Back to Journals » International Journal of Nanomedicine » Volume 15
Doxycycline-Embedded Nanofibrous Membranes Help Promote Healing of Tendon Rupture
Authors Weng CJ, Lee D, Ho J, Liu SJ
Received 30 May 2019
Accepted for publication 15 November 2019
Published 9 January 2020 Volume 2020:15 Pages 125—136
DOI https://doi.org/10.2147/IJN.S217697
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Thomas Webster
Chun-Jui Weng,1,2 Demei Lee,2 Jui Ho,2 Shih-Jung Liu1,2
1Department of Orthopedic Surgery, Musculoskeletal Research Center, Chang Gung Memorial Hospital-Linkou, Taoyuan, Taiwan; 2Department of Mechanical Engineering, Chang Gung University, Taoyuan, Taiwan
Correspondence: Shih-Jung Liu
Biomaterials Lab, Mechanical Engineering, Chang Gung University, 259, Wen-Hwa 1st Road, Kwei-Shan, Tao-Yuan 333, Taiwan
Tel +886-3-2118166
Fax +886-3-2118558
Email [email protected]
Background: Despite recent advancements in surgical techniques, the repair of tendon rupture remains a challenge for surgeons. The purpose of this study was to develop novel doxycycline-loaded biodegradable nanofibrous membranes and evaluate their efficacy for the repair of Achilles tendon rupture in a rat model.
Materials and Methods: The drug-loaded nanofibers were prepared using the electrospinning process and drug release from the prepared membranes was investigated both in vitro and in vivo. Furthermore, the safety and efficacy of the drug-loaded nanofibrous membranes were evaluated in rats that underwent tendon surgeries. An animal behavior cage was employed to monitor the post-surgery activity of the animals.
Results: The experimental results demonstrated that poly(D,L-lactide-co-glycolide) (PLGA) nanofibers released effective concentrations of doxycycline for more than 40 days post-surgery, and the systemic plasma drug concentration was low. Rats receiving implantation of doxycycline-loaded nanofibers also showed greater activities and stronger tendons post-operation.
Conclusion: Nanofibers loaded with doxycycline may have great potential in the repair of Achilles tendon rupture.
Keywords: doxycycline, tendon, nanofibrous membrane
Introduction
The Achilles tendon is the strongest and largest tendon in human body. Acute Achilles tendon rupture is one of the most commonly seen injuries around ankle joints and can cause severe disability, especially in athletes. The incidence rate of Achilles tendon rupture ranges from 11 to 37 per 10,000 people.1–3 The mechanism of Achilles tendon injuries can be predominantly attributed to a contracted gastrocnemius-soleus complex against forced dorsiflexion of ankle. Furthermore, Achilles tendon rupture can occur not only through a single traumatic event, but also via tendon degeneration, poor vascularity, and corticosteroid/fluoroquinolone use.1,4–6 Treatment options include surgical repair or conservative treatment, although there is no consensus on these treatment options, and re-rupture is the main concern for acute Achilles tendon injuries. It has previously been reported that the re-rupture rate ranged from 1.7% to 8.0% in a surgically treated group,7–10 whereas this rate ranged from up to 4.0% to 20.8%7–11 in patients treated conservatively.
Matrix metalloproteinase (MMP) is an enzyme that involves degradation and remodeling of extracellular matrix (ECM) and plays an important role in the tendon repair process.12–17 Inhibition of matrix metalloproteinase (MMP) has been the treatment of choice for tendinopathy.18–21 Systemic daily administration of doxycycline orally has been demonstrated to improve collagen fibril organization through inhibition of local MMP activity, which accelerates matrix remodeling, increases equilibrium modulus, and decreases creep strain of surgically repaired Achilles tendons in rats.20–22 Common side effects of doxycycline include esophageal erosion, heartburn, nausea, vomiting, diarrhea, and gastritis.23 This may reflect patients’ inability to tolerate the high systemic drug concentrations required to achieve adequate exposure of the drug at the target site. Local drug delivery may potentially provide the advantage of achieving high and sustained local concentrations with a low systemic dose, thus minimizing systemic side effects.
The purpose of our study was to evaluate the efficacy of novel doxycycline-loaded biodegradable membranes that provide sustained-release drug at the target site. We hypothesized that doxycycline-loaded biodegradable membrane would enhance the healing process and improve the biomechanical properties of surgically repaired Achilles tendons. To the best knowledge of the authors, no one has ever analyzed the effect of local slow-released doxycycline in surgically repaired Achilles tendons.
