Back to Journals » OncoTargets and Therapy » Volume 12
Downregulation of survivin by adenovirus-mediated shRNA promotes apoptosis in skin cancer cells
Authors Hao Y, Bai X, Liu X, Kang S, Zhang X, Liu C, Li Z
Received 10 January 2018
Accepted for publication 9 June 2018
Published 17 April 2019 Volume 2019:12 Pages 2921—2930
DOI https://doi.org/10.2147/OTT.S162150
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr XuYu Yang
Yuqin Hao,1 Xuefeng Bai,2 Xia Liu,1,3 Shuxia Kang,1,3 Xin Zhang,1,3 Caiyun Liu,4 Zhehai Li3,5
1Department of Dermatology, Third Affiliated Hospital of Inner Mongolia Medical University, Baotou, 014010, People’s Republic of China; 2Department of Pathology, Baotou Cancer Hospital, Baotou, 014030, People’s Republic of China; 3Inner Mongolia Medical University, Hohhot, 010000, People’s Republic of China; 4Hunan Youcheng Biotechnology Co. Ltd, Changsha, 410000, People’s Republic of China; 5Department of Orthopedics, Beijing Northern Hospital, China North Industries, Beijing, 100089, People’s Republic of China
Background: Survivin, a member of the inhibitor of apoptosis protein family, is highly expressed in many cancers and has important roles in inhibiting apoptosis by blocking caspase activation. However, its antitumor effects remain largely unknown. Here we explore the function of survivin in skin cancer.
Methods: We used qPCR and Western blot to examine survivin expression in skin cancer patients and cell line. We generated several survivin shRNA constructs and tested the effects of survivin shRNA on cancer cell viability using MTT assay, flow cytometry, and TUNEL assay.
Results: We found that survivin was upregulated in both skin cancer patients and skin cancer cell line A431. Knockdown survivin via shRNA inhibited cancer cell proliferation and promoted apoptosis in both A431 cell and in vivo xenograft tumor mouse model. The antitumor effect is comparable to resveratrol, a drug known to inhibit cancer progression. Moreover, we showed that inhibition of survivin was able to increase the expression of cleaved caspase 7/caspase 9 and activate the ataxia-telangiectasia mutated-NF-κB pathway in A431 cells.
Conclusion: Survivin-shRNA possesses antitumor abilities in vitro and in vivo by inhibiting the proliferation and promoting apoptosis of A431 cells. It may serve as a potential anticancer target for skin cancer therapy in the future.
Keywords: survivin, skin cancer, apoptosis, NF-κB, caspases 7/9
Introduction
The cutaneous squamous cell carcinoma (cSCC) is the second most common non-melanoma skin cancer, accounting for about 20% of all cutaneous malignancies.1–3 Its incidence has increased worldwide in the past years. At present, the pathogenesis of cSCC is not entirely clear, but excessive proliferation and inhibition of apoptosis have been identified to be related.4 While most cSCCs can be successfully managed with excision, there is a subset of lesions that metastasize, leading to severe morbidity and mortality. Therefore, more effective therapies are needed.
Survivin is the smallest member of the inhibitor of apoptosis (IAP) protein family.5,6 It functions to inhibit caspase activation, thereby resulting in negative regulation of apoptosis. The survivin protein has been implicated in mitosis as well.7 Among those IAPs,8 survivin shows the most striking overexpression in various tumors, but its expression is at low level or completely undetectable in most of the normal tissues.9,10 For cSSC patients, almost all of them had high levels of survivin. Moreover, overexpression of survivin is generally associated with poor prognosis, tumor aggressiveness, drug resistance, bad clinical outcome, and overall low rate of survival in cSCC patients.11 Moreover, many studies have shown survivin overexpression in cervical SCC compared to the normal tissues, and its expression positively associated with lesion size, lymphovascular invasion, tumor grade, and clinical stages. All these findings suggest survivin participate in the onset and progression of cervical carcinoma.12,13 In addition, it also has been shown that downregulation of survivin strongly induces cancer cell apoptosis, thereby suppressing tumor growth such as cervical SCC.11 These features of survivin make it an ideal target for cancer therapy.
