Back to Journals » Cancer Management and Research » Volume 13
Downregulation of MicroRNA-130a Inhibits Oral Squamous Cell Carcinoma Proliferation and Metastasis via the Hippo-YAP Pathway
Authors Peng Y, Hu S, Zhang K, Wang Y, Rouzi M, Zhou D, Yang R
Received 21 October 2020
Accepted for publication 18 March 2021
Published 17 June 2021 Volume 2021:13 Pages 4829—4840
DOI https://doi.org/10.2147/CMAR.S287575
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
This paper has been retracted.
Yiran Peng, 1, 2 Shoushan Hu, 1 Kun Zhang, 1 Yuru Wang, 1 Maierdanjiang Rouzi, 1 Dan Zhou, 1 Ran Yang 1, 2
1State Key Laboratory of Oral Diseases & National Clinical Research Center for Oral Diseases, Chengdu, Sichuan, 610041, People’s Republic of China; 2Department of Pediatric Dentistry, West China Hospital of Stomatology, Sichuan University, Chengdu, Sichuan, 610041, People’s Republic of China
Correspondence: Ran Yang
Department of Pediatric Dentistry, West China Hospital of Stomatology, Sichuan University, No. 14, Renmin South Road, Chengdu, 610041, Sichuan, People’s Republic of China
Tel/Fax +86-028-85503527
Email [email protected]
Introduction: Oral squamous cell carcinoma (OSCC) means oral epithelial cell injury caused by multiple genetic mutations of the cells. Dysregulation of microRNAs (miRs) can disrupt the progression of OSCC. This study explored the mechanism of miR-130a in OSCC progression.
Methods: miR-130a expression in OSCC cell lines was analyzed. Functional assays were utilized to test the alterations of OSCC cell proliferation, apoptosis and epithelial–mesenchymal transition (EMT) with downregulated miR-130a, shRNA-PTEN or/and YAP inhibitor verteporfin. Then, dual-luciferase reporter gene assay was performed to clarify the targeting relation between miR-130a and PTEN. After that, Hippo-YAP pathway-related protein levels were tested. Moreover, xenograft transplantation was applied to confirm the in vitro experiments.
Results: Highly expressed miR-130a was observed in OSCC cell lines. Silenced miR-130a reduced OSCC proliferation, metastasis, invasion and EMT while propelled apoptosis. Furthermore, miR-130a targeted PTEN to promote the OSCC progression. Downregulation of PTEN reversed the inhibition of silencing miR-130a on proliferation and migration of SCC-4 cells. miR-130a targeted PTEN to inactivate the Hippo-YAP axis. OSCC progression was notably promoted by a combination of YAP inhibitor verteporfin and miR-130a inhibitor. Additionally, silenced miR-130a inhibited OSCC progression in vivo.
Discussion: Silencing miR-130a inhibited OSCC progression by targeting PTEN and activating the Hippo-YAP axis. This investigation may provide novel insight for OSCC treatment.
Keywords: oral squamous cell carcinoma, microRNA-130a, Hippo-YAP pathway, PTEN, epithelial–mesenchymal transition
Introduction
Oral squamous cell carcinoma (OSCC) emerges as a deforming and fatal disease when oro-facial destruction, tumor invasion, blood-borne dissemination and cervical lymph node metastasis occur.1 Tobacco, areca nut use, alcohol consumption and human papillomavirus (HPV) infection account for the majority of oral cancer.2 Patients with oral cavity cancers have 55% five-year survival and 38% incidence of relapse.3 Most patients with oral cancer are often asymptomatic at the time of diagnosis, and until pain, persistent ulceration, or unexplained bleeding is discovered at which time the disease is very advanced.2 In this context, biomarkers in the early stage and novel therapeutic strategies for OSCC are in urgent need. Dysregulation of microRNAs (miRs) has been found to disrupt the progression of oral cancer, which provides an underlying treatment of OSCC.4,5 Towards this, this study was designed to identify a miR-based approach for OSCC treatment, in order to develop novel intervention strategies.
