Back to Journals » OncoTargets and Therapy » Volume 13
Downregulation of miR-575 Inhibits the Tumorigenesis of Gallbladder Cancer via Targeting p27 Kip1
Authors Qin Y, Mi W, Huang C, Li J, Zhang Y, Fu Y
Received 2 September 2019
Accepted for publication 18 February 2020
Published 1 May 2020 Volume 2020:13 Pages 3667—3676
DOI https://doi.org/10.2147/OTT.S229614
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Nicola Silvestris
Yiyu Qin, 1,* Wunan Mi, 2,* Cheng Huang, 1 Jian Li, 1 Yizheng Zhang, 2 Yang Fu 2
1Clinical Medical College, Jiangsu Vocational College of Medicine, Yancheng, Jiangsu 224005, People’s Republic of China; 2Department of Gastrointestinal Surgery, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan 450052, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yiyu Qin
Clinical Medical College, Jiangsu Vocational College of Medicine, 283 Jiefang South Road, Yancheng, Jiangsu 224005, People’s Republic of China
Email [email protected]
Yang Fu
Department of Gastrointestinal Surgery, The First Affiliated Hospital of Zhengzhou University, 1 Jianshe East Road, Zhengzhou, Henan 450052, People’s Republic of China
Email [email protected]
Background: Gallbladder cancer (GBC) is the most common biliary tract malignant cancer worldwide. It has been reported that microRNA-575 (miR-575) was involved in the tumorigenesis of many cancers. However, the role of miR-575 during the progression of GBC remains largely unknown.
Methods: The expression of miR-575 in GBC cells was detected by quantitative real-time polymerase chain reaction. The proliferation of GBC cells was examined by CCK-8 assay and Ki-67 staining. Apoptosis of GBC cells was measured by flow cytometry, and cell invasion was tested by transwell assay. Moreover, protein expressions in GBC cells were evaluated using Western blot. The target gene of miR-575 was predicted using Targetscan and miRDB. Finally, xenograft tumor model was established to verify the function of miR-575 in GBC in vivo.
Results: Our findings indicated that miR-575 antagonist decreased the proliferation and invasion of GBC cells. In addition, miR-575 antagonist significantly induced apoptosis of GBC cells via inducing G1 arrest. Meanwhile, p27 Kip1 was found to be a direct target of miR-575 with luciferase reporter assay. Moreover, miR-575 antagonist significantly decreased the expressions of CDK1 and cyclin E1 and upregulated the levels of cleaved caspase3 and p27 Kip1 in GBC cells. Finally, miR-575 antagonist notably suppressed GBC tumor growth in vivo.
Conclusion: Downregulation of miR-575 significantly inhibited the tumorigenesis of GBC via targeting p27 Kip1. Thus, miR-575 might be a potential novel target for the treatment of GBC.
Keywords: miR-575, p27 Kip1, gallbladder cancer
Corrigendum for this paper has been published
Introduction
Gallbladder cancer is the most common biliary tract malignant cancer worldwide.1 Patients with GBC are always been diagnosed at an advanced stage due to the ignorance of early symptoms.2 Nowadays, surgery or chemotherapy is regarded as the major treatment of GBC, while only 10% patients with GBC have good prognosis.3 Moreover, large number of patients with GBC have suffered from severe side effects after chemotherapy and surgical operation.4 Therefore, it is necessary to explore novel therapeutic methods for the treatment of GBC.
MicroRNAs (miRNAs) are endogenic noncoding small RNAs which are generous in body. Aberrant miRNA expression has found to be related with the progression of multiple diseases recently.5 In addition, it has been confirmed that miRNAs play key roles in the tumorigenesis of malignancy.6 MiR-575 is one of the miRNAs which has been firstly reported in 2009.7 MiR-575 was involved in the progression of Kawasaki disease.8 Meanwhile, a previous study has indicated that BH3-like motif-containing protein (BLID) was a direct target of miRNA-575 in the tumorigenesis of non-small cell lung cancer (NSCLC) in vitro,9 indicating its key role during the progression of malignancies. Yao et al revealed that miR-575 together with the other miRNAs were significantly overexpressed in tumor tissues of patients with GC.7 However, the role of miR-575 during the progression of GBC remains unclear.