Poly(D,L-lactide-co-glycolide) (PLGA) polymers were selected as the delivery vehicles, mainly due to their excellent biocompatibility, controllable biodegradability, tunable degradation rates, mechanical properties, and thermal processibility.24 Biodegradable doxycycline-loaded nanofibrous membranes were fabricated using the electrospinning technique, which is a fiber production process that adopts electric force to draw charged threads of polymer solutions up to fiber diameters in the order of some hundred nanometers. The technique can also be used to generate nanofiber assemblies for mimicking the special fiber organizations in musculoskeletal tissues. Nanofibrous scaffolds can provide the required mechanical properties that minimize the risk of re-rupture associated with the movement of the tendon gap defect following surgical repair.25 After electrospinning technique, the extent of drug release from the prepared membranes was measured both in vitro and in vivo. Furthermore, animal models of rats that underwent tendon repair surgeries were used to evaluate the efficacy and safety of the drug-loaded nanofibrous membranes. Animal activities post-operation was also compared by employing an animal behavior cage (ABC) developed by our group.
Materials and Methods
Preparation of Doxycycline-Incorporated Nanofibrous Membranes
The polymeric materials included PLGA polymer (lactide/glycolide: 50/50, molecular weight Mw=33,000 Da) (Sigma-Aldrich, MO, U.S.A.). Doxycycline and hexafluoroisopropanol (HFIP) were also acquired from Sigma-Aldrich.
To manufacture the nanofibrous membranes, PLGA and doxycycline were primarily dissolved in HFIP at concentrations of 30% (w/v) and 7.5% (w/v) respectively.26,27 Electrospinning of the PLGA/doxycycline solution was performed on a lab-made setup consisting of a syringe and needle (the internal diameter is 0.42 mm), a ground collection plate, and a high voltage supply. The flow rate of the solution was 0.1 mL/h, while the applied voltage was 15 kV. The solution was gathered by the collection plate distanced 15 cm from the syringe in a nonwoven form. All procedures were performed at a temperature of 26°C and a relative humidity of 64%.
Characterization of Electrospun Nanofibrous Membranes
To quantify the doxycycline-incorporated nanofibers, we used a field emission scanning electron microscope (FE-SEM) (JSM – 7500F, Joel, Japan). The diameter compositions of nanofibers were obtained by characterizing the micro-images of 50 arbitrarily picked fibers for each specimen.
The spectra of electrospun nanofibrous membranes were characterized using a Fourier Transform Infrared (FTIR) spectrometry. We used Nicolet iS5 spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) at a resolution of 4 cm−1 and 32 scans to perform FTIR analysis. Nanofibrous membranes were pressed as KBr discs, and spectra were analyzed over the 400–4000 cm−1 range.
The tensile strength of electrospun nanofibrous scaffolds was measured with a Lloyd tensiometer (AMETEK, Berwyn, PA, USA) that was equipped with a 100 N load cell, in accordance with the ASTM D638 standard. A strip cut from the membrane that had dimensions of 10 mm by 50 mm was held between two clamps that were separated by 3 cm. During the tests, the membrane was pulled by the top clamp at a rate of 60 mm/minthrough a distance of 10 cm before the clamp was returned to its starting point. The force and elongation before the membrane was broken were recorded. Measurements were made five times on each membrane.
A contact angle measuring apparatus (First Ten Angstroms, Portsmouth, VA, USA) was used to assess the water contact angles of the scaffolds that have a dimension of 1 cm × 1 cm (N= 5).
Evaluation of Doxycycline Levels in vitro
The amount doxycycline released from the drug-incorporated membranes was evaluated. Specimens with an approximate size of 2.0 × 3.0 mm and weight of 1 g were cut from the electrospun membrane and then kept in glass tubes. In each tube, 1 mL of buffered solution (0.15 mol/L, pH 7.4) was added to the specimen. Then, tubes were maintained at 37°C for 24 hrs. Then, the eluent was collected for analysis with another 1 mL of fresh-buffered solution added into each tube. Every 24 hrs, the procedure was repeated, until the specimen was fully dissolved. The experiment was performed in triplicate (N=3). The doxycycline levels in the eluents were quantified using a high-performance liquid chromatography (HPLC) assay on a Hitachi L-2200R system (Hitachi Inc., Tokyo, Japan).
In vivo Animal-Related Procedure
Eighteen Sprague Dawley (SD) rats, weighing 250±25 g each, were enrolled for the in vivo study. All animal-related procedures received institutional approval of Chang Gung University (CGU 107-168), and all of the animals were cared for under the supervision of a licensed veterinarian in a manner that was consistent with the regulations of the Department of Health and Welfare, Taiwan.
The rats were sedated under general anesthesia in an anesthesia chamber (40cm × 20cm × 28cm, transparent acrylic box), with isoflurane (Aesica-Queenborough, Queenborough, Kent, UK) by a vaporizer (Matrix, USA). Isoflurane inhalation was used throughout the entire surgical procedure to maintain anesthesia. Before the surgical procedure, the rat received a local injection over right leg with 0.5 mL 2% Xylestesin-A containing epinephrine at a concentration of 1:100,000. As shown in Figure 1, a 3 cm longitudinal skin incision lateral to Achilles tendon was made over right leg of each rat using a #15 blade. The Achilles tendon was exposed by dissecting the surrounding soft tissue. The mid-portion of Achilles tendon was then cut via scalpel and sutured end-to-end with a 5-0 Dexon suture (Johnson & Johnson, USA), and the 18 rats were randomly separated into two groups. In the test group, the repaired Achilles tendon was surrounded with biodegradable nanofibrous membrane and suture fixation with 5-0 suture, and the wound was closed with 3-0 Nylon suture (Johnson & Johnson, USA). In the control group, the repaired tendon was not covered with nanofibrous membrane.