Gain of assays certify that since dysregulated miRs are importantly involved in cancer progression, they serve as oncogenes or cancer suppressors, making them possible targets for tumor therapies.6 In a recent research, miR-130a-3p regulates esophageal squamous cell carcinoma (ESCC) through reducing migration and adherence as well as promoting apoptosis.7 As there are few researches about the interaction between miR-130a and OSCC, our experiment aims to investigate it to explore new targets for OSCC therapy. In addition, the Hippo signaling pathway remains a promising target in tissue engineering and cell biology by modulating cell development and division.8 When the Hippo signaling pathway is activated, it serves as a tumor suppressor.9 As a transcriptional regulator often upregulated in malignant tumors, yes-associated protein (YAP) induces cancer cell biological behaviors including proliferation, metastasis and chemoresistance, modulating oncogenic signaling and inflammation.10 Crucially involved in cell balanced development, Hippo-YAP signaling pathway results in pathological aftermath including tissue overgrowth and tumorigenesis when it is abnormally regulated.11 It is suggested in a prior study that knockdown of YAP efficiently limits OSCC cell migration.12 In liver diseases, silenced miR-130a counteracted the negative impacts from of the inactivated Hippo-YAP signaling pathway by helping liver recuperated to normal size and preventing tumorigenesis.13 From all above, it is reasonable to hypothesize that there may be interaction between miR-130a and the Hippo-YAP signaling pathway in OSCC progression. Thus, we conducted a series of experiments to verify the hypothesis.
Materials and Methods
Cell Culture and Transfection
Human OSCC cell lines HSC-2, Cal-27, Tca8113 and SCC-4 purchased from American Type Culture Collection (ATCC, Manassas, VA, USA) were cultured in Roswell Park Memorial Institute 1640 medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA) consisting of 10% fetal bovine serum (FBS), 100 IU/mL penicillin and 100 mg/mL streptomycin (ThermoFisher Scientific) in a 37°C moist incubator with 5% CO2. The normal human oral keratinocytes (HOK) brought from ScienCell Research Laboratories (San Diego, CA, USA) were incubated in oral keratinocyte media (Thermo Fisher Scientific).
Cal-27 cells were assigned into control group, negative control (NC) group (Cal-27 cells were transfected with 50 nM miR-130a mimic NC (Ambion, ThermoFisher Scientific)), and miR-130a mimic group (Cal-27 cells were transfected with 50 nM miR-130a mimic). SCC-4 cells were assigned into control group, NC group (SCC-4 cells were transfected 50 nM miR-130a inhibitor NC) (Ambion), miR-130a inhibitor group (SCC-4 cells were transfected with 50 nM miR-130a inhibitor), miR-130a inhibitor + sh-NC group (transfected with 50 nM miR-130a inhibitor and 40 nM shRNA-NC), miR-130a inhibitor + sh-PTEN group (transfected with 50 nM miR-130a inhibitor and 40 nM shRNA-PTEN), miR-130a inhibitor + verteporfin (Ver) group (transfected with 50 nM miR-130a inhibitor and treated with 10 μmoL/L YAP inhibitor Ver (MedChemExpress, NJ, USA)), and miR-130a inhibitor + PBS group (transfected with 50 nM miR-130a inhibitor and treated with equal volume of phosphate buffer saline). All the transfection was performed according to the instructions of Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA).
3-(4, 5-Dimethylthiazol-2-yl)-2, 5-Diphenyltetrazolium Bromide (MTT) Assay
Differentially treated cells were planted into 96-well microplates at 5×103 cells/well. After incubation for 24, 48 and 72 h, respectively, 20 μL MTT solutions (5 mg/mL) were added into each well. To expedite crystal dissolution, 150 μL dimethyl sulfoxide (DMSO) was added into each well after 4-h incubation. Cell viability was detected at 0, 24, 48 and 72 h and the optical density at 490 nm was observed using a microplate reader.
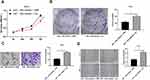
Colony Formation Assay
After the cells in logarithmic growth period were detached with 0.25% trypsin and dispersed into single cells, the cell suspension was diluted into gradient concentrations and seeded in dishes containing 10 mL 37°C pre-heated culture medium. Then, cells were gently rotated to be dispersed evenly, and were cultured at 37°C with 5% CO2 and saturated humidity for 2–3 weeks. When the visible clone appeared in the dishes, the culture was stopped and cells were fixed with 4% paraformaldehyde for 15 min. Next, cells were stained with appropriate amount of Giemsa for 10–30 min. Afterward, the staining fluid was washed away with running water slowly, dried in air, and colonies more than 50 cells were counted under the microscope (Olympus Optical Co., Ltd., Tokyo, Japan).
