cells by modulation of p53. Cancer Biol Ther. 2013;14(6):511–520. doi:10.4161/cbt.24345
73. Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19(4):491–505. doi:10.1016/j.devcel.2010.09.011
74. Chan EHY, Nousiainen M, Chalamalasetty RB, et al. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase LATS1. Oncogene. 2005;24(12):2076–2086. doi:10.1038/sj.onc.1208445
75. Wu L, Yang X. Targeting the Hippo pathway for breast cancer therapy. Cancers. 2018;10(11):422. doi:10.3390/cancers10110422
76. Shou J, Massarweh S, Osborne CK, et al. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu crosstalk in ER/HER2–positive breast cancer. J Natl Cancer Inst. 2004;96(12):926–935. doi:10.1093/jnci/djh166
77. Kim HB, Myung SJ. Clinical implications of the Hippo-YAP pathway in multiple cancer contexts. BMB Rep. 2018;51(3):119–125. doi:10.5483/bmbrep.2018.51.3.018
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