Cyclin-dependent kinase (CDK) inhibitor p27 Kip1 was found to be a member of the CIP/KIP family.10 In addition, p27 Kip1 has been mostly studied for its roles in inhibiting G1 progression and maintaining cell quiescence in response to anti-proliferative signals or terminal differentiation.11–13 It can be regarded that p27 Kip1 may act as an important regulator in the growth of malignant tumors by regulation of CDK2 and cyclin E1.14 Besides, downregulation of p27 Kip1 has been regarded as an independent prognostic factor in GBC.15 In the current study, we aimed to investigate the function of miR-575 during the tumorigenesis of GBC and explore the correction between miR-575 and p27 Kip1.
Materials and Methods
Cell Culture
GBC-SD and G415 cell lines were purchased from Cell Bank of the Chinese Academy of Science (Shanghai, China) and RIKEN Cell Engineering Division-Cell Bank (Tokyo, Japan), respectively. All GBC cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Thermo Fisher Scientific) with 10% FBS (Thermo Fischer Scientific), 1% penicillin and streptomycin (Thermo Fisher Scientific) at 37°C, 5% CO2.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted from GBC-SD or G415 cell lines using TRIzol reagent (TaKaRa, Tokyo, Japan) according to the manufacturer’s protocol. cDNA was synthesized using the reverse transcription kit (TaKaRa, Ver.3.0). Real-Time qPCRs were performed in triplicate under the following protocol: 2 min at 94°C, followed by 35 cycles (30 s at 94°C and 45 s at 55°C). The primers for miR-575 and U6 were obtained from GeneCreate Biological Engineering Co., Ltd (Wuhan, China). miR-575: forward, 5ʹ-GTCCACCGCAAATGCTTCTA-3ʹ and reverse 5ʹ- CCATCAGTCCCGTCTTGAAAC-3ʹ. U6: forward, 5ʹ- CTCGCTTCGGCAGCACAT-3ʹ and reverse 5ʹ- AACGCTTCACGAATTTGCGT-3ʹ. The relative fold changes were calculated using the 2−ΔΔCt method by the formula: 2−(sample ΔCt – control ΔCt), where ΔCt is the difference between the amplification fluorescent thresholds of the gene of interest and the internal reference gene (U6) used for normalization.
MiR-575 Transfection
MiR-575 agonist, miR-575 antagonist, or negative control RNAs (all at 10 nM) were transfected into GBC cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA).16 MiR-575 agonist, antagonist, and negative control RNAs were purchased from GenePharma (Shanghai, China). The sequences of miR-575 agonist/antagonist were as follows: miR-575 agonist, GAGCCAGUUGGACAGGAGC and miR-575 antagonist, CUCGGUCAACCUGUCCUCG.
CCK-8 Assay
GBC cells were seeded in 96-well plates (5×103 per well) overnight. Then, cells were treated with negative control (NC) or miR-575 antagonist for 0, 24, 48, and 72 h, respectively. Ten microliters of CCK-8 reagent (Beyotime, Shanghai, China) were added to each well and further incubated for 2 h at 37°C. Finally, the absorbance of GBC cells was measured at 450 nm using a microplate reader (Thermo Fisher Scientific).
Immunofluorescence
GBC-SD cells were seeded in 24-well plates overnight. Then, cells were treated with NC or miR-575 antagonist for 72 h. Next, cells were blocked with 10% goat serum for 30 min at room temperature and then incubated with anti-Ki67 antibody (Abcam, Cambridge, MA, USA; 1:1000) at 4°C overnight, followed by incubation with goat anti-rabbit IgG (Abcam; 1:5000) at 37°C for 1 h. Then, the nuclei were stained with DAPI (Beyotime, Shanghai, China) for 5 min. Finally, cells were observed under a fluorescence microscope (Olympus CX23, Tokyo, Japan).
Cell Apoptosis Analysis
GBC-SD cells were trypsinized, washed with phosphate-buffered saline and resuspended in Annexin V Binding Buffer, followed by staining with 5 μL FITC and 5 μL propidium (PI) in the system for 15 min. Cells were analyzed using flow cytometer (BD, Franklin Lake, NJ, USA) to test the cell apoptosis rate.