Cell Apoptosis Assay
Cells were gathered after 48-hour transfection and were resuspended with 500 μL buffer solution. Then, 5 μL Annexin-V-fluorescein isothiocyanate and propidium iodide (both from Invitrogen) were cultivated with cell suspension for 20 min at room temperature. Stained cells were analyzed on a flow cytometer (Beckman Coulter, Inc., Pasadena, CA, USA). With Annexin-V as the horizontal axis and PI as the vertical axis, mechanically damaged cells were located in the upper left quadrant, apoptotic or necrotic cells in the upper right quadrant, negative normal cells in the left lower quadrant, and early apoptotic cells in the right lower quadrant.
Wound Healing Assay
Cell migration was detected with wound-healing assay. Cal-27 or SCC-4 cells (2 × 106 cells/well) were seeded into 6-well microplates at 37°C for 48 h. Then, cell migration was estimated by measuring its movement into the scraped region created by a micropipette tip. Next, 0 or 48 h later, cells were fixed with 4% paraformaldehyde (Cat. No. 15714, Electron Microscopy Sciences, Hatfield, PA, USA) for 1 h at room temperature and were observed using a microscope. Six random visual fields of the wounds were analyzed with an IX71 inverted microscope (Olympus).
Transwell Assay
Transwell chambers were added with the mixture of Matrigel (Shanghai Ponsure Biotech Co., Ltd., Shanghai, China) and serum-free media. When the Matrigel was clotted, cells were planted into the apical chamber at 1×105 cells/mL, while 600 μL 10% FBS Dulbecco’s modified Eagle medium was paved in the basolateral chamber. After 24-h incubation, the chambers were fixed with 70% ethanol and stained with 0.1% crystal violet. Then, the cells across the membrane were measured in 6 randomly chosen visual fields under the microscope.
Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
TRIzol (Invitrogen) was utilized to extract total RNA and the ultramicro spectrophotometer (Shanghai Puyuan Instrument Co., Ltd., Shanghai, China) was employed to measure the RNA concentration and purity. Reverse transcription kit (Thermo Fisher Scientific, Inc.) reverse transcribed 1μg RNA to cDNA. mRNA expression was detected using fluorescence qPCR and calculated using 2−ΔΔCt method with U6 or β-actin as the internal reference. All the primers used in the experiment were designed and synthesized by Shanghai Bioengineering Company (Shanghai, China) (Table 1).
 |
Table 1 Primer Sequences for RT-qPCR |
Western Blot Analysis
The cells were added with radio-immunoprecipitation assay lysis buffer (KeyGEN Biotech Corp., Ltd., Nanjing, Jiangsu, China) and were homogenized and centrifuged to extract the proteins. The protein concentration was examined with bicinchoninic acid method. Afterward, 30 μg proteins were transferred onto the polyvinylidene fluoride membranes after sodium dodecyl sulfate polyacrylamide gel electrophoresis. Next, the membranes were sealed by 5% skim milk powder at room temperature and cultured with the primary antibodies (Table 2) at 4°C overnight. After washes, the membranes were incubated with diluted goat anti-mouse secondary antibody (1:10000, ab205719, Abcam Inc., Cambridge, MA, USA) 1 h at room temperature. Then, the membranes were washed again, soaked in exposure liquid, and gently percussed for exposure. The exposure condition was collected for gray value analysis.
 |
Table 2 Antibodies Used in Experiment |
Dual-Luciferase Reporter Gene Assay
PTEN 3ʹuntranslated region (UTR) sequence consisting of binding site with miR-130a was synthesized and PTEN 3ʹUTR wild-type plasmid (PTEN-WT) and PTEN 3ʹUTR mutant-type plasmid (PTEN-MUT) were constructed. Then, the constructed plasmids were, respectively, mixed with NC and miR-130a mimic and transfected into 293T cells (ATCC). After transfection for 48 h, the cells were collected and lysed. Luciferase activity was detected using a luciferase assay kit (BioVision, Mountain View, CA, USA) and Glomax 20/20 luminometer (Promega, Madison, Wisconsin, USA).
Xenograft Tumors in Nude Mice
Stably transfected transduced SCC-4 cells (2 × 107 cells in 100 μL) were subcutaneously injected into athymic BALB/c nude mice (4 week-old) (Hunan SJA Laboratory Animal Co., Ltd., Changsha, Hunan, China). The tumor volume was calculated according to the formula V = πAB2/6 (V: tumor volume, A: the largest diameter, B: the perpendicular diameter). Six weeks later, the mice were euthanized and the tumors were removed and weighed. Tumor samples were treated correspondingly to perform immunohistochemistry.
