Back to Journals » OncoTargets and Therapy » Volume 10
Downregulation of lncRNA SDPR-AS is associated with poor prognosis in renal cell carcinoma
Authors Ni W, Song E, Gong M, Li Y, Yao J , An R
Received 21 March 2017
Accepted for publication 6 May 2017
Published 19 June 2017 Volume 2017:10 Pages 3039—3047
DOI https://doi.org/10.2147/OTT.S137641
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Samir Farghaly
Wenjun Ni,1,2 Erlin Song,1 Mancheng Gong,3 Yongxiang Li,4 Jie Yao,5 Ruihua An1
1Department of Urology Surgery, The First Affiliated Hospital of Harbin Medical University, 2Department of Urology, Heilongjiang Provincial Hospital, Harbin, Heilongjiang, 3Department of Urology, The People’s Hospital of Zhongshan, Zhongshan, Guangdong, 4Department of Urology, The People’s Hospital of Weifang, Weifang, Shandong, 5Department of Oncology, the 161th Hospital of PLA, Wuhan, Hubei, People’s Republic of China
Abstract: Renal cell carcinoma (RCC) is a common type of kidney cancer. Normally, surgical treatment can prolong life, but only for patients with early stage tumors. However, it is difficult for early detection strategies to distinguish between benign and malignant kidney tumors. Therefore, potential biomarkers for early diagnosis and prognosis of RCC are needed. Intriguingly, mounting evidence has demonstrated that many long noncoding RNAs (lncRNAs) are strongly linked to cancers. Indeed, promising RCC-associated lncRNA biomarkers have also been identified. However, the functional and prognostic roles of the antisense (AS) serum deprivation response (SDPR) lncRNA (SDPR-AS) in RCC remain largely unknown. The aims of this study were to investigate the expression and prognostic relevance of SDPR-AS in RCC. We uncovered the downregulated expressions of both lncRNA SDPR-AS and its protein-coding gene, SDPR, in RCC tissues compared to the matched normal tissues. Furthermore, SDPR-AS and SDPR expressions were positively correlated. Overexpression and knockdown experiments suggested that SDPR-AS and SDPR were coregulated in RCC cell lines. In addition, overexpression of SDPR-AS suppressed cell migration and invasion, but not cell growth. Furthermore, expression of SDPR-AS was associated with tumor differentiation and lymphatic metastasis. Kaplan–Meier survival and log-rank tests demonstrated the association of elevated expression of SDPR-AS with increased overall survival. In conclusion, our results suggest that the SDPR-AS may serve as a prognostic biomarker and therapeutic target of RCC.
Keywords: long noncoding RNAs, metastasis, prognosis, renal cell carcinoma, suppressor
Introduction
Renal cell carcinoma (RCC) accounts for approximately 2%–4% of all human malignancies and is one of the most common cancers in urology.1,2 Because kidney diseases are mostly asymptomatic during the early stages, many RCC patients are diagnosed with advanced stage metastatic diseases that are often resistant to chemotherapy, and therefore this is associated with high mortality.3 Despite the great advances made in the diagnosis and treatment of RCC, therapeutic options are limited and prognosis remains unfavorable.4 Therefore, there is a need to identify effective biomarkers for predicting the progression and prognosis of RCC and to advance the development of new targeted therapies.
During the past decades, numerous studies have revealed that a large portion of the human genome is universally transcribed into noncoding RNAs (ncRNAs).5 Long ncRNAs (lncRNAs) are RNA molecules that are larger than 200 nucleotides in length and with no protein coding capabilities.6,7 Recently, many studies have shown that lncRNAs are involved in carcinogenesis, including invasion and metastasis of kidney, breast, and colorectal cancers.8–10 Particularly in RCC, it has been reported that high expression of lncRNA ATB promotes cell migration and invasion, and correlates with metastases,11 while low expression of lncRNA NBAT-1 increases cell proliferation, migration, and invasion, and is also associated with poor prognosis.12 In addition, many other lncRNAs, including TUG1,13 BX357664,14 and MEG3,15 have been demonstrated to participate in the development and progression of RCC. Despite the identification of numerous lncRNAs in RCC, the majority remain unexplored, and this is therefore the reason for conducting this study.