Cell Cycle Detection
GBC-SD cells were treated with NC or miR-575 antagonist for 72 h, respectively. Then, the cell cycle distribution was detected by flow cytometry (BD) as previously described.17 Briefly, cell cycle detection was performed using Cycletest Plus DNA Reagent Kit (BD, NJ, USA). GBC-SD cells were harvested by accutase treatment and counted with a hemocytometer. A total of 5×105 cells were fixed, permeabilized, and stained in accordance with the manufacturers’ instructions, and the sample was analyzed by flow cytometry using a FACSCalibur measuring FL2 area versus total counts. The data were analyzed using ModFit (http://mycyte.org/) and FlowJo (http://mycyte.org/) softwares to generate the percentages of cells in G1, S, and G2 to M phases of the cell cycle.
Transwell Assay
For cell invasion analysis, transwell assay was performed in this study. The upper chamber is pre-treated with 100 μL of Matrigel. GBC-SD cells were seeded into the upper chamber in media with 1% FBS, and the density was adjusted to about 1.0×106 cells per chamber. DMEM with 10% FBS was added in the lower chamber. After 48 h of incubation at 37°C, the non-invading cells in the upper chamber were removed with a cotton swab. Then, cells in the lower chamber were stained with 0.1% crystal violet and counted at 3 different fields under a microscope (LEICADMLB2, Frankfurt, Germany).
Western-Blot Detection
Total protein was isolated from tissue or cell lysates by using RIPA buffer and quantified by BCA protein assay kit (Beyotime, Shanghai, China). Proteins were resolved on 10% SDS-PAGE and transferred to PVDF (Bio-Rad) membranes. After blocking with 5% skim milk, the membranes were incubated with primary antibodies at 4°C overnight. Then, the membranes were incubated with secondary anti-rabbit antibody (Abcam; 1:5000) at room temperature for 1 h. After that, membranes were scanned by using an Odyssey Imaging System and analyzed with Odyssey v2.0 software (LICOR Biosciences, Lincoln, NE, USA). The primary antibodies used in this study: anti-CDK2 (Abcam, Cambridge, MA, USA; 1:1000), anti-cyclin E1 (Abcam; 1:1000), anti-p27 Kip1 (Abcam; 1:1000), anti-cleaved caspase3 (Abcam; 1:1000) and anti-β-actin (Abcam; 1:1000). β-actin was used as an internal control.
Luciferase Reporter Assay
MiR-575 targeted gene prediction was performed using 2 publicly available programs (miRDB and TargetScan). The results were selected for further analysis. Then, the wild type (wt) or mutate type (mt) of p27 3ʹ-UTR was built based on pmiRGLO vector (Promega, Fitchburg, WI, USA) and named as wt-p27 or mt-p27. Cells were co-transfected with synthetic miRNAs and wt or mt-p27 using Lipofectamine 2000. After 48 h of transfection, luciferase activity was measured using dual-luciferase reporter assay system (Promega).
In vivo Experiments
Eighteen BALB/c nude mice (6–8 weeks old) were purchased from Vital River (Beijing, China). The mice were housed within a dedicated SPF facility (six mice per group). GBC-SD cells were subcutaneously transplanted into mouse according to the previous reference.18 When the tumor volume reached about 150 mm3, vector-control or miR-575 antagonist (50 nM) was injected intra-tumor twice weekly. The tumor volume was measured weekly according to the formula: Length×Width×Width/2.19 At the end of the experiments, mice were sacrificed and the tumors were collected and weighted. All in vivo experiments were performed in accordance with National Institutes of Health guide for the care and use of laboratory animals, following a protocol approved by the Ethics Committees of Clinical Medical College, Jiangsu Vocational College of Medicine (No. 20190516030).
Statistical Analysis
Each group were performed at least three independent experiments and all data were expressed as the mean ± standard deviation (SD). Differences were analyzed using Student’s t-test (only 2 groups) or one-way analysis of variance (ANOVA) followed by Tukey’s test (more than 2 groups, Graphpad Prism7). P<0.05 was considered to indicate a statistically significant difference.
Results
Downregulation of miR-575 Promoted the Proliferation of GBC Cells
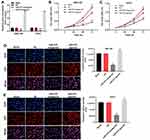
To detect the efficacy of transfections, the expressions of miR-575 in GBC cells were measured with qRT-PCR. As indicated in Figure 1A, the level of miR-575 in GBC cells was significantly increased in the presence of miR-575 agonist. In contrast, miR-575 antagonist notably downregulated the expression of miR-575 in GBC cells. This data revealed that GBC cells were stably transfected with miR-575 agonist or antagonist. Next, to explore the role of miR-575 during the tumorigenesis of GBC, CCK-8 assay was performed. As revealed in Figure 1B and C, the OD value of GBC cells was significantly decreased by miR-575 antagonist but increased in the presence of miR-575 agonist. In Ki-67 immunofluorescence assay, downregulation of miR-575 obviously inhibited the proliferation ability of GBC cells compared with control. However, overexpression of miR-575 significantly promoted the proliferation of GBC cells (Figure 1D and E). Thus, these results revealed that downregulation of miR-575 could inhibit the proliferation of GBC cells, while upregulation of miR-575 exhibited the opposite effect.