Resveratrol (Res) is a naturally occurring polyphenol suggested to have beneficial health effects, including improved metabolism, cardioprotection, and cancer prevention.14,15 In 1997, its cancer chemopreventive properties were first discovered by Jang et al, when they demonstrated the anti-initiation, anti-promotion, and anti-progression activities of Res in different tumors.16 Following that, a body of studies has shown that Res inhibits tumor growth in vivo against multiple cancer types, which are dose- and duration-dependent. It is also believed to work as a chemopreventive agent by producing its effect on cell apoptosis, antiproliferation, and anti-inflammation.17 Currently, there are many clinical trials going on to confirm its potential as a therapeutic agent for cancers, neurological disorders, and cardiovascular diseases in human beings.18
In the present study, we fully investigated the role of survivin in skin cancer and the effect of survivin shRNA on skin cancer progression. We found that survivin expression was greatly increased in both skin cancer patients and a human epidermoid carcinoma cell line termed A431. Furthermore, knockdown of survivin expression through survivin shRNA inhibited cancer cell proliferation and promoted apoptosis through regulation of caspases and NF-κB signaling in both in vitro cancer cell line model and in vivo mouse model. Noticeably, the antitumor effect of survivin shRNA is comparable to Res. Altogether, our data suggest that survivin plays a key role in skin cancer and that it could serve as a molecular target for therapeutic interventions in SCC.
Materials and methods
Cell culture and drug treatments
A431 cells were purchased from the American Tissue Culture Collection (ATCC:CRL-1555™) and cultured in Dulbecco’s Modified Eagle’s Medium (GE Healthcare Bio-Sciences Corp., Piscataway, NJ, USA) containing 10% fetal bovine serum and penicillin-streptomycin (100 IU/mL) at 37°C in a humidified atmosphere containing 5% CO2. The cells were seeded and treated with shRNA or Res (15 mg/L) for indicated time. qPCR, Western blotting, and flow cytometry were performed at 48 hours posttreat; MTT assays were did at 24, 48, and 72 hours after treatment or transfection.
Tissue samples
We obtained 15 paired SCC samples and adjacent non-tumor normal epidermal tissues (located more than 5 cm away from the tumors) from Inner Mongolia Medical University Third Hospital in 2016. These 15 patients were all diagnosed as SCC and no other treatments were received before surgery. Both tumor and non-tumor samples were confirmed by pathological examinations. These samples were snap-frozen in liquid nitrogen after resection. Written informed consent was obtained from each patient and the study was approved by the Ethics Committee of Inner Mongolia Medical University.
Interference vector transfection
The shRNA oligos for survivin gene knockdown were designed and synthesized as previously described.19 They were synthesized from GenePharm Co., Ltd (Shanghai, China). The survivin shRNA targeting sequences were as follows: sh1, 5′-GGCTGGCTTCATCCACTGC-3′; sh2, 5′-GCAGTTTGAAGAATTAACC-3′; sh3, 5′-GAATTAACCCTTGGTGAAT-3′. sh-NC was used as negative control. The A431 cells were seeded in six-well plates and transfected with constructed vectors. Transfection was performed using Lipofectamine 2000 according to the manufacturer’s protocol. The transfection efficiency was examined under florescence microscopy 48 hours after transfection. RT-PCR and Western blot analysis were employed to evaluate the inhibitory activity.
RNA extraction and q-PCR
Total RNA was isolated from the cells using Trizol according to the manufacturer’s instructions. DNaseI was used to avoid DNA contamination; ~1 μg total RNA from each sample was subjected to reverse transcription using QuantiTect Reverse Transcription Kit (QIAGEN). qPCRs were performed in triplicate for each sample using the Maxima SYBR Green qPCR Master Mix (TOYOBO) and the following primers: survivin forward: 5′-AGATGACGACCCCATGCAAA-3′ and survivin reverse: 5′-CGCACTTTCTCCGCAGTTTC-3′; and GAPDH forward: 5′-TGCACCACCAACTGCTTAGC-3′; GAPDH reverse: 5′-GGCATGGACTGTGGTCATGAG-3′, which was used as reference gene. The data were quantified using the ΔΔCq method.