Through in vitro experiments, Burgener et al16 in 1990 discovered serum deprivation response (SDPR), also named cavin-2, located on chromosome 2q32-q33 as a substrate for protein kinase C isoforms. SDPR has been confirmed to be involved in inducing membrane curvature and caveolae formation, and it is strongly linked to gut electrophysiological pacing functions, cell proliferation, and migration.17–19 Importantly, previous studies have indicated that SDPR is downregulated in kidney, prostate, and breast cancers.20,21 SDPR has also been reported to be a possible biomarker for early detection and discrimination of malignant kidney tumors.22 Through bioinformatic analysis, we uncovered an lncRNA, termed AC098617.1, which is located at chromosome 2q32.3, ranging from 192,711,265 to 192,901,485, with the full length up to about 190,221 bp (data from UCSC website). AC098617.1 (Ensembl Gene ID: ENSG00000233766) with 13 transcripts is also known as LOC105373813 Gene or Uncharacterized LOC105373813. Of particular note is that the lncRNA is the antisense (AS) transcript of SDPR (SDPR-AS) and is located on the upstream of the gene. The expression and functions of lncRNAs are often associated with their adjacent protein coding genes.23 It is therefore tempting to speculate that the expression and functions of SDPR-AS may also be associated with the progression and prognosis of RCC. In this study, we first investigated the expression profiles of SDPR-AS and SDPR in RCC. Subsequently, the functional roles of SDPR-AS in RCC cells were evaluated. Additionally, the correlations between SDPR-AS expression and clinical variables, as well as prognosis, were examined.
Materials and methods
Statement of ethics
The tissue specimens and clinical materials used in this study were collected after each participant gave written informed consent, which was in accordance with our institutional ethical guidelines. The ethical committee of the Chinese People’s Liberation Army (PLA) General Hospital approved the utilization of tumor tissues for this study.
Patients and samples
In total, 56 RCC patients who underwent initial surgery at the Chinese PLA General Hospital between 2005 and 2007 were enrolled into this research. Tumor and adjacent normal tissues were immediately snap-frozen in liquid nitrogen after resection, then maintained at −80°C until use.
Cell culture
The human RCC cell lines, OS-RC-2, 786-O, 769-P, Caki-1, Caki-2, and ACHN, as well as normal renal cell line HKC were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). All cells were cultured in Dulbecco’s Modified Eagle’s Medium (Hyclone, UT, USA), containing 10% heat-inactivated fetal bovine serum, penicillin (100 U/mL), and streptomycin (100 μg/mL) in a 5% CO2 and humidified atmosphere at 37°C.
SDPR-AS overexpression experiments
The universally available pcDNA3.1 vector was used for cDNA-SDPR-AS plasmid construction. Following the manufacturer’s instructions, the SDPR-AS low-expressing OS-RC-2 cells were transfected with pcDNA-SDPR-AS using Lipofectamine 2000 (Invitrogen). The transfected cells were harvested for RNA isolation and functional assays.
SDPR-AS knockdown experiments
Small interfering RNAs (siRNAs) for the target SDPR-AS (SDPR-AS-si) and negative-control siRNAs (NC-si) were obtained from GenePharma (Shanghai, People’s Republic of China), and the sequences are listed in Table 1. The SDPR-AS high-expressing 769-P cell line was selected for transfection with SDPR-AS-si. Prior to transfection, 12-well plates were seeded with approximately 50% of the cells and cultured for 24 h. Then, siRNA transfections were carried out with X-treme GENE transfection reagents (Roche) according to the manufacturer’s instructions. RNA was isolated after 48 h of transfection and used to perform functional assays.
RNA isolation and reverse transcription
Total RNA was isolated from cells and tissues using Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. NanoDrop ND2000 (Thermo Scientific Inc., Santa Clara, CA, USA) was used to determine the purity and quantify the concentration of RNA. A First Strand cDNA Synthesis Kit (TakaRa, Dalian, People’s Republic of China) was used to perform reverse transcription.
Real-time quantitative polymerase chain reaction
Primers used for real-time quantitative polymerase chain reaction (RT-qPCR) were obtained from GenePharma (Shanghai, People’s Republic of China), and the sequences are listed in Table 1. According to the manufacturer’s instructions, RT-qPCR was performed using the SYBR PrimeScript RT-PCR kit (Takara) in an Applied Biosystems 7500 fluorescent quantitative PCR system (Applied Biosystems, Foster City, CA, USA). The reaction started at 95°C for 5 min, followed by 40 cycles of 95°C for 30 s, 59°C for 30 s, and 72°C for 30 s. GAPDH was used as the internal reference to normalize qPCR results. Relative gene expression levels were measured using cycle threshold (CT) in the ΔΔCT calculation.