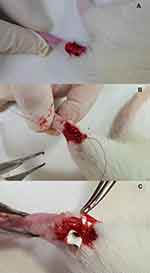 |
Figure 1 (A) Exposing the Achilles tendon, (B) suturing the tendon with sutures, and (C) enveloping the tendon with doxycycline-loaded nanofibrous membrane. |
Post-Operative Activity Test
After the surgery, general activity level was recorded daily by housing each rat in a lab-developed animal behavior cage (ABC) for 1 week, after which the rats were sent back to their original cages for animal care. As shown in Figure 2, the dimensions of the cage were 50 cm × 50 cm × 50 cm. The movement of the rats was monitored and recorded by nine diffusion-scan type photoelectric switch sensors with self-contained amplifiers (HP100-A1, Azbil Corp., Tokyo, Japan). The cage was sub-divided into nine symmetric areas, each with one sensor placed at the top of the cage with 16.7 cm between each sensor. Each sensor has the ability to scan up to 1 m of distance. As the rat moves, the corresponding “arriving” sensor will be triggered by activity and start recording the movement. The total triggered count was recorded on a personal computer equipped with an acquisition interface, with the entire monitoring process lasting 7 days.
 |
Figure 2 Schematic of the animal behavior cage. |
Analysis of in vivo Levels of Doxycycline
In each group, the rats were divided into three sub-groups (N=3) and euthanized at 1.5 weeks, 3 weeks, and 6 weeks. At each time point, 1 mL of blood was sampled following the injection of 1 mL lidocaine to euthanize the rats. Meanwhile, tiny tissue near the membranes was also sampled. The levels of doxycycline in tissue and in blood were characterized by the HPLC assay. An additional six rats undergoing Achilles tendon repair with doxycycline-loaded nanofibrous membrane were euthanized on days 1 and 3 (three in each day). Through skin incision, Achilles tendon was also dissected and sampled. In each sub-group, one Achilles tendon sample was sent for histologic analysis and the other two tendon samples were sent for biomechanical analysis.
Biomechanical Test
The biomechanical properties of retrieved tendons were assessed with a Lloyd tensiometer (AMETEK, USA) equipped with a 100 N load cell, in a manner consistent with the ASTM D638 standard. Before testing, tendon was wrapped with gauze and immersed in normal saline solution in order to simulate the in vivo condition. During measurement, the repaired Achilles tendon was pulled by the top clamp at a rate of 60 mm/minute for a 10 cm distance before the clamp was returned to its starting point.
Histological Analysis
The harvested tendon for histological assay was fixed in 10% formalin and embedded in paraffin. The embedded tendon was cut into 4 μm frontal section and stained by hematoxylin and eosin (H&E) staining. An independent examiner completed the histologic interpretation at ×10 and ×20 magnification.
Statistical Analysis
The statistical analysis between groups was evaluated by a paired sample t-test using commercially available SPSS software (Version 12.0; SPSS Inc, Chicago, IL, USA). Data are expressed as mean ± SD (standard deviation), and the threshold for significance was established at a p-value of less than 0.05.
Results
Characterization of Electrospun Nanofibers
Doxycycline-embedded nanofibers were prepared using the electrospinning technique. Figure 3 shows the SEM images of the doxycycline-embedded and pure PLGA nanofibers, as well as the fiber size distributions. The calculated diameters were 1.07±0.73 μm and 104±25.8 nm for pure PLGA nanofibers and doxycycline-embedded nanofibers, respectively. The fiber diameter decreased significantly upon the addition of doxycycline.
 |
Figure 3 SEM images and fiber diameter distribution of electrospun (A) pure PLGA nanofibers and (B) doxycycline-loaded PLGA nanofibers. |
Figure 4 shows the FTIR spectra of pure PLGA nanofibers versus doxycycline-embedded PLGA nanofibers. In the spectra of the drug-loaded nanofibers, a new vibration peak at 3400 cm−1 was observed and can be attributed to the N–H bonds of doxycycline. In contrast, the vibration at 1725 cm−1 (C=O bond) was increased upon drug loading. Furthermore, the absorbance peak at around 1300 cm−1 might be a result of the increase in C–O bonds in doxycycline.23,24 The FTIR spectra analysis verified that the drugs were incorporated into the PLGA matrices successfully.