Western blotting
Total protein was extracted from the cells with RIPA lysis buffer (Beyotime Institute of Biotechnology, Nantong, China), and the concentration of the protein was determined with Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA). Protein samples were separated by 12% SDS-polyacrylamide gels and then transferred to PVDF membranes (EMD Millipore, Billerica, MA, USA). The membranes were blocked for 1 hour at room temperature. Subsequently, primary antibodies were added and incubated overnight at 4°C. Antibodies against survivin, caspase 3, 7 and 9, p53, and NF-κB were from Cell Signaling Technology (Danvers, MA, USA) and diluted by 1:1,000, β-actin (Santa Cruz Biotechnology Inc., Dallas, TX, USA) were diluted by 1:3,000. Following washes with TBST, the membranes were incubated with a secondary antibody (anti-mouse and anti-rabbit IgG [H+L] antibodies diluted by 1:1,000, KPL, USA) for 1 hour at room temperature. Protein bands were visualized using the ECL kit.
Cell proliferation assay
Cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)–2,5-diphenyltetrazolium bromide (MTT) assay (Sigma-Aldrich Co., St Louis, MO, USA). A431 cells were seeded in a 96-well plate (Corning Incorporated, Corning, NY, USA) at a density of 5×103 cells each well and treated with Res (15 mg/L) or transfected with survivin-shRNA or shRNA-NC. MTT (5 mg/mL) was added at 24, 48, and 72 hours post-transfection, and the cells were incubated for additional 4 hours. After the supernatant was removed, 150 μL dimethyl sulfoxide was added. The absorbance of each well at 490 nm was measured using a plate reader.
Cell apoptosis analysis
The effect of survivin knockdown on cell apoptosis was evaluated by flow cytometry, using an FITC Annexin V apoptosis kit (BD, Franklin Lakes, NJ, USA) as previously described.20 In brief, cells were washed with ice-cold PBS and resuspended in binding buffer at a concentration of 106 cells/mL. Cells were then stained with Annexin V-FITC and propidium (PI) for 15 minutes before being analyzed with flow cytometer: Annexin V-FITC (-) and PI (-), living cells; Annexin V-FITC (+) and PI (-), early apoptotic cells; and Annexin V-FITC (+) and PI (+), late apoptotic cells and necrotic cells. Both Annexin V-FITC (+)/PI (-) and Annexin V-FITC (+)/PI (+) cells were quantified as apoptotic cells.20,21
Tumor growth in mice
Four-week-old nude mice were acquired from Hunan Slack King Laboratory Animal Co., Ltd. (Changsha, China) and kept in the laboratory conditions. Experiments were conducted with the approval of the Animal Care and Use Committee of Inner Mongolia Medical University. Further, we conducted this experiment based on the welfare according to Inner Mongolia Medical University guidelines. Eight-week-old nude mice were implanted with unilateral subaxillary subcutaneous injection of 1×107 A431 cells to induce primary tumors. Mice were divided randomly into four groups (ie, control group, survivin-shRNA group, shRNA-NC group, and the Res group; five mice per group) on the 8th day and received an intratumor injection of survivin-shRNA (2×1010 pfu), shRNA-NC (2×1010 pfu), or Res (50 mg/kg) every 2 days at total of three times. The control mice received 150 μL PBS. Tumor volume was measured and calculated according to the formula: length/2 × width2.
TUNEL assay
Assessment of cell death was performed by TUNEL method using an in situ cell death detection kit (Hoffman-La Roche Ltd., Basel, Switzerland) according to the manufacturer’s instructions. Five equal-sized fields were randomly chosen and analyzed under microscope.