Immunohistochemistry
Paraffined sections at 4 μm were dewaxed in xylene, rehydrated in gradient ethanol and incubated with 3% hydrogen peroxide for 10 min. When antigen was retrieved and blocked, the sections were cultivated with antibody against E-cadherin (1:100; ab1416; Abcam). Subsequently, the sections were cultured with horseradish peroxidase-polymer anti-mouse/rabbit antibody and 2,4-diaminobutyric acid detection kit (Fuzhou Maixin Biotech, Fuzhou, China) before they were counterstained by hematoxylin. Finally, all stained sections were dehydrated with gradient alcohol, cleaned in xylene and mounted with neutral gum. Image J program (version 1.49, NIH) was used to measure and analyze DAB staining sites, with the data in control group as 1.
Statistical Analysis
SPSS 21.0 (IBM Corp. Armonk, NY, USA) was employed for data analysis. Kolmogorov–Smirnov test indicated whether the data were in normal distribution. The data are shown in mean ± standard deviation. Kaplan–Meier assay was applied for analyzing survival rate. The t-test was used for analyzing comparisons between two groups, one-way or two-way analysis of variance (ANOVA) for comparing multiple groups, and Tukey’s multiple comparison test for pairwise comparisons after ANOVA. The p value was attained using a two-tailed test and p < 0.05 indicated a significant difference.
Results
miR-130a is Upregulated in OSCC Cell Lines and miR-130a Downregulation Inhibits OSCC Cell Proliferation
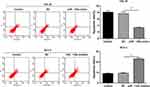
To understand the role of miR-130a in OSCC development, we detected miR-130a expression in OSCC cell lines (HSC-2, Cal-27, Tca8113 and SCC-4), and found that OSCC cell lines showed higher miR-130a expression than oral epithelial cells (HOK cells) (Figure 1A) (p < 0.01). It was indicated that miR-130a might play a significant role in OSCC progression. Hence, we transfected miR-130a mimic into Cal-27 cells with relatively lower miR-130a expression and miR-130a inhibitor into SCC-4 cells with highest miR-130a expression, and mimic NC/inhibitor-NC was acted as negative control. The results of RT-qPCR confirmed that miR-130a mimic and miR-130a inhibitor were transfected into Cal-27 and SCC-4 cells successfully (Figure 1B). MTT assay and colony formation assay suggested that after miR-130a overexpression, SCC-4 cell proliferation and colonies were remarkably higher than those in the control cells, while miR-130a inhibition resulted in opposite trends (Figure 1C and D) (all p < 0.01).
miR-130a Downregulation Enhances Apoptosis of OSCC Cell
We adopted flow cytometry to evaluate the apoptosis of Cal-27 cells transfected with miR-130a mimic and SCC-4 cells transfected with miR-130a inhibitor. Overexpression of miR-130a decreased the apoptosis rate of Cal-27 cells, while inhibition of miR-130a increased the apoptosis rate of SCC-4 cells (both p < 0.01) (Figure 2). It was indicated that inhibition of miR-130a promoted apoptosis of OSCC cells.
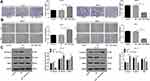
miR-130a Downregulation Inhibits OSCC Cell Invasion, Migration and Epithelial–Mesenchymal Transition (EMT)
As transwell and wound-healing assays showed, miR-130a downregulation obviously inhibited SCC-4 cell invasion and migration, while Cal-27 cells overexpressing miR-130a led to reversed consequences (Figure 3A and B) (all p < 0.01). Western blot analysis detected EMT-related proteins and suggested that SCC-4 cells with miR-130a downregulation showed elevated E-cadherin expression and reduced N-cadherin and snail protein levels (Figure 3C) (all p < 0.01).
miR-130a Targets PTEN to Promote OSCC Cell Proliferation and Migration
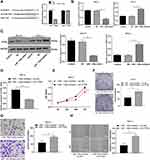
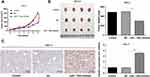
To explore the downstream mechanism of miR-130a in OSCC, we found that PTEN may serve as a potential target of miR-130a in OSCC cells through bioinformatics website. Dual-luciferase reporter gene assay confirmed the targeting relation between miR-130a and PTEN (p < 0.01) (Figure 4A). Overexpressed miR-130a greatly suppressed PTEN mRNA expression and protein level, while downregulated miR-130a contributed to opposite results (p < 0.01) (Figure 4B and C).