Downregulation of miR-575 Significantly Induced Apoptosis of GBC Cells
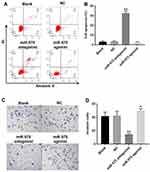
Next, to assess the effect of miR-575 on cell apoptosis of GBC-SD, flow cytometry was performed. As showed in Figure 2A and B, miR-575 antagonist notably induced apoptosis of GBC-SD cells. As expected, upregulation of miR-575 had no effect on cell apoptosis. Consistently, as showed in Supplementary Figure 1A and B, downregulation of miR-575 notably induced the apoptosis of G415 cells. These data suggested that downregulation of miR-575 could induce apoptosis of GBC cells.
Downregulation of miR-575 Notably Inhibited the Invasion of GBC Cells
To examine the effect of miR-575 on invasion of GBC cells, transwell was performed. As expected, downregulation of miR-575 significantly inhibited the invasion of GBC-SD cells, while miR-575 agonist promoted cell invasion (Figure 2C and D). Similarly, miR-575 antagonist significantly inhibited the invasion of G415 cells (Supplementary Figure 1C and D). These data revealed that downregulation of miR-575 significantly downregulated the invasion of GBC cells, while upregulation of miR-575 exhibited the opposite effect in vitro.
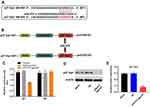
p27 Kip1 Was a Direct Binding Target of miR‑575
TargetScan and miRDB were used to search target genes of miR-575. The result suggested that p27 Kip1 might be a potential target of miR-575 (Figure 3A and B). In addition, we applied dual-luciferase reporter assay to evaluate whether p27 Kip1 was a binding target for miR-575. The results demonstrated that miR-575 agonist inhibited the luciferase activity of psiCHECK-2-p27 Kip1-WT; however, it did not affect the luciferase activity of psiCHECK-2-p27 Kip1-MT (Figure 3C). Additionally, the result of Western blot showed that the protein expression of p27 Kip1 in GBC-SD cells was notably decreased in the presence of miR-575 agonist (Figure 3D and E). All these data suggested that p27 Kip1 was a direct downstream target of miR‑575.
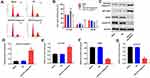
Downregulation of miR-575 Obviously Induced G1 Arrest in GBC Cells
Next, flow-cytometry was applied to detect the cell cycle distribution in GBC-SD. As illustrated in Figure 4A and B, downregulation of miR-575 notably induced G1 arrest in GBC-SD cells, while miR-575 agonist exhibited the opposite effect. Since miR-575 antagonist exhibited significantly regulatory effect on cell apoptosis and cycle of GBC-SD, apoptosis and cell cycle-related proteins were detected by Western blot. The results indicated that miR-575 antagonist markedly enhanced the expressions of cleaved caspase3 and p27 Kip1 and decreased the expressions of CDK2 and cyclin E1 in GBC cells (Figure 4C–G). Moreover, miR-575 antagonist greatly induced G1-arrest in G415 cells (Supplementary Figure 1E and F). Taken together, downregulation of miR-575 could significantly induce G1 arrest in GBC-SD cells.
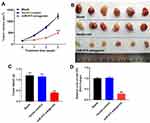
MiR-575 Antagonist Significantly Inhibited GBC Tumor Growth in vivo
To further evaluate the effect of miR-575 on tumor growth of GBC in vivo, xenograft model was established. As indicated in Figure 5A and B, downregulation of miR-575 markedly inhibited the tumor sizes in mice. In addition, miR-575 antagonist significantly decreased the weight of tumors, compared with control (Figure 5C). Meanwhile, the expression of miR-575 was significantly downregulated in tumor tissues after miR-575 antagonist injection (Figure 5D). All these data revealed that miR-575 antagonist could inhibit tumor growth of GBC in vivo.