Statistical analysis
A two-tailed Student’s t-test was applied to analyze statistical differences between two groups. For multiple comparisons, the one-way ANOVA was used to analyze the difference. P<0.05 was considered to be statistically significant. Each test data were repeated at least three times. All data are expressed as the mean ± SD.
Results
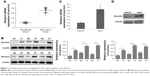
Survivin expression is upregulated in skin cancer patients and cell lines
Many studies have demonstrated the overexpression of survivin in various types of human tumors.11 To study the function of survivin in skin cancer, we first analyzed survivin expression in skin cancer patients. Compared with normal control samples, we found that survivin mRNA level was elevated to ˜3.7-fold in skin cancer patients (Figure 1A). Moreover, we randomly selected six pairs of tissues from 15 patients and detected the protein level of survivin by Western blot. We also found that survivin protein level was significantly increased in skin cancer tissues as compared to nearby normal tissues (Figure 1B). To further confirm our patient study, we used a skin cancer cell line A431. Consistently, we detected a higher expression of survivin in A431, compared with normal skin cells HaCaT. Both mRNA and protein levels were significantly increased (Figure 1C and D). These results indicate that survivin expression is upregulated in both skin cancer patients and cell line.
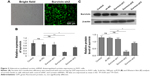
Survivin shRNA inhibits survivin expression in A431 cell
To manipulate the level of survivin in A431 cells, we generated several survivin shRNA constructs. After A431 cells were transfected with shRNAs for 48 hours, the levels of mRNA were determined by q-PCR while the protein expression of survivin was assessed using Western blot analysis. First, we examined the transfection efficiency with microscopy and observed that more than 80% of cells expressed green fluorescent protein (GFP), which indicated a very high transfection rate (Figure 2A). Moreover, we detected an obvious survivin expression decrease in both mRNA and protein levels in cells transfected with survivin-sh2 and survivin-sh3 compared with those transfected with sh-NC while survivin-sh1 had little effect (Figure 2B and C). The knockdown effect of survivin-sh2 was better than that of survivin-sh3. Therefore, survivin-sh2 was used in subsequent experiments.
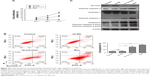
Survivin is involved in skin cancer cell proliferation and apoptosis through regulation of caspase 7, 9
To determine the function of survivin in skin cancer, we tested the effect of survivin-sh2 on the proliferation ability of A431 cells. MTT assay was used to measure cell viability. We used the Res drug as a positive control since it can induce cell apoptosis through suppression of antiapoptotic proteins such as BCL-2, BCL-xL, and survivin.22,23 Inhibition of cell proliferation was noticeable in A431 cells transfected with survivin-sh2 or Res at 48 hours (20% and 30%, respectively) and 72 hours (40% and 60%, respectively) post-transfection, whereas no inhibitory effect was observed in the shRNA-NC group (Figure 3A). Next, the apoptotic population was evaluated by flow cytometer. As shown in Figure 3B, the population of apoptotic cells was significantly increased when cells were transfected with survivin-sh2 or treated with Res. These data suggest that knockdown of survivin induces apoptosis in skin cancer cells, thereby inhibiting cell proliferation.
To investigate the underlying molecular mechanisms, we then measured protein levels of apoptosis-associated caspases after downregulation of survivin. Res treatment inhibited survivin expression and activated caspases 3, 7 and 9. However, knockdown of survivin increased caspase 7 and 9 levels but not caspase 3 (Figure 3C). These data demonstrate that knockdown of survivin inhibits cancer cell apoptosis and proliferation through regulation of caspase 7 and 9.