In order to verify the effect of PTEN, the shRNA-PTEN was transfected into the cells with low expression of miR-130a, and successfully downregulated the expression of PTEN (Figure 4D). Further detection showed that the proliferation and migration of SCC-4 cells were significantly increased after downregulating PTEN at the same time, which indicated that downregulating PTEN could reverse the inhibition of miR-130a on the proliferation and migration of SCC-4 cells to a certain extent (all p < 0.01) (Figure 4E–H). These results suggest that miR-130a can inhibit PTEN and promote the proliferation and migration of OSCC cells.
miR-130a Targets PTEN to Activate the Hippo-YAP Pathway in OSCC Cells
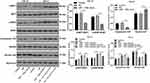
It is reported that the inactivation of PTEN lipid phosphatase is related to Hippo.14 Hippo-Yap signaling pathway affects the proliferation and metastasis of tumor cells.15 Western blot was used to detect the expression of Hippo-Yap signaling pathway-related proteins. The results showed that the Hippo-Yap signaling pathway was significantly inhibited in Cal-27 cells overexpressing miR-130a (miR-130a mimic vs NC group, p < 0.01); the Hippo-Yap signaling pathway was activated in SCC4 cells with miR-130a inhibition (miR-130a inhibitors vs NC group, p < 0.01). To further investigate whether PTEN can affect the Hippo-Yap pathway, we silenced PTEN in SCC4 cells with low expression of miR-130a, and the activation of Hippo-Yap signaling pathway was inhibited to a certain extent (miR-130a inhibitor + sh-NC vs miR-130a inhibitor + sh-PTEN, p < 0.05) (Figure 5). These results indicate that miR-130a inhibits the activity of Hippo-Yap signaling pathway through PTEN.
Inactivated Hippo-YAP Axis Inhibits the Promotion of miR-130a Overexpression on OSCC Cell Proliferation and Invasion
To verify that miR-130a/PTEN can regulate biological behaviors of OSCC cells through the Hippo-YAP pathway, OSCC cells were treated with verteporfin (a YAP inhibitor) and miR-130a inhibitor. Compared with the miR-130a inhibitor + PBS group, the proliferation and invasion of SCC-4 cells in miR-130a inhibitor + Ver group were significantly promoted (Figure 6A–D) (all p < 0.01), indicating that inhibition of Hippo-YAP signaling pathway can reverse the effect of miR-130a inhibition in preventing the growth and invasion of OSCC cells.
miR-130a Downregulation Inhibits Xenograft Tumor Growth in Nude Mice and EMT
To verify the in vitro results, we injected SCC-4 cells stably low-expressing miR-130a into nude mice to establish xenograft model, and to further study the effect of miR-130a on OSCC in vivo. We measured the volume and weight of tumors, and found that compared with the control group, the tumor volume and weight were lower in mice with downregulated miR-130a (all p < 0.01) (Figure 7A and B). Moreover, E-cadherin positive expression was importantly elevated in nude mice with downregulated miR-130a (p < 0.01) (Figure 7C).
Discussion
OSCC marked one of the most prevailing killers to patients with neck and head cancers.16 It was unveiled that differentially expressed miRs served as oncogenes or tumor suppressors in oral tumorigenesis by necessarily participating in cellular processes, including differentiation, proliferation, survival and death, indicating their valuable role in oral carcinoma diagnosis and prognosis.17 miR-130a was highly expressed in salivary gland adenoid cystic carcinoma (SACC), hinting an evident role in exaggerating SACC metastasis.18 In this study, we hypothesized that there can be role of miR-130a in OSCC via the Hippo-YAP signaling pathway. Consequently, our data showed that silenced miR-130a mitigated OSCC by targeting PTEN and upregulating the Hippo-YAP signaling pathway.
Dysregulation of miRs has been associated with the carcinogenesis and progression of oral cavity cancer.19 The results of RT-qPCR showed that miR-130a was expressed higher in OSCC cells than that in HOK cells. Highly expressed miR-130a-3p was also observed in aggressive OSCC cells.20 In addition, we noticed that survival rate in patients with poorly expressed miR-130a was higher than those strongly expressed miR-130a. The Kaplan–Meier curves demonstrated in cholangiocarcinoma, the miR-130a-3p high group had a significantly lower overall survival and disease-free survival than the miR-130a-3p low group.21 Our study complemented the diagnostic value of miR-130a in OSCC. Then, we noticed that downregulated miR-130a inhibited OSCC cell proliferation and enhanced apoptosis. Interestingly, the destructive effects of knockdown of miR-130a on tumor development were also verified in hemangioma cells.22 Most of the studies reported that miR-130a acts as a tumor promoter but, in some cases, it acts as a tumor suppressor.21 In this study, we unveiled that miR-130a was a promoter for OSCC progression.