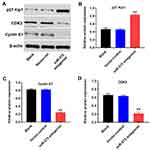
Downregulation of miR-575 Significantly Decreased the Expression of Cell Cycle-Related Proteins in vivo
To investigate the effect of miR-575 on the expression of cycle-related proteins in tumor tissues, Western blot assay was performed. As indicated in Figure 6A–D, miR-575 antagonist notably increased the level of p27 Kip1 and decreased the expressions of cyclin E1 and CDK2 in tumor tissues. These data were consistent with in vitro result and further revealed that miR-575 antagonist inhibited GBC tumor growth via modulating the expression of cell cycle-related proteins.
Discussion
Recent studies have reported that the miRNA was involved in the growth of GBC, and dysregulation of miRNAs plays a critical role during the tumorigenesis of GBC.20–22 In this research, we found that the cell growth of GBC was significantly inhibited by miR-575 antagonist. Our study firstly found the effect of miR-575 on GBC, indicating that miR-575 could function as an oncogene in GBC. A previous study found that miR-575 knockdown significantly downregulated the progression of gastric cancer cells.23 Additionally, miR-575 was notably upregulated in hepatic carcinoma.24 Our finding was consistent with these data suggesting that miR-575 might act as an oncogene.
It has been confirmed that miRNAs exert their biological functions due to their target genes.25,26 In this study, luciferase reporter assay indicated that p27 Kip1 was identified as a downstream target of miR-575 in GBC. P27 Kip1 is a cell cycle regulator firstly regarded as a cyclin-dependent kinase antagonist.27 It has been reported that Rooibos decreased the proliferation of castration-resistant prostate cancer cells via upregulation of p27 Kip1.28 Additionally, Higd-1a inhibited the progression of pancreatic cancer cells through a pERK/p27 Kip1/pRB pathway.29 In our research, downregulation of miR-575 significantly increased the protein level of p27 Kip1. Our findings were consistent with the previous studies. Collectively, our findings revealed that miR-575 exhibited an oncogenic effect on GBC cells via targeting p27 Kip1. Otherwise, a previous report has suggested that miR-575 could mediate the progression of gastric cancer via targeting PTEN.23 This difference may be due to the different tumor types.
Additionally, in vitro experiments indicated that downregulation of miR-575 significantly induced G1 arrest in GBC cells via downregulating the expression of CDK2 and cyclin E1. CDK2 and cyclin E1 have been regarded to be cell cycle regulators in malignant tumors.30–33 Moreover, Liu et al found that NF-κB induced cell cycle arrest in laryngeal squamous cell cancer via downregulation of CDK2.34 Overexpression of p38γ could promote the progression of human colorectal cancer via upregulation of cyclin E1.35 Our research was consistent with these data, revealing that miR-575 antagonist notably induced G1 arrest in GBC cells through inactivating CDK2 and cyclin E1. On the other hand, this study found that downregulation of miR-575 notably activated cleaved caspase 3 in GBC cells. It has been confirmed that cleaved caspase3 is a pro-apoptotic protein.36 Our result further confirmed the role of cleaved caspase 3, indicating that miR-575 antagonist induced the apoptosis of GBC cells through upregulation of cleaved caspase 3. Frankly speaking, we only focused on the effect of miR-575 on cell cycle-related proteins so far. Since Lupeol inhibited the tumorigenesis of GBC via suppression of EGFR/MMP-9 signaling pathway,37 we will further investigate the effect of miR-575 on EGFR/MMP-9 signaling.
Taken together, downregulation of miR-575 significantly suppressed the tumorigenesis of GBC via targeting p27 Kip1, which may be used as a potential target for the treatment of GBC.
Acknowledgment
This research was supported by National Natural Science Foundation of China (81702422 and 81871995), Project of Jiangsu Provincial Commission of Education (17KJB320024), Project of Jiangsu Provincial Commission of Health and Family Planning (H2017086) and Technology Research Projects of Henan Science and Technology Department (182107000054).
Disclosure
The authors declare no competing interests in this research.