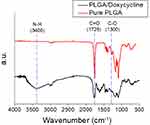 |
Figure 4 FTIR spectra of pure PLGA and drug-loaded PLGA nanofibers. |
We observed that the water contact angles of pure PLGA and doxycycline-embedded nanofibers in Figure 5 were 138.3±3.5° and 85.6±5.2°, respectively. Furthermore, the addition of doxycycline increased the hydrophilicity of PLGA nanofibers.
 |
Figure 5 Water contact angle of (A) pure PLGA nanofibers (138.3±3.5°) and (B) doxycycline-loaded PLAG nanofibers (85.6±5.2°). |
The tensile test results in Figure 6 suggest that the maximum tensile strength and the elongation at the break of the pure PLGA and doxycycline-embedded nanofibers were 1.74±0.10 MPa (136.14%) and 4.90±1.18 MPa (94.39%), respectively. Moreover, the mechanical strength of the nanofibers increased with the incorporation of drugs, whereas the elongation at the break decreased.
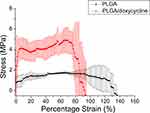 |
Figure 6 Tensile test results of PLGA nanofibers and doxycycline-loaded PLGA nanofibers (maximum strengths: PLGA nanofibers 1.74±0.10 MPa, doxycycline-loaded PLGA nanofibers 4.89±1.18 MPa). |
In vitro and in vivo Release Profiles of PLGA/Doxycycline Nanofibrous Membrane
The daily and accumulated release profiles of doxycycline from the nanofibrous membranes in vitro are shown in Figure 7. The daily doxycycline release curve showed a high concentration released on day 1, and a second peak was observed on day 13 (Figure 7A). Meanwhile, the cumulative release profiles suggested that 21.1% of the doxycycline was released on day 1 and 58.3% was eluted on day 13 (Figure 7B). At 42 days (6 weeks), the accumulated released of doxycycline reached 69.6%. Overall, the PLGA/doxycycline nanofibrous membranes could provide sustained drug release for more than 40 days in vitro.
 |
Figure 7 In vitro (A) daily and (B) accumulated release of doxycycline from the nanofibers. |
Figure 8 shows the in vivo release patterns of doxycycline in tissue near the nanofibers. A burst release was observed at day 1. After that, the doxycycline level decreased gradually with time. On the other hand, the release profile in blood exhibited similar pattern, but with much lower concentrations.
 |
Figure 8 In vivo release profiles of doxycycline in tissue and in blood. |
Animal Activity
The total activity counts for the control and doxycycline-treated groups over 7 days post-operation are shown in Figure 9. The total triggered counts recorded from the photoelectric switch sensors in doxycycline-treated and control groups were 12,977±10,223 and 9220±708, respectively. The total number of triggered counts was significantly greater in the doxycycline group than in the control group (P<0.05). Therefore, rats that underwent Achilles tendon repair with doxycycline-loaded biodegradable nanofibrous membrane demonstrated higher activity levels than those who received Achilles tendon repair alone.
 |
Figure 9 Activity counts of the rats (*P<0.05). |
Biomechanical Study
The rats were euthanized at 1.5, 3, and 6 weeks after surgery. Repaired tendons were cut from the musculotendinous junction proximally and bony insertion sites distally, and sent for biomechanical testing. In addition, healthy and normal tendons from the left leg were obtained for comparison. At 1.5 weeks (Figure 10A), the maximum strength levels of the tendons in both doxycycline and control groups were lower than those of the healthy tendons (P<0.01).
 |
Figure 10 Mechanical strength of tendons at (A) 1.5, (B) 3, and (C) 6 weeks post-surgery. |
At 3 weeks (Figure 10B), the doxycycline group exhibited maximum tendon strength levels that were comparable with the healthy tendon group. The control group, which did not receive doxycycline-loaded nanofibrous membranes, had inferior maximum strengths compared to other two groups (P<0.05).
At 6 weeks (Figure 10C) post-operation, all three groups showed comparable tendon strengths (non-significant, P>0.05), while the doxycycline group exhibited a slightly larger value compared to the other groups. In conclusion, doxycycline-loaded nanofibrous membrane can significantly improve tendon healing at 3 weeks, and to a lesser extent at 6 weeks, post-surgery.
Histology Study
There was no obvious difference between two groups in tendon cell organization and cellularity at 3 weeks post-surgery. However, there was only mild inflammation with a few mononuclear inflammatory cells noted (Figure 11A). On the contrary, severe inflammation was observed in the control group (Figure 11B).
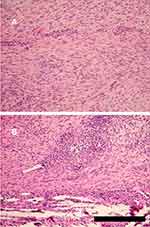 |
Figure 11 H&E stain of Achilles tendon in the (A) doxycycline group and (B) control group, at 3 weeks post-surgery (scale bar: 250 μm). |
Discussion
Achilles tendon rupture commonly occurs in athletes and individuals performing leisure activities. The spectrum of Achilles tendon injuries varies from tendonitis to partial tear and eventually complete rupture. Treatment options for an Achilles tendon rupture range from non-operative brace protection to surgical repair. The different treatment choices to each patient are dependent on their age, general medical condition, and desired function of activity. Non-surgical treatment includes initial brace or cast protection for months, then followed by gentle passive range of motion rehabilitation and muscle strengthening. For those with young age or high functional demand, surgery is indicated. Most surgical procedures are primary repair of the ruptured Achilles tendon. A curved or straight longitudinal skin incision is made medial to the Achilles tendon. Deep dissection into the subcutaneous tissue gently and identify both ends of the ruptured tendon. Clamps are used to approximate each ends together then primary repair of the tendon is performed by suturing two ends of the tendon together using a variety of different stitching techniques. In addition to the traditional open surgery, a minimal invasive technique has also been used to reduce the risk of complications during wound healing. The type of surgery and repair techniques used will depend on the surgeon, the type of rupture, and tissue quality.