EMT is a biological activity often accompanied by tumor metastasis and invasion.23 Results from transwell and wound-healing assays as well as Western blot analysis suggested that downregulated miR-130a inhibited OSCC invasion, metastasis and EMT, with decreased N-cadherin and snail protein levels. N-cadherin expression was higher in OSCC tissues than in non-tumor tissues, making it a useful target in OSCC diagnosis.24 As a major modulator in EMT, snail encouraged head and neck squamous cell carcinoma progression.25 It was suggested that in osteosarcoma cells, miR-130a encouraged cell migration, invasion as well as EMT.26 In a word, downregulated miR-130a was conducive in blocking OSCC progression.
Additionally, dual-luciferase reporter gene assay certified the targeting relation between miR-130a and PTEN. Associations between pathological consequences and PTEN levels engender the effect on tumor inhibition.27 PTEN acts as a tumor suppressor gene and inactivation of PTEN has been reported in various types of cancers.28 In order to verify the effect of PTEN, the shRNA-PTEN was transfected into the cells with low expression of miR-130a, and the proliferation and migration of SCC-4 cells were significantly increased after downregulating PTEN at the same time, indicating that downregulating PTEN could reverse the inhibition of miR-130a on the proliferation and migration of SCC-4 cells. PTEN expression was seen to be downregulated in OSCC cells.29 The targeting relation between miR-130a and PTEN was observed in a prior research in gastric cancer.30 Many anti-tumor drugs inhibit tumor growth by limiting YAP and supporting PTEN expression,31 hinting a negative relation between YAP and PTEN. Abnormal YAP expression, negatively related to Hippo, was related to tumor occurrence and progression, expediting biological behaviors like proliferation, invasion, metastasis and apoptosis.32,33 The Hippo pathway has been discovered to control organ size, tissue homeostasis, cell growth, tumorigenesis and metastasis.15 During this research, the Hippo-YAP axis was activated in SCC-4 cells with downregulated miR-130a but inhibited in Cal-27 cells with overexpressed miR-130a. In cells treated with miR-130a inhibitor and shRNA-PTEN, the Hippo-YAP axis was inhibited to some degree. The high expression of YAP in OSCC was greatly associated with the differentiation degree and tumor diameter.34 Furthermore, we discovered that inhibition of Hippo-YAP pathway using verteporfin reversed the miR-130a downregulation in inhibiting the growth and metastasis of OSCC cells. YAP influenced OSCC cell activities and intrinsic apoptotic axis, therefore augmenting OSCC cell proliferation and retarding cell apoptosis.35 Finally, downregulated miR-130a in vivo refrained OC growth and EMT as the E-cadherin expression was improved. E-cadherin represents an essential part in adherent junctions and cell epithelial phenotype, leading to decreased cell motility and relieved terminal cancer.36 Similarly, poorly expressed E-cadherin in OSCC indicated a malignant biomarker of high risk which was dangerously related to cell survival, recurrence, metastasis, and tumor differentiation.37 Overall, our data shown that downregulated miR-130a was helpful in blocking OSCC progression.
In summary, our study supported that downregulation of miR-130a reduced OSCC by targeting PTEN and activating the Hippo-YAP signaling pathway. These results discovered a novel approach for OSCC treatment. In the future, we will further explore the underlying mechanism of other targets of miR-130a. More attention will be paid on seeking reliable therapeutic targets of OSCC. Still, this is just a preclinical research, although our findings provide therapeutic implication in OSCC treatment, the experimental results and effective applications into clinical practice need further validation. In the future, we will study the clinical effect of miR-130a on OSCC to provide clinical relevance of the current findings in clinical practice.
Data Sharing Statement
All the data generated or analyzed during this study are included in this published article.
Ethics Statement
This study was approved and supervised by the ethics committee of the West China Hospital of Stomatology. All the subjects signed the informed consent. The protocol was also approved by the Institutional Animal Care and Use Committee of the West China Hospital of Stomatology. The animal experiments were implemented by the relevant regulations of International Association for the Study of Pain. Significant efforts were made to minimize animals and their suffering.
Funding
This study was supported by the National Natural Science Foundation of China (Grant no. 81800989).
Disclosure
All authors declare that there is no conflict of interest in this study.