References
1. Zhong Y, Wu X, Li Q, et al. Long noncoding RNAs as potential biomarkers and therapeutic targets in gallbladder cancer: a systematic review and meta-analysis. Cancer Cell Int. 2019;19:169. doi:10.1186/s12935-019-0891-1
2. Wang JW, Peng SY, Li JT, et al. Identification of metastasis-associated proteins involved in gallbladder carcinoma metastasis by proteomic analysis and functional exploration of chloride intracellular channel 1. Cancer Lett. 2009;281(1):71–81. doi:10.1016/j.canlet.2009.02.020
3. Hoyos S, Navas MC, Restrepo JC, Botero RC. Current controversies in cholangiocarcinoma. Biochim Biophys Acta Mol Basis Dis. 2018;1864(4 Pt B):1461–1467. doi:10.1016/j.bbadis.2017.07.027
4. Liu TY, Tan ZJ, Jiang L, et al. Curcumin induces apoptosis in gallbladder carcinoma cell line GBC-SD cells. Cancer Cell Int. 2013;13(1):64. doi:10.1186/1475-2867-13-64
5. Pu M, Chen J, Tao Z, et al. Regulatory network of miRNA on its target: coordination between transcriptional and post-transcriptional regulation of gene expression. Cell Mol Life Sci. 2019;76(3):441–451. doi:10.1007/s00018-018-2940-7
6. Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16(3):203–222. doi:10.1038/nrd.2016.246
7. Yao Y, Suo AL, Li ZF, et al. MicroRNA profiling of human gastric cancer. Mol Med Rep. 2009;2(6):963–970. doi:10.3892/mmr_00000199
8. Zhang X, Xin G, Sun D. Serum exosomal miR-328, miR-575, miR-134 and miR-671-5p as potential biomarkers for the diagnosis of Kawasaki disease and the prediction of therapeutic outcomes of intravenous immunoglobulin therapy. Exp Ther Med. 2018;16(3):2420–2432. doi:10.3892/etm.2018.6458
9. Wang H, Yan C, Shi X, et al. MicroRNA-575 targets BLID to promote growth and invasion of non-small cell lung cancer cells. FEBS Lett. 2015;589(7):805–811. doi:10.1016/j.febslet.2015.02.013
10. Roskoski R
11. Barbacid M, Ortega S, Sotillo R, et al. Cell cycle and cancer: genetic analysis of the role of cyclin-dependent kinases. Cold Spring Harb Symp Quant Biol. 2005;70:233–240. doi:10.1101/sqb.2005.70.005
12. Denicourt C, Dowdy SF. Cip/Kip proteins: more than just CDKs inhibitors. Genes Dev. 2004;18(8):851–855. doi:10.1101/gad.1205304
13. Jasinski S, Perennes C, Bergounioux C, Glab N. Comparative molecular and functional analyses of the tobacco cyclin-dependent kinase inhibitor NtKIS1a and its spliced variant NtKIS1b. Plant Physiol. 2002;130(4):1871–1882. doi:10.1104/pp.008573
14. Zhou N, Huang Q, Cheng W, et al. p27kip1 haploinsufficiency preserves myocardial function in the early stages of myocardial infarction via Atg5mediated autophagy flux restoration. Mol Med Rep. 2019;20(4):3840–3848. doi:10.3892/mmr.2019.10632
15. Filipits M, Puhalla H, Wrba F. Low p27Kip1 expression is an independent prognostic factor in gallbladder carcinoma. Anticancer Res. 2003;23(1B):675–679.
16. Rippa E, La Monica G, Allocca R, et al. Overexpression of gastrokine 1 in gastric cancer cells induces Fas-mediated apoptosis. J Cell Physiol. 2011;226(10):2571–2578. doi:10.1002/jcp.22601
17. Menbari MN, Rahimi K, Ahmadi A, et al. miR-483-3p suppresses the proliferation and progression of human triple negative breast cancer cells by targeting the HDAC8 oncogene. J Cell Physiol. 2019.