Complications following Achilles tendon rupture are not uncommon. Among them, re-rupture of tendon is most widely seen. Re-rupture rate of the Achilles tendon after surgical repair is around 1.7–5.6%.28 Wound dehiscence and infection are also commonly seen complications after surgical intervention. Surgical treatment of Achilles tendon ruptures is reported to have lower rates of re-rupture compared to non-operative treatment, despite the risk of adverse events such as wound dehiscence and infections.29
The risk of wound-related complications, such as infection or dehiscence of wound, can be minimized through minimal invasive technique or even arthroscopic surgery.28 In contrast, the issue of poor tendon healing and re-rupture is difficult to address. Sufficient initial mechanical stability of repaired tendon can be achieved through various suture techniques. Due to the relatively deficient vascularity around the Achilles tendon, poor tendon healing may result in tendon re-rupture. Various agents have been proposed and employed to improve tendon healing, including platelet-rich-plasma (PRP),29 mesenchymal stem cells,30,31 simvastatin32 and doxycycline.21
Matrix metalloproteinases (MMP) have been reported to play an important role in tendon healing process.16,33,34 Increased MMP activity is associated with deterioration of collagen quality after tendon rupture.35–37 Doxycycline is considered as the most potent MMP inhibitor among tetracyclines group and has various clinical applications.38–41 Several previous studies had demonstrated the effect of doxycycline in enhancing Achilles tendon repair. In 2007, Pasternak et al42 showed that improved suture-holding capacity occurs in rats with Achilles tendon sutures who have been administered systemic doxycycline (100 mg/kg/day). Furthermore, the authors also demonstrated that sutures coated with doxycycline had improved suture-holding capacity at 3 days after surgery. Nguyen et al21 used oral administration of 10 mg/kg doxycycline in a rat model of Achilles tendon laceration. The authors concluded that oral administration of doxycycline accelerates matrix remodeling and enhances biomechanical properties of surgical repaired Achilles tendon. The enhancements were observed most significantly at 3 to 6 weeks after operation.
However, the exact mechanism of doxycycline in the healing process of Achilles tendon remains controversial. A negative effect of doxycycline on Achilles tendon healing was also reported by Pasternak et al.43 In their study, the Achilles tendon was transected and left unrepaired. They found significantly decreased mechanical strength at 5, 8, and 14 days after transection of Achilles tendon in rats when doxycycline was given orally at the dosage of 130 mg/kg body weight/day. The biomechanical tests were performed until 2 weeks after surgery; however, no data were available later than 2 weeks post-operation.
Biodegradable polymers can be applied for the delivery of drugs in a controlled and targeted manner. We adopted the PLGA as the material for scaffolding and the delivery vehicle for doxycycline. PLGA is one of the most characterized biodegradable copolymers that decomposes to non-toxic products (H2O and CO2) during the degradation process. In the development of devices for controlled delivery of proteins, small molecules, and other macromolecules, PLGA has been studied extensively.44 Local drug therapy with pharmaceuticals incorporated within the implants possess the benefit of delivering high concentration of drug directly to the target site with extremely low drug concentration systemically, thus minimizing associated side effects. Additionally, the highly porous scaffold is able to accommodate mammalian cells and guide their growth and tissue regeneration in three dimensions. The incorporation of doxycycline increased the hydrophilicity of PLGA nanofibers, which in turn help promote the healing of tendons. It has been reported45 that human skin fibroblasts showed significantly higher proliferation on electrospun PLGA fiber matrices possessing fiber size in the range of 350–1100 nm. Despite the diameter of the doxycycline-incorporated nanofibers in this study (range of 80–130 nm) is less than the reported optimum value, the electrospun nanofibrous scaffolds show good capability in promoting tendon healing.
This study exploited doxycycline-embedded nanofibrous membranes that provide local and sustainable release of drugs to the target site for tendon repair. The results presented here highlight that the doxycycline-embedded nanofibrous membranes exhibit a three-stage drug-release process in vitro, namely a primary blast at day 1, steady release thereafter until day 12, at which point a second peak was observed, followed a steadily diminishing release for more than 40 days. In general, drug release from a pharmaceutical-embedded biodegradable device can be divided into three phases, including a burst liberation, a diffusion-dominated elution, and a degradation-dominated release. After the electrospinning process, most drugs are dispersed into the volume of the PLGA/doxycycline membranes. However, a few pharmaceutical compounds located on the surface of nanofibers may result in initial burst release of medication. Following the initial burst, the drug-release profiles were mainly dominated by diffusion. A relatively constant slow elution of doxycycline was thus observed. Finally, the drug-loaded nanofibers swell due to the water absorption during elution, leading to disruption of the polymer matrix to form openings for doxycycline release. Consistent with this, a second peak was observed at day 12. From day 12 onwards, the liberation rate of doxycycline decelerated gradually.