Survivin knockdown upregulates NF-κB via a conserved pathway of ATM-NF-κB in A431 cells
Previous studies have shown that NF-κB pathway is regulated by survivin during apoptosis.24,25 To test whether this pathway is also involved in skin cancer, we measured NF-κB level after transfection with survivin-sh2. We found that NF-κB was significantly increased in cells transfected with survivin-shRNA or treatment with Res compared with sh-NC and control groups (Figure 4A). We also detected an increase of phosphorylated ataxia-telangiectasia mutated (p-ATM) level but not total ATM level after survivin was knocked down by shRNA (Figure 4A). Moreover, p-ATM inhibitor KU-55933 partly reversed the effect of survivin-sh2, on both NF-κB and p-ATM expression but did not affect survivin level, indicating that survivin shRNA increases NF-κB via a conserved pathway of ATM-NF-κB (Figure 4B). To further investigate the underlying mechanism, we used FACS to analyze the effects of NF-κB pathway on cell apoptosis. Consistently, after survivin was knocked down, we saw a significantly increase of cell apoptosis percentage. Moreover, p-ATM inhibitor KU-55933 further downregulated the percentage of cell apoptosis (Figure 4C). This suggests that activation of NF-κB is a negative feedback mechanism for cancer cells when survivin is downregulated. Altogether, these data demonstrate that survivin knockdown upregulates NF-κB via a conserved pathway of ATM-NF-κB.
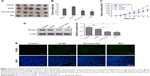
Survivin shRNA inhibits tumor growth in vivo
To further confirm the antitumor activity of survivin shRNA in skin cancer, we evaluated the effect in vivo by using an A431 xenograft tumor model. As shown in Figure 5A, transfection of sh-NC did not inhibit tumor growth when compared with control group. In contrast, transfection of survivin-shRNA or administration of Res inhibited tumor growth significantly in mice bearing A431 xenograft tumors. Tumor weight and volume were both reduced in survivin-sh2 and Res groups (Figure 5B and C). Moreover, we also measured the protein level of survivin in each group and found that survivin-sh2 and Res treatment decreased survivin expression while sh-NC did not (Figure 5D), suggesting that the antitumor effects of survivin-sh2 and Res were related to survivin level. TUNEL results indicated that survivin-sh2 could specifically inhibit tumor growth via activation of cell apoptosis (Figure 5E). Taken together, these results show that survivin shRNA can prevent skin tumor growth in vivo.
Discussion
Despite advances in therapy and research, the prognosis of metastatic cSCC remains very poor, with several large studies demonstrating a mortality of >70%. At present, surgery, radiation therapy, and chemotherapy are the main treatments for cSCC.1–3,26 However, each approach has limited scope of application and side effects as well. For example, surgical excision cannot prevent recurrences, which generally become evident within 2 years after treatment; Radiotherapy is highly expensive and requires multiple visits. Furthermore, tumors that recur after radiotherapy or chemotherapy are likely to be highly regressive. Therefore, new approaches with better clinical outcomes are in demand. Short interfering RNA (siRNA) is one of the members of the family of RNA interference (RNAi), which is able to trigger the cleavage of target RNAs, resulting in the inhibition of protein translation.27,28 Since its discovery, RNAi approaches have been used as an experimental tool in vitro in cell biology. Meanwhile, due to its high specificity and efficiency, sufficient preclinical and clinical studies supported that siRNA could be a potential medicine for targeted therapy of various diseases in the near future. In this study, we further elucidate the role of survivin in skin cancer and evaluate the treatment effects of survivin siRNA on SCC. We demonstrate that downregulation of survivin through siRNA could inhibit cancer cell proliferation and promote apoptosis through regulation of caspases and NF-κB signaling in both in vitro and in vivo and that the effect of survivin siRNA is comparable to Res. Based on these data, we propose a working model shown in Figure 6. Our study identified a novel protein implicated in skin cancer, which might help provide a target for future therapeutic development.
