Back to Journals » Cancer Management and Research » Volume 11
Downregulation of Proline Hydroxylase 2 and Upregulation of Hypoxia-Inducible Factor 1α are Associated with Endometrial Cancer Aggressiveness
Authors Zhu C, Ding H, Yang J, Zhou Y, Luo Y, Shi S, Zhang Y, Wei Y, Ni G
Received 16 July 2019
Accepted for publication 30 October 2019
Published 22 November 2019 Volume 2019:11 Pages 9907—9912
DOI https://doi.org/10.2147/CMAR.S223421
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Chengcheng Zhu,1 Huafeng Ding,1 Junwen Yang,1 Yihui Zhou,1 Yonghong Luo,1 Suhua Shi,1 Ying Zhang,1 Yalan Wei,2 Guantai Ni1
1Departments of Gynecology and Obstetrics, Yi Jishan General Hospital of Wannan Medical College, Wuhu, Anhui, People’s Republic of China; 2Departments of Gynecology and Obstetrics, General Hospital of Wuhu Second People’s Hospital, Wuhu, Anhui, People’s Republic of China
Correspondence: Guantai Ni; Yalan Wei
Departments of Gynecology and Obstetrics, Yi Jishan General Hospital of Wannan Medical College, 2 Zheshan West Road, Wuhu, Anhui 241000, People’s Republic of China
Tel +86-138 5532 0259
Email [email protected]; [email protected]
Introduction: Proline hydroxylase 2 (PHD2) is involved in tumorigenesis. This study aimed to examine PHD2 and hypoxia-inducible factor 1α (HIF-1α) expression in different endometrial tissues and explore the correlations between PHD2 and HIF-1α expression with clinicopathological characteristics of endometrial cancer.
Methods: We collected 50 tissue sections of endometrial adenocarcinoma, 30 of atypical endometrial hyperplasia, and 30 of control normal endometrium. The expression of PHD2 was detected by PCR, Western blot, and immunohistochemical analysis.
Results: PHD2 mRNA and protein levels reduced in endometrial cancer tissues compared to normal endometrium (p<0.05). In contrast, HIF-1α expression levels increased in endometrial cancer tissues compared to normal endometrium (p<0.05). In addition, PHD2 and HIF-1α levels were correlated with lymphovascular stromal invasion (LVSI), postoperative FIGO stage, and lymph node metastasis of endometrial cancer (p<0.05).
Conclusion: Our findings suggest that reduced expression of PHD2 and increased expression of HIF-1α are associated with endometrial cancer aggressiveness. PHD2 might be a novel biomarker and a potential target for endometrial cancer management.
Keywords: proline hydroxylase, hypoxia-inducible factor, endometrial cancer, prognosis
Introduction
Endometrial cancer (EC) is the sixth most common malignancy in females worldwide.1 Endometrioid adenocarcinoma is the most common subtype of EC, accounting for 80% of all EC.2 Most early-stage cancers respond well to radiotherapy and chemotherapy. However, radiotherapy and chemotherapy failure are sometimes observed and are attributed to hypoxia as a consequence of cell proliferation and angiogenesis.3
The hypoxia-inducible factor (HIF)-1 pathway regulates the expression of genes that promote angiogenesis, invasion, glycolysis, and pH regulation.4,5 HIF-1 and Proline hydroxylase (PHD) 1–3, and the four human HIF-α hydroxylases belong to a family of 2-oxoglutarate-dependent, non-heme iron-binding dioxygenases.6 Among them, PHD2 is considered to play an important role in the regulation of HIF.7–9 The stability of HIF-1α is affected by PHD2, which in turn can accelerate or decelerate cell proliferation and angiogenesis through multiple pathways.10 PHD2 is expressed in several types of cancers, but the clinical significance of PHD2 in EC remains unclear. Therefore, this study aimed to examine PHD2 expression in EC and explore the correlations of PHD2 and HIF-1α expression with clinicopathologic characteristics of EC, including lymph node metastasis, LVSI, and postoperative International Federation of Gynecology and Obstetrics (FIGO) stage.