Cell proliferation assay
The CCK8 assay was used to examine the proliferation of RCC cells. After 48 h of transfection, cells were seeded into 96-well plates together with the CCK8 reagent and incubated for 1 h at 37°C. The Tecan infinite M200 multimode microplate reader (Tecan, Mechelen, Belgium) was then used to detect cell density at 450 nm absorption wavelength. All experiments were performed in triplicate.
Scratch wound-healing assay
The scratch wound-healing assay was used to evaluate the migratory properties of RCC cells. Cells were seeded into 12-well plates to achieve a confluent monolayer after 48 h of transfection. Then, uniform wounds were scraped with sterile 200 μL pipette tips. To remove floating cells, each well was washed thrice with PBS. The initial distance (0 h) and the distances traveled by cells after 24, 48, and 72 h of scratching were detected microscopically.
Cell invasion assay
Transwell assays were conducted with 8 mm pore size Matrigel Invasion Chambers (Millipore Corporation, Billerica, MA, USA) to evaluate the invasive properties of RCC cells. After 48 h of transfection, single-cell suspensions were seeded into the upper chamber, with the lower chamber filled with 600 μL Dulbecco’s Modified Eagle’s Medium containing 10% fetal bovine serum. After 24–48 h of incubation, the noninvading cells were wiped off from the upper surface of the membranes, whereas cells that had invaded were stained with 0.5% crystal violet solution after cell fixation in 95% methyl aldehyde. The images of migrated cells per well were captured and counted in random fields using a microscope. Each experiment was conducted in triplicate.
Statistical analyses
Statistical analyses were performed with SPSS version 18.0 (SPSS, Chicago, IL, USA). All experiments mentioned above were repeated three times, and data were presented as mean ± standard deviation. Two-tailed t-test and χ2 test were used for comparisons between different groups. Kaplan–Meier estimator was used to perform survival analysis. P<0.05 was considered statistically significant.
Results
Downregulation of both SDPR-AS and SDPR expression in RCC tissue samples
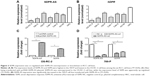
The expression levels of SDPR-AS and its protein coding gene SDPR were examined by RT-qPCR in panel-paired specimens obtained from the 56 patients with RCC. Compared to the matched normal tissues, the expression levels of SDPR-AS (Figure 1A, ***P<0.001) and SDPR (Figure 1B, ***P<0.001) in tumor tissues were significantly downregulated.
Expressions of SDPR-AS and SDPR are positively correlated
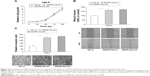
To investigate the relationship between SDPR-AS and SDPR, we assessed the correlation of their expression levels by RT-qPCR. The result showed a positive correlation in the levels of their expression (Figure 2, N=56, R=0.66, P<0.01).
  | Figure 2 Expressions of SDPR-AS and SDPR are positively correlated. |
SDPR expression was coregulated with SDPR-AS overexpression or knockdown in RCC cell lines
RT-qPCR was performed to determine the expression levels of SDPR-AS and SDPR in six RCC cell lines (OS-RC-2, 786-O, 769-P, Caki-1, Caki-2, and ACHN) and the normal cell line (HKC). The results indicated that the expression levels of SDPR-AS and SDPR were significantly lower in the six RCC cell lines compared to the normal HKC cell line (Figure 3A and B, *P<0.05). In addition, as shown in Figure 3A and B, among the six RCC cell lines, the expression levels of SDPR-AS and SDPR were highest in the 769-P and lowest in the OS-RC-2 cell lines. After being transfected with pcDNA-SDPR-AS plasmid, SDPR-AS was overexpressed in the OS-RC-2 cell line, while the expression level of SDPR was apparently upregulated (Figure 3C, *P<0.05). On the contrary, SDPR and SDPR-AS expressions were significantly decreased in the 769-P cell line transfected with SDPR-AS-si (Figure 3D, *P<0.05). The data indicated that SDPR and SDPR-AS were coregulated in the RCC cell lines.
Overexpression of SDPR-AS suppressed the migration and invasion of OS-RC-2 cell line but had no evident association with proliferation
To further investigate the functional role of SDPR-AS, we examined its effects on OS-RC-2 cells transfected with pcDNA-SDPR-AS or the pcDNA control. There was no significant difference in the growth of OS-RC-2 cells transfected with either pcDNA-SDPR-AS or pcDNA control (Figure 4A). However, the wound healing assay showed significant inhibition of cell migration by pcDNA-SDPR-AS compared to pcDNA control (Figure 4B, *P<0.05). The matrigel invasion assay also revealed remarkable suppression of cell invasion