Survivin is a unique member of the IAP family that is expressed in most human tumors including SCC but is barely detected in most of the normal tissues.11 This feature makes survivin an ideal candidate for tumor therapy. Survivin orchestrates various important mechanisms to support tumor cell survival including inhibition of apoptosis and regulation of cell division.6,11 In the case of skin cancer, using cell line and tumor tissues from patients, we first confirmed that both mRNA and protein levels of survivin were greatly upregulated. Then we used in vitro assays to test the possibility that survivin-shRNA could be a potential approach to treat skin cancer. We found that knockdown of survivin induced cancer cell apoptosis and inhibited cell proliferation, similar to Res. These data highly suggest that survivin-shRNA could be used to prevent the progression of skin cancer.
Molecularly, we show that survivin-shRNA induce cell apoptosis through the regulation of caspases 7 and 9. These data are consistent with previous studies, indicating that survivin can regulate apoptosis signaling. In addition to regulation of caspases, we also observed an upregulation of transcription factor NF-κB when survivin level was knocked down in skin cancer cells. This increase resulted from p-ATM and inhibition of p-ATM could block the increase of NF-κB. Previously, it has been shown that NF-κB could mediate hepatocyte apoptosis through upregulation of survivin.24 In this case, we think downregulation of survivin through shRNA could cause some DNA damage, which then activates cellular repairing signaling including the p-ATM-NF-κB pathway. Using the specific ATM inhibitor KU-55933, we confirm the essential role of ATM in the induction of NF-κB for inhibiting cell apoptosis and partly reversed the effect of survivin-sh2 on A431 cells apoptosis. Altogether, these data suggest that survivin plays a key role in the development of skin cancer and knockdown of its expression through siRNA may be a way to prevent tumor progression.
In the end, we evaluated the effects of survivin-shRNA on skin cancer growth in a mouse skin cancer model. The results indicated that intratumoral injection of survivin-shRNA caused obvious tumor growth inhibition. Both tumor weight and size were greatly reduced. Using TUNEL and DAPI methods, we confirmed that survivin-shRNA induced cancer cell apoptosis in vivo. This anticancer effect is similar to what we saw with the positive control drug Res, indicating that survivin could be used as a potential target for skin cancer treatment. One caveat of this study is that we examined only one cancer line and further characterizations in other cancer lines are needed to provide more evidence.
Conclusion
The results of our study reveal that adenovirus-mediated survivin-shRNA possesses antitumor abilities in vitro and in vivo, by inhibiting the proliferation and promoting apoptosis of A431 skin cancer cells. Compared with chemical drugs, shRNAs are more specific and less costly.27 Therefore, knocking down survivin by shRNA may be considered as a potential therapeutic approach for the treatment of cutaneous squamous cell carcinoma.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Acknowledgment
This study was supported by the Natural Science Foundation of China (grant number 81260407).
Disclosure
Caiyan Liu is employed by Hunan Youcheng Biotechnology Co. Ltd. The authors report no other conflicts of interest in this work.
References
Burton KA, Ashack KA, Khachemoune A. Cutaneous squamous cell carcinoma: a review of high-risk and metastatic disease. Am J Clin Dermatol. 2016;17(5):491–508. | ||
Brougham ND, Tan ST. The incidence and risk factors of metastasis for cutaneous squamous cell carcinoma – implications on the T-classification system. J Surg Oncol. 2014;110(7):876–882. | ||
Parekh V, Seykora JT. Cutaneous squamous cell carcinoma. Clin Lab Med. 2017;37(3):503–525. | ||