Materials and Methods
Patients
Endometrium tissue samples were collected from 30 volunteers, 30 patients with atypical endometrial hyperplasia, and 50 patients with EC at the Department of Obstetrics and Gynecology, Yi Jishan Hospital of Wannan Medical College, between Jan 2017 and December 2018. All samples were resected before any treatment. This study was approved by the Scientific research IRB of Wannan Medical College Yi Jishan Hospital and was conducted in accordance with the Declaration of Helsinki. Informed written consent was obtained from all participants.
Real-Time PCR
Total RNA was extracted from endometrial tissue samples with the RNeasy Kit (Qiagen) following the manufacturer’s instructions. cDNA synthesis was performed using a first-strand cDNA synthesis kit (Thermo Scientific), and PCR was performed using SYBR Green PCR Master Mix (Qiagen), 2 μL of diluted cDNA, and 200 nmol/L oligonucleotide primers as follows: PHD2 5ʹGGGACATTCATTGCCTCACTCTC3ʹ (forward) and 5ʹGCTTGCTGTTATGTGCCCAATC3ʹ (reverse); HIF-1α 5ʹACTTCTGGATGCTGGTGATT3ʹ (forward) and 5ʹTCCTCGGCTAGTTAGGGTAC3ʹ (reverse). Real-time PCR was performed in triplicate and relative mRNA levels of target genes were calculated as the difference of reaction cycle thresholds (Ct) between GAPHD and each of the target genes (2−ΔCt).
Western Blot Analysis
Endometrial tissues were homogenized in RIPA lysis buffer and centrifuged at 12,000 rpm/min and 4°C for 5 mins. The supernatant was collected, and protein extract (20 µg) was separated by 10% SDS polyacrylamide gel electrophoresis and then transferred to nitrocellulose membranes. The membranes were blocked and incubated with antibody for PHD2, HIF-1α, or β-actin (Affinity Biosciences, diluted at 1:1000) overnight at 4°C and then with horseradish peroxidase conjugated secondary antibody (Affinity Biosciences, diluted at 1:4000) for 1 hr at room temperature. The membranes were developed using chemiluminescence substrate (Affinity Biosciences, China) and exposed to X-ray films. The intensity of the bands from triplicate experiments was quantified with Image.plus5.1 software (Media Cybernetics, Rockville, MD, USA).
Immunohistochemistry
Endometrial tissues were fixed in 40 g/L paraformaldehyde, embedded and then cut into 4 μm serial sections. The sections were then subjected to deparaffinization, heat-induced antigen retrieval with EDTA pH 8.0, hydrogen peroxide quenching, and then incubated with PHD2 or HIF-1α antibody (1:100 dilution), biotinylated secondary antibody, streptavidin-biotin-peroxidase complex, and DAB. Staining for PHD2 and HIF-1α was evaluated by three experienced pathologists in a blind manner, and scored positive if the staining was observed in the cytoplasm or nuclei. Scores were calculated in a semiquantitative fashion by multiplying the intensity (negative scored as 0, weak scored as 1, moderate scored at 2, and strong scored as 3) with the percentage of stained cells (0, no cells; 1, 1–10%; 2, 10–50%; 3, 50–75%; and 4, 75–100%).
Statistical Analysis
The data were processed by GraphPad Prism 7.0 software and analyzed by statistical software SPSS Version 20.0. The comparison of all categorical variables was made by chi-square test, and p<0.05 was considered statistically significant.
Results
Reduced Expression of PHD2 in Endometrial Tissues from EC Patients
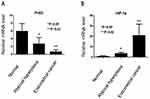
RT-PCR showed that PHD2 mRNA level was the lowest in endometrial tissues from EC patients and the highest in normal endometrium tissues (Figure 1A), while HIF-1α mRNA level was the highest in endometrial tissues from EC patients and the lowest in normal endometrium tissues (Figure 1B).
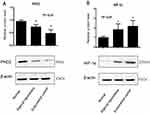
Western blot analysis showed that PHD2 protein level was the lowest in endometrial tissues from EC patients and the highest in normal endometrium tissues (Figure 2A), while HIF-1α protein level was the highest in endometrial tissues from EC patients and the lowest in normal endometrium tissues (Figure 2B).