The in vivo release profile differed from that of in vitro release, and we observed a burst of liberation followed by a gradually diminishing release profile. This might be due to the fact that in vivo metabolism is slower than that in the glass tube. Meanwhile, the levels of drug in the blood remained much lower, which in turn resulted in minimal side effects. Most importantly, our in vivo analysis demonstrated that the biodegradable drug-embedded nanofibers resulted in sustained liberation of doxycycline for more than 40 days, which would be advantageous for promoting tendon healing.
Both the animal activity and the mechanical strength tests suggested that rats who received doxycycline-embedded nanofibrous membranes showed superior outcome than the control rats. Additionally, the nanofibers significantly enhanced the biomechanical strength of tendons at 3 weeks post-surgery in a rat model with Achilles tendon laceration. In other words, doxycycline embedded nanofibrous membranes improved tendon healing most significantly at mid-term and this result is similar to oral administration of doxycycline.21 This also highlights that doxycycline, when administered locally, promotes healing of the Achilles tendon.
Several limitations existed in our study. First, we did not examine the dose-dependent effects of various doxycycline concentrations loaded onto nanofibrous membranes and further studies will be needed to elucidate these effects. Second, Achilles tendon rupture in humans mainly results from repetitive micro-trauma to the tendon. Moreover, the quality of tendon cell differs from that of the Achilles tendons surgically excised in our experiment. Third, there were only control group and doxycycline loaded with PLGA nanofibrous membrane group. There was no pure PLGA group proving that there is no effect on tendon healing of PLGA. Future studies may be needed to further clarify the mechanism and role of doxycycline in the repair of Achilles tendon rupture.
Conclusion
In this work, we fabricated biodegradable doxycycline-embedded nanofibrous membranes and evaluated their efficacy in Achilles tendon repair, utilizing PLGA as the delivery vehicle. The in vitro release patterns of doxycycline were evaluated by HPLC. A rat model of Achilles tendon rupture was created and used for the in vivo efficacy assessment of drug-eluting nanofibers. The empirical results revealed that PLGA nanofibers eluted effective concentrations of doxycycline for more than 40 days post-surgery and the systemic drug concentration in plasma was low. Rats receiving implantation of doxycycline-loaded nanofibers were more active and had stronger tendons post-operation. Nanofibers loaded with doxycycline, therefore, display great potential for the repair of Achilles tendon rupture.
Acknowledgements
The authors would like to thank the Ministry of Science and Technology, Taiwan (Contract No. 107-2221-E-182-017) and the Chang Gung Memorial Hospital (Contract No. CMRPD2G0252) for providing financial support on this study.
Disclosure
The authors declare that there are no conflicts of interest in this work.
References
1. Jarvinen TA, Kannus P, Maffulli N, Khan KM. Achilles tendon disorders: etiology and epidemiology. Foot Ankle Clin. 2005;10(2):255–266. doi:10.1016/j.fcl.2005.01.013
2. Leppilahti J, Puranen J, Orava S. Incidence of Achilles tendon rupture. Acta Orthop Scand. 1996;67(3):277–279. doi:10.3109/17453679608994688
3. Suchak AA, Bostick G, Reid D, Blitz S, Jomha N. The incidence of Achilles tendon ruptures in Edmonton, Canada. Foot Ankle Int. 2005;26(11):932–936. doi:10.1177/107110070502601106
4. McQuillan R, Gregan P. Tendon rupture as a complication of corticosteroid therapy. Palliat Med. 2005;19(4):352–353. doi:10.1177/026921630501900412
5. Seeger JD, West WA, Fife D, Noel GJ, Johnson LN, Walker AM. Achilles tendon rupture and its association with fluoroquinolone antibiotics and other potential risk factors in a managed care population. Pharmacoepidemiol Drug Saf. 2006;15(11):784–792. doi:10.1002/(ISSN)1099-1557
6. Kraemer R, Wuerfel W, Lorenzen J, Busche M, Vogt PM, Knobloch K. Analysis of hereditary and medical risk factors in Achilles tendinopathy and Achilles tendon ruptures: a matched pair analysis. Arch Orthop Trauma Surg. 2012;132(6):847–853. doi:10.1007/s00402-012-1476-9