References
1. Thomson PJ. Perspectives on oral squamous cell carcinoma prevention-proliferation, position, progression and prediction. J Oral Pathol Med. 2018;47(9):803–807. doi:10.1111/jop.12733
2. McCullough MJ, Prasad G, Farah CS. Oral mucosal malignancy and potentially malignant lesions: an update on the epidemiology, risk factors, diagnosis and management. Aust Dent J. 2010;55(Suppl 1):61–65. doi:10.1111/j.1834-7819.2010.01200.x
3. Abrahao R, Anantharaman D, Gaborieau V, et al. The influence of smoking, age and stage at diagnosis on the survival after larynx, hypopharynx and oral cavity cancers in Europe: the ARCAGE study. Int J Cancer. 2018;143(1):32–44. doi:10.1002/ijc.31294
4. El-Sakka H, Kujan O, Farah CS. Assessing miRNAs profile expression as a risk stratification biomarker in oral potentially malignant disorders: a systematic review. Oral Oncol. 2018;77:57–82. doi:10.1016/j.oraloncology.2017.11.021
5. Patil S, Warnakulasuriya S. Blood-based circulating microRNAs as potential biomarkers for predicting the prognosis of head and neck cancer-a systematic review. Clin Oral Investig. 2020;24(11):3833–3841. doi:10.1007/s00784-020-03608-7
6. Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16(3):203–222. doi:10.1038/nrd.2016.246
7. Eichelmann AK, Matuszcak C, Lindner K, Haier J, Hussey DJ, Hummel R. Complex role of miR-130a-3p and miR-148a-3p balance on drug resistance and tumor biology in esophageal squamous cell carcinoma. Sci Rep. 2018;8(1):17553. doi:10.1038/s41598-018-35799-1
8. Zygulska AL, Krzemieniecki K, Pierzchalski P. Hippo pathway - brief overview of its relevance in cancer. J Physiol Pharmacol. 2017;68(3):311–335.
9. Plouffe SW, Hong AW, Guan KL. Disease implications of the Hippo/YAP pathway. Trends Mol Med. 2015;21(4):212–222. doi:10.1016/j.molmed.2015.01.003
10. Zanconato F, Cordenonsi M, Piccolo S. YAP/TAZ at the roots of cancer. Cancer Cell. 2016;29(6):783–803. doi:10.1016/j.ccell.2016.05.005
11. Lee M, Goraya N, Kim S, Cho SH. Hippo-yap signaling in ocular development and disease. Dev Dyn. 2018;247(6):794–806. doi:10.1002/dvdy.24628
12. Qi L, Shi C, Li J, et al. Yes-associated protein promotes cell migration via activating Wiskott-Aldrich syndrome protein family member 1 in oral squamous cell carcinoma. J Oral Pathol Med. 2019;48(4):290–298. doi:10.1111/jop.12833
13. Shen S, Guo X, Yan H, et al. A miR-130a-YAP positive feedback loop promotes organ size and tumorigenesis. Cell Res. 2015;25(9):997–1012. doi:10.1038/cr.2015.98
14. Xu W, Yang Z, Xie C, et al. PTEN lipid phosphatase inactivation links the hippo and PI3K/Akt pathways to induce gastric tumorigenesis. J Exp Clin Cancer Res. 2018;37(1):198. doi:10.1186/s13046-018-0795-2
15. Wang D, He J, Dong J, Meyer TF, Xu T. The HIPPO pathway in gynecological malignancies. Am J Cancer Res. 2020;10(2):610–629.
16. Gharat SA, Momin M, Bhavsar C. Oral squamous cell carcinoma: current treatment strategies and nanotechnology-based approaches for prevention and therapy. Crit Rev Ther Drug Carrier Syst. 2016;33(4):363–400. doi:10.1615/CritRevTherDrugCarrierSyst.2016016272
17. Manasa VG, Kannan S. Impact of microRNA dynamics on cancer hallmarks: an oral cancer scenario. Tumour Biol. 2017;39(3):1010428317695920. doi:10.1177/1010428317695920
18. Feng X, Matsuo K, Zhang T, et al. MicroRNA profiling and target genes related to metastasis of salivary adenoid cystic carcinoma. Anticancer Res. 2017;37(7):3473–3481. doi:10.21873/anticanres.11715
19. Wang H, Guo Y, Mi N, Zhou L. miR-101-3p and miR-199b-5p promote cell apoptosis in oral cancer by targeting BICC1. Mol Cell Probes. 2020;52:101567. doi:10.1016/j.mcp.2020.101567
20. Hilly O, Pillar N, Stern S, et al. Distinctive pattern of let-7 family microRNAs in aggressive carcinoma of the oral tongue in young patients. Oncol Lett. 2016;12(3):1729–1736. doi:10.3892/ol.2016.4892
21. Asukai K, Kawamoto K, Eguchi H, et al. Micro-RNA-130a-3p regulates gemcitabine resistance via PPARG in cholangiocarcinoma. Ann Surg Oncol. 2017;24(8):2344–2352. doi:10.1245/s10434-017-5871-x
22. Gao F, Wang FG, Liu RR, et al. Epigenetic silencing of miR-130a ameliorates hemangioma by targeting tissue factor pathway inhibitor 2 through FAK/PI3K/Rac1/mdm2 signaling. Int J Oncol. 2017;50(5):1821–1831. doi:10.3892/ijo.2017.3943
23. Chen T, You Y, Jiang H, Wang ZZ. Epithelial-mesenchymal transition (EMT): a biological process in the development, stem cell differentiation, and tumorigenesis. J Cell Physiol. 2017;232(12):3261–3272. doi:10.1002/jcp.25797
24. Chandolia B, Rajliwal JP, Bajpai M, Arora M. Prognostic potential of N-cadherin in oral squamous cell carcinoma via immunohistochemical methods. J Coll Physicians Surg Pak. 2017;27(8):475–478.