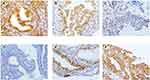
Furthermore, immunohistochemistry analysis showed that PHD2 expression was mainly localized in the cytoplasm of normal endometrial cells and HIF-1α expression was mainly localized in the cytoplasm of endometrial tumor (Figure 3). Quantitative analysis of immunohistochemical staining showed that PHD2 staining was the strongest in normal endometrium tissues while HIF-1α staining was the strongest in endometrial tissues from EC patients (Table 1).
 |
Table 1 PHD2 Staining in Endometrial Tissues |
Association of PHD2/HIF-1α Expression and Clinicopathological Characteristics of EC Patients
The correlation between PHD2/HIF-1α expression and clinicopathological characteristics in endometrial cancer samples was shown in Table 2. Reduced PHD2 expression correlated with FIGO stage (p=0.012), LVSI (p=0.015), and lymph node metastasis (p=0.012). Elevated HIF-1α expression was associated with FIGO stage (p=0.015), LVSI (p=0.032), and lymph node metastasis (p=0.015). In contrast, no significant correlation was found between PHD2/HIF-1α expression and tumor size, histologic grade, and stromal infiltration depth (p>0.05).
 |
Table 2 Relationship of PHD2 and HIF-1α Expression with Clinicopathologic Characteristics of EC |
Discussion
PHD2 expression in mRNA and protein levels was reduced in EC samples compared with normal endometrium. In contrast, HIF-1α expression was elevated in EC samples compared with normal endometrium. Therefore, PHD2 downregulation and HIF-1α upregulation were significantly associated with EC tumorigenesis.
We explored the correlation between PHD2/HIF-1α expression and clinicopathological characteristics of EC. There was an obvious association between low PHD2 expression and lymph node metastasis, LVSI, and FIGO stage. PHD2 was markedly correlated with malignant transformation and tumor progression. Recent studies have shown that elevated PHD2 expression might serve as a valuable biomarker for poor prognosis in patients with lung and gastric adenocarcinomas.11–13
PHD2 regulates oxygen metabolism, producing a slight decrease in proline hydroxylation and a rapid increase in HIF-1α level.14 As PHD2 negatively regulates HIF-1α, reduced PHD2 expression is considered to promote tumorigenesis and metastasis of various cancers. Kato et al showed that uterine sarcoma was associated with a high frequency of PHD2 gene abnormalities, and had a poor prognosis.15 In pancreatic cancer, PHD2 functioned as a tumor suppressor gene that inhibited tumor growth and reduced tumor invasion by inhibiting angiogenesis.16 In colorectal cancer, low expression of PHD2 predicted poor survival, independent of HIF-1α.17 Moreover, reduced activity or expression of PHD2 can be associated with tumorigenesis and metastasis of various cancers.18 We assessed PHD2 and HIF-1α mRNA and protein levels from endometrial tissues of different histological subtypes. Our results showed the downregulation of PHD2 and the upregulation of HIF-1α in EC tissues are consistent with previous reports on the opposite role of PHD2 and HIF-1α in tumorigenesis. In addition, low levels of PHD2 expression correlated with LVSI, lymph node metastasis, and FIGO stage of EC patients.
Reduced PHD2 expression could contribute to tumorigenesis and lymph node metastasis by negatively regulating HIF-1α. Therefore, PHD2 might be a novel biomarker for poor prognosis and metastasis in patients with endometrial cancer. In addition, PHD2 could be utilized as a target to screen novel components with anti-tumor activity.19 Nevertheless, our study has several limitations. The sample sizes of the groups were small, and we did not get follow-up data on the prognosis of patients.
In conclusion, these results show that reduced expression of PHD2 is associated with EC aggressiveness. PHD2 might be a novel biomarker and a potential target for EC management.
Acknowledgment
This study was supported by Science-Technology Foundation for Middle-aged and Young Scientist of Wannan Medical College (WK2017F30).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.v68.6
2. Lai-Tiong F. Metastatic recurrence after a pT1a grade 1 endometrioid endometrial adenocarcinoma. Eur J Gynaecol Oncol. 2018;39(2):314–315.