7. Moller M, Movin T, Granhed H, Lind K, Faxen E, Karlsson J. Acute rupture of tendon Achillis. A prospective randomised study of comparison between surgical and non-surgical treatment. J Bone Joint Surg Br. 2001;83(6):843–848. doi:10.1302/0301-620X.83B6.0830843
8. Twaddle BC, Poon P. Early motion for Achilles tendon ruptures: is surgery important? A randomized, prospective study. Am J Sports Med. 2007;35(12):2033–2038. doi:10.1177/0363546507307503
9. Metz R, Verleisdonk EJ, van der Heijden GJ, et al. Acute Achilles tendon rupture: minimally invasive surgery versus nonoperative treatment with immediate full weightbearing – a randomized controlled trial. Am J Sports Med. 2008;36(9):1688–1694. doi:10.1177/0363546508319312
10. Nilsson-Helander K, Silbernagel KG, Thomee R, et al. Acute achilles tendon rupture: a randomized, controlled study comparing surgical and nonsurgical treatments using validated outcome measures. Am J Sports Med. 2010;38(11):2186–2193. doi:10.1177/0363546510376052
11. Keating JF, Will EM. Operative versus non-operative treatment of acute rupture of tendo Achillis: a prospective randomised evaluation of functional outcome. J Bone Joint Surg Br. 2011;93(8):1071–1078. doi:10.1302/0301-620X.93B8.25998
12. Gong F, Cui L, Zhang X, Zhan X, Gong X, Wen Y. Piperine ameliorates collagenase-induced Achilles tendon injury in the rat. Connect Tissue Res. 2018;59(1):21–29. doi:10.1080/03008207.2017.1289188
13. Fernandes de Jesus J, Spadacci-Morena DD, Rabelo N, Pinfildi CE, Fukuda TY, Plapler H. Photobiomodulation of matrix metalloproteinases in rat calcaneal tendons. Photobiomodul Photomed Laser Surg. 2019;37(7):421–427. doi:10.1089/photob.2019.4633
14. Molloy TJ, Wang Y, Horner A, Skerry TM, Murrell GA. Microarray analysis of healing rat Achilles tendon: evidence for glutamate signaling mechanisms and embryonic gene expression in healing tendon tissue. J Orthop Res. 2006;24(4):842–855. doi:10.1002/(ISSN)1554-527X
15. Thomopoulos S, Parks WC, Rifkin DB, Derwin KA. Mechanisms of tendon injury and repair. J Orthop Res. 2015;33(6):832–839. doi:10.1002/jor.22806
16. Gotoh M, Mitsui Y, Shibata H, et al. Increased matrix metalloprotease-3 gene expression in ruptured rotator cuff tendons is associated with postoperative tendon retear. Knee Surg Sports Traumatol Arthrosc. 2013;21(8):1807–1812. doi:10.1007/s00167-012-2209-x
17. Jacob J, Eisemon E, Sheibani-Rad S, Patel A, Jacob T, Choueka J. Matrix metalloproteinase levels as a marker for rotator cuff tears. Orthopedics. 2012;35(4):e474–478. doi:10.3928/01477447-20120327-18
18. Baldwin SJ, Kreplak L, Lee JM. MMP-9 selectively cleaves non-D-banded material on collagen fibrils with discrete plasticity damage in mechanically-overloaded tendon. J Mech Behav Biomed Mater. 2019;95:67–75. doi:10.1016/j.jmbbm.2019.03.020
19. Bedi A, Fox AJ, Kovacevic D, Deng XH, Warren RF, Rodeo SA. Doxycycline-mediated inhibition of matrix metalloproteinases improves healing after rotator cuff repair. Am J Sports Med. 2010;38(2):308–317. doi:10.1177/0363546509347366
20. Kessler MW, Barr J, Greenwald R, et al. Enhancement of Achilles tendon repair mediated by matrix metalloproteinase inhibition via systemic administration of doxycycline. J Orthop Res. 2014;32(4):500–506. doi:10.1002/jor.v32.4
21. Nguyen QT, Norelli JB, Graver A, et al. Therapeutic effects of doxycycline on the quality of repaired and unrepaired achilles tendons. Am J Sports Med. 2017;45(12):2872–2881. doi:10.1177/0363546517716637
22. Rooney SI, Torino DJ, Baskin R, et al. Doxycycline improves cage activity, but not exercised, supraspinatus tendon and muscle in a rat model. J Biomech. 2018;80:79–87. doi:10.1016/j.jbiomech.2018.08.027
23. Smith K, Leyden JJ. Safety of doxycycline and minocycline: a systematic review. Clin Ther. 2005;27(9):1329–1342. doi:10.1016/j.clinthera.2005.09.005
24. Sun X, Xu C, Wu G, Ye Q, Wang C. Poly(lactic-co-glycolic acid): applications and future prospects for periodontal tissue regeneration. Polymers (Basel). 2017;9:6. doi:10.3390/polym9060189
25. Ma B, Xie J, Jiang J, Shuler FD, Bartlett DE. Rational design of nanofiber scaffolds for orthopedic tissue repair and regeneration. Nanomedicine. 2013;8(9):1459–1481. doi:10.2217/nnm.13.132