25. Ota I, Masui T, Kurihara M, et al. Snail-induced EMT promotes cancer stem cell-like properties in head and neck cancer cells. Oncol Rep. 2016;35(1):261–266. doi:10.3892/or.2015.4348
26. Chen J, Yan D, Wu W, Zhu J, Ye W, Shu Q. MicroRNA-130a promotes the metastasis and epithelial-mesenchymal transition of osteosarcoma by targeting PTEN. Oncol Rep. 2016;35(6):3285–3292. doi:10.3892/or.2016.4719
27. Malaney P, Uversky VN, Dave V. PTEN proteoforms in biology and disease. Cell Mol Life Sci. 2017;74(15):2783–2794. doi:10.1007/s00018-017-2500-6
28. Lapthanasupkul P, Klongnoi B, Mutirangura A, Kitkumthorn N. Investigation of PTEN promoter methylation in ameloblastoma. Med Oral Patol Oral Cir Bucal. 2020;e481–e487. doi:10.4317/medoral.23498
29. Baghaei F, Abdollahi A, Mohammadpour H, et al. PTEN and miR-26b: promising prognostic biomarkers in initiation and progression of oral squamous cell carcinoma. J Oral Pathol Med. 2019;48(1):31–35. doi:10.1111/jop.12794
30. Liu T, Liu S, Xu Y, et al. Circular RNA-ZFR inhibited cell proliferation and promoted apoptosis in gastric cancer by sponging miR-130a/miR-107 and modulating PTEN. Cancer Res Treat. 2018;50(4):1396–1417. doi:10.4143/crt.2017.537
31. Tian Y, Tang B, Wang C, et al. Metformin mediates resensitivity to 5-fluorouracil in hepatocellular carcinoma via the suppression of YAP. Oncotarget. 2016;7(29):46230–46241. doi:10.18632/oncotarget.10079
32. Cheng Y, Hou T, Ping J, Chen T, Yin B. LMO3 promotes hepatocellular carcinoma invasion, metastasis and anoikis inhibition by directly interacting with LATS1 and suppressing Hippo signaling. J Exp Clin Cancer Res. 2018;37(1):228. doi:10.1186/s13046-018-0903-3
33. Wierzbicki PM, Rybarczyk A. The Hippo pathway in colorectal cancer. Folia Histochem Cytobiol. 2015;53(2):105–119. doi:10.5603/FHC.a2015.0015
34. Tu MS, Li YS, Zhang X. [Expression and significance of YAP and TAZ proteins in oral squamous cell carcinoma]. Shanghai Kou Qiang Yi Xue. 2018;27(4):415–418. Chinese.
35. Chen X, Gu W, Wang Q, et al. C-MYC and BCL-2 mediate YAP-regulated tumorigenesis in OSCC. Oncotarget. 2018;9(1):668–679. doi:10.18632/oncotarget.23089
36. Mendonsa AM, Na TY, Gumbiner BM. E-cadherin in contact inhibition and cancer. Oncogene. 2018;37(35):4769–4780. doi:10.1038/s41388-018-0304-2
37. Lopez-Verdin S, Martinez-Fierro ML, Garza-Veloz I, et al. E-Cadherin gene expression in oral cancer: clinical and prospective data. Med Oral Patol Oral Cir Bucal. 2019;24(4):e444–e451. doi:10.4317/medoral.23029
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.