3. Muz B, de la Puente P, Azab F, Azab AK. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia (Auckl). 2015;3:83–92. doi:10.2147/HP
4. Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9(6):677–684. doi:10.1038/nm0603-677
5. Park Y, Moon YJ, Kwon JY, Kim AL, Kim YH. The expression of sirtuin1 in normal and preeclamptic villous explants under hypoxia. Clin Exp Obstet Gynecol. 2018;45(1):75–80.
6. Nguyen LK, Cavadas MA, Scholz CC, et al. A dynamic model of the hypoxia-inducible factor 1α (HIF-1α) network. J Cell Sci. 2013;126(Pt 6):1454–1463. doi:10.1242/jcs.119974
7. Appelhoff RJ, Tian YM, Raval RR, et al. Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J Biol Chem. 2004;279(37):38458–38465. doi:10.1074/jbc.M406026200
8. Berra E, Benizri E, Ginouvès A, Volmat V, Roux D, Pouysségur J. HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia. EMBO J. 2003;22(16):4082–4090. doi:10.1093/emboj/cdg392
9. Na YR, Woo DJ, Kim SY, Yang EG. Pyrithione Zn selectively inhibits hypoxia-inducible factor prolyl hydroxylase PHD3. Biochem Biophys Res Commun. 2016;472(2):313–318. doi:10.1016/j.bbrc.2016.02.115
10. Niecknig H, Tug S, Reyes BD, Kirsch M, Fandrey J, Berchner-Pfannschmidt U. Role of reactive oxygen species in the regulation of HIF-1 by prolyl hydroxylase 2 under mild hypoxia. Free Radic Res. 2012;46(6):705–717. doi:10.3109/10715762.2012.669041
11. Xu XL, Gong Y, Zhao DP. Elevated PHD2 expression might serve as a valuable biomarker of poor prognosis in lung adenocarcinoma, but no lung squamous cell carcinoma. Eur Rev Med Pharmacol Sci. 2018;22(24):8731–8739.
12. Kamphues C, Wittschieber D, Klauschen F, et al. Prolyl hydroxylase domain 2 protein is a strong prognostic marker in human gastric cancer. Pathobiology. 2012;79(1):11–17. doi:10.1159/000330170
13. Andersen S, Donnem T, Stenvold H, et al. Overexpression of the HIF hydroxylases PHD1, PHD2, PHD3, and FIH are individually and collectively unfavorable prognosticators for NSCLC survival. PLoS One. 2011;6(8):e23847. doi:10.1371/journal.pone.0023847
14. Hirsilä M, Koivunen P, Günzler V, Kivirikko KI, Myllyharju J. Characterization of the human prolyl 4-hydroxylases that modify the hypoxia-inducible factor. J Biol Chem. 2003;278(33):30772–30780. doi:10.1074/jbc.M304982200
15. Kato H, Inoue T, Asanoma K, Nishimura C, Matsuda T, Wake N. Induction of human endometrial cancer cell senescence through modulation of HIF-1alpha activity by EGLN1. Int J Cancer. 2006;118(5):1144–1153. doi:10.1002/ijc.21488
16. Su Y, Loos M, Giese N, et al. Prolyl hydroxylase-2 (PHD2) exerts tumor-suppressive activity in pancreatic cancer. Cancer. 2012;118(4):960–972. doi:10.1002/cncr.26344
17. Xie G, Zheng L, Ou J, et al. Low expression of prolyl hydroxylase 2 is associated with tumor grade and poor prognosis in patients with colorectal cancer. Exp Biol Med (Maywood). 2012;237(7):860–866. doi:10.1258/ebm.2012.011331
18. Singh L, Aldosary S, Saeedan AS, Ansari MN, Kaithwas G. Prolyl hydroxylase 2: a promising target to inhibit hypoxia-induced cellular metabolism in cancer cells. Drug Discov Today. 2018;23(11):1873–1882. doi:10.1016/j.drudis.2018.05.016
19. Montes FQ, Vázquez-Hernández A, Fenton-Navarro B. Active compounds of medicinal plants, mechanism for antioxidant and beneficial effects. Phyton, Int J Exp Botany. 2019;88(1):1–10.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.