18. Zhang X, Zhang L, Chen M, Liu D. miR-324-5p inhibits gallbladder carcinoma cell metastatic behaviours by downregulation of transforming growth factor beta 2 expression. Artif Cells Nanomed Biotechnol. 2020;48(1):315–324. doi:10.1080/21691401.2019.1703724
19. Zhou T, Yu L, Huang J, et al. GDF10 inhibits proliferation and epithelial-mesenchymal transition in triple-negative breast cancer via upregulation of Smad7. Aging (Albany NY). 2019;11(10):3298–3314. doi:10.18632/aging.101983
20. Wu XS, Wang F, Li HF, et al. LncRNA-PAGBC acts as a microRNA sponge and promotes gallbladder tumorigenesis. EMBO Rep. 2017;18(10):1837–1853. doi:10.15252/embr.201744147
21. Chandra V, Kim JJ, Mittal B, Rai R. MicroRNA aberrations: an emerging field for gallbladder cancer management. World J Gastroenterol. 2016;22(5):1787–1799. doi:10.3748/wjg.v22.i5.1787
22. Ma Q, Zhang Y, Liang H, et al. EMP3, which is regulated by miR-663a, suppresses gallbladder cancer progression via interference with the MAPK/ERK pathway. Cancer Lett. 2018;430:97–108. doi:10.1016/j.canlet.2018.05.022
23. Wang YN, Xu F, Zhang P, et al. MicroRNA-575 regulates development of gastric cancer by targeting PTEN. Biomed Pharmacother. 2019;113:108716. doi:10.1016/j.biopha.2019.108716
24. Yan S, Tang Z, Chen K, et al. Long noncoding RNA MIR31HG inhibits hepatocellular carcinoma proliferation and metastasis by sponging microRNA-575 to modulate ST7L expression. J Exp Clin Cancer Res. 2018;37(1):214. doi:10.1186/s13046-018-0853-9
25. Backes C, Meese E, Keller A. Specific miRNA disease biomarkers in blood, serum and plasma: challenges and prospects. Mol Diagn Ther. 2016;20(6):509–518. doi:10.1007/s40291-016-0221-4
26. Bernardo BC, Ooi JY, Lin RC, McMullen JR. miRNA therapeutics: a new class of drugs with potential therapeutic applications in the heart. Future Med Chem. 2015;7(13):1771–1792. doi:10.4155/fmc.15.107
27. Bencivenga D, Caldarelli I, Stampone E, et al. p27(Kip1) and human cancers: A reappraisal of a still enigmatic protein. Cancer Lett. 2017;403:354–365. doi:10.1016/j.canlet.2017.06.031
28. Huang SH, Tseng JC, Lin CY, et al. Rooibos suppresses proliferation of castration-resistant prostate cancer cells via inhibition of Akt signaling. Phytomedicine. 2019;64:153068. doi:10.1016/j.phymed.2019.153068
29. An HJ, Ryu M, Jeong HJ, et al. Higd-1a regulates the proliferation of pancreatic cancer cells through a pERK/p27(KIP1)/pRB pathway. Cancer Lett. 2019;461:78–89. doi:10.1016/j.canlet.2019.07.007
30. Bo L, Wei B, Wang Z, et al. Bioinformatics analysis of the CDK2 functions in neuroblastoma. Mol Med Rep. 2018;17(3):3951–3959. doi:10.3892/mmr.2017.8368
31. Ji X, Humenik J, Yang D, Liebhaber SA. PolyC-binding proteins enhance expression of the CDK2 cell cycle regulatory protein via alternative splicing. Nucleic Acids Res. 2018;46(4):2030–2044. doi:10.1093/nar/gkx1255
32. Pellerano M, Tcherniuk S, Perals C, et al. Targeting conformational activation of CDK2 kinase. Biotechnol J. 2017;12(8). doi:10.1002/biot.201600531.
33. Karst AM, Jones PM, Vena N, et al. Cyclin E1 deregulation occurs early in secretory cell transformation to promote formation of fallopian tube-derived high-grade serous ovarian cancers. Cancer Res. 2014;74(4):1141–1152. doi:10.1158/0008-5472.CAN-13-2247
34. Liu JL, Ma HP, Lu XL, et al. NF-kappaB induces abnormal centrosome amplification by upregulation of CDK2 in laryngeal squamous cell cancer. Int J Oncol. 2011;39(4):915–924. doi:10.3892/ijo.2011.1125
35. Su C, Sun Q, Liu S, et al. Targeting p38gamma to inhibit human colorectal cancer cell progression. Biochem Biophys Res Commun. 2019;517(1):172–179. doi:10.1016/j.bbrc.2019.07.038
36. Huang M, Zhong Y, Lin L, et al. 1,2-Dichloroethane induces cerebellum granular cell apoptosis via mitochondrial pathway in vitro and in vivo. Toxicol Lett. 2020;322:87–97. doi:10.1016/j.toxlet.2020.01.004
37. Liu Y, Bi T, Shen G, et al. Lupeol induces apoptosis and inhibits invasion in gallbladder carcinoma GBC-SD cells by suppression of EGFR/MMP-9 signaling pathway. Cytotechnology. 2016;68(1):123–133. doi:10.1007/s10616-014-9763-7
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.