26. Kao CW, Tseng YY, Liu KS, et al. Anesthetics and human epidermal growth factor incorporated into anti-adhesive nanofibers provide sustained pain relief and promote healing of surgical wounds. Int J Nanomedicine. 2019;14:4007–4016. doi:10.2147/IJN.S202402
27. Chen YP, Liu YW, Lee D, Qiu JT, Lee TY, Liu SJ. Biodegradable andrographolide-eluting nanofibrous membranes for the treatment of cervical cancer. Int J Nanomedicine. 2019;14:421–429. doi:10.2147/IJN.S186714
28. Cerrato R, Switaj P. Using arthroscopic techniques for Achilles pathology. Foot Ankle Clin. 2017;22(4):781–799. doi:10.1016/j.fcl.2017.07.007
29. Schlussel MM, Keene DJ, Wagland S, et al. Platelet-rich plasma in Achilles tendon healing 2 (PATH-2) trial: statistical analysis plan for a multicentre, double-blinded, parallel-group, placebo-controlled randomised clinical trial. Trials. 2018;19(1):464. doi:10.1186/s13063-018-2840-z
30. Oliva F, Maffulli N, Gissi C, et al. Combined ascorbic acid and T3 produce better healing compared to bone marrow mesenchymal stem cells in an Achilles tendon injury rat model: a proof of concept study. J Orthop Surg Res. 2019;14(1):54. doi:10.1186/s13018-019-1098-9
31. Xie S, Zhou Y, Tang Y, et al. Book-shaped decellularized tendon matrix scaffold combined with bone marrow mesenchymal stem cells-sheets for repair of Achilles tendon defect in Rabbit. J Orthop Res. 2019. doi:10.1002/jorr.v37.4
32. Jeong C, Kim SE, Shim KS, et al. Exploring the in vivo anti-inflammatory actions of simvastatin-loaded porous microspheres on inflamed tenocytes in a collagenase-induced animal model of Achilles Tendinitis. Int J Mol Sci. 2018;19:3. doi:10.3390/ijms19030820
33. Del Buono A, Oliva F, Longo UG, et al. Metalloproteases and rotator cuff disease. J Shoulder Elbow Surg. 2012;21(2):200–208. doi:10.1016/j.jse.2011.10.020
34. Garofalo R, Cesari E, Vinci E, Castagna A. Role of metalloproteinases in rotator cuff tear. Sports Med Arthrosc. 2011;19(3):207–212. doi:10.1097/JSA.0b013e318227b07b
35. Riley GP, Curry V, DeGroot J, et al. Matrix metalloproteinase activities and their relationship with collagen remodelling in tendon pathology. Matrix Biol. 2002;21(2):185–195. doi:10.1016/S0945-053X(01)00196-2
36. Pasternak B, Schepull T, Eliasson P, Aspenberg P. Elevation of systemic matrix metalloproteinases 2 and 7 and tissue inhibitor of metalloproteinase 2 in patients with a history of Achilles tendon rupture: pilot study. Br J Sports Med. 2010;44(9):669–672. doi:10.1136/bjsm.2008.049411
37. Karousou E, Ronga M, Vigetti D, Passi A, Maffulli N. Collagens, proteoglycans, MMP-2, MMP-9 and TIMPs in human achilles tendon rupture. Clin Orthop Relat Res. 2008;466(7):1577–1582. doi:10.1007/s11999-008-0255-y
38. Dong M, Zhong L, Chen WQ, et al. Doxycycline stabilizes vulnerable plaque via inhibiting matrix metalloproteinases and attenuating inflammation in rabbits. PLoS ONE. 2012;7(6):e39695. doi:10.1371/journal.pone.0039695
39. Golub LM, Lee HM, Ryan ME, Giannobile WV, Payne J, Sorsa T. Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Adv Dent Res. 1998;12(2):12–26. doi:10.1177/08959374980120010501
40. Griffin MO, Ceballos G, Villarreal FJ. Tetracycline compounds with non-antimicrobial organ protective properties: possible mechanisms of action. Pharmacol Res. 2011;63(2):102–107. doi:10.1016/j.phrs.2010.10.004
41. Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol. 2008;75(2):346–359. doi:10.1016/j.bcp.2007.07.004
42. Pasternak B, Missios A, Askendal A, Tengvall P, Aspenberg P. Doxycycline-coated sutures improve the suture-holding capacity of the rat Achilles tendon. Acta Orthop. 2007;78(5):680–686. doi:10.1080/17453670710014392
43. Pasternak B, Fellenius M, Aspenberg P. Doxycycline impairs tendon repair in rats. Acta Orthop Belg. 2006;72(6):756–760.
44. Makadia HK, Siegel SJ. Poly Lactic-co-Glycolic Acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers-Basel. 2011;3(3):1377–1397. doi:10.3390/polym3031377
45. Kumbar SG, Nukavarapu SP, James R, Nair LS, Laurencin CT. Electrospun poly(lactic acid-co-glycolic acid) scaffolds for skin tissue engineering. Biomaterials. 2008;29(30):4100–4107. doi:10.1016/j.biomaterials.2008.06.028
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