Yu X, Li Z. The role of miRNAs in cutaneous squamous cell carcinoma. J Cell Mol Med. 2016;20(1):3–9. | ||
Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3(8):917–921. | ||
Peery RC, Liu JY, Zhang JT. Targeting survivin for therapeutic discovery: past, present, and future promises. Drug Discov Today. 2017;22(10):1466–1477. | ||
Mita AC, Mita MM, Nawrocki ST, Giles FJ. Survivin: key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin Cancer Res. 2008;14(16):5000–5005. | ||
Philchenkov A, Miura K. The IAP protein family, SMAC mimetics and cancer treatment. Crit Rev Oncog. 2016;21(3–4):185–202. | ||
Bongiovanni L, Müller EJ, della Salda L. Survivin in skin pathologies. Exp Dermatol. 2011;20(6):457–463. | ||
Cheung CH, Cheng L, Chang KY, Chen HH, Chang JY. Investigations of survivin: the past, present and future. Front Biosci. 2011;16:952–961. | ||
Khan Z, Khan AA, Yadav H, Prasad G, Bisen PS. Survivin, a molecular target for therapeutic interventions in squamous cell carcinoma. Cell Mol Biol Lett. 2017;22:8. | ||
Liu HQ, Wang YH, Wang LL, Hao M. P16INK4A and survivin: diagnostic and prognostic markers in cervical intraepithelial neoplasia and cervical squamous cell carcinoma. Exp Mol Pathol. 2015;99(1):44–49. | ||
Lee JP, Chang KH, Han JH, Ryu HS. Survivin, a novel anti-apoptosis inhibitor, expression in uterine cervical cancer and relationship with prognostic factors. Int J Gynecol Cancer. 2005;15(1):113–119. | ||
Singh CK, Ndiaye MA, Ahmad N. Resveratrol and cancer: challenges for clinical translation. Biochim Biophys Acta. 2015;1852(6):1178–1185. | ||
Carter LG, D’Orazio JA, Pearson KJ. Resveratrol and cancer: focus on in vivo evidence. Endocr Relat Cancer. 2014;21(3):R209–R225. | ||
Jang M, Cai L, Udeani GO, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275(5297):218–220. | ||
Aluyen JK, Ton QN, Tran T, et al. Resveratrol: potential as anticancer agent. J Diet Suppl. 2012;9(1):45–56. | ||
Berman AY, Motechin RA, Wiesenfeld MY, Holz MK. The therapeutic potential of resveratrol: a review of clinical trials. NPJ Precis Oncol. 2017;1(1):35. | ||
Lee JS, Oh E, Yoo JY, et al. Adenovirus expressing dual c-Met-specific shRNA exhibits potent antitumor effect through autophagic cell death accompanied by senescence-like phenotypes in glioblastoma cells. Oncotarget. 2015;6(6):4051–4065. | ||
Yu H, Xiong J, Zhang R, et al. Ndk, a novel host-responsive regulator, negatively regulates bacterial virulence through quorum sensing in Pseudomonas aeruginosa. Sci Rep. 2016;6:28684. | ||
Li Y, Wang XQ, Zhang L, et al. A SNP in pri-miR-10a is associated with recurrent spontaneous abortion in a Han-Chinese population. Oncotarget. 2016;7(7):8208–8222. | ||
Aggarwal BB, Bhardwaj A, Aggarwal RS, et al. Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res. 2004;24(5A):2783–2840. | ||
Jiang Z, Chen K, Cheng L, et al. Resveratrol and cancer treatment: updates. Ann N Y Acad Sci. 2017;1403(1):59–69. | ||
Wang K, Brems JJ, Gamelli RL, Holterman AX. Survivin signaling is regulated through nuclear factor-kappa B pathway during glycochenodeoxycholate-induced hepatocyte apoptosis. Biochim Biophys Acta. 2010;1803(12):1368–1375. | ||
Zhang Y, Huang H, Zhou H, et al. Activation of nuclear factor κB pathway and downstream targets survivin and livin by SHARPIN contributes to the progression and metastasis of prostate cancer. Cancer. 2014;120(20):3208–3218. | ||
Parikh SA, Patel VA, Ratner D. Advances in the management of cutaneous squamous cell carcinoma. F1000 Prime Rep. 2014;6:70. | ||
Tam C, Wong JH, Cheung RCF, Zuo T, Ng TB. Therapeutic potentials of short interfering RNAs. Appl Microbiol Biotechnol. 2017;101(19):7091–7111. | ||
Selvam C, Mutisya D, Prakash S, Ranganna K, Thilagavathi R. Therapeutic potential of chemically modified siRNA: recent trends. Chem Biol Drug Des. 2017;90(5):665–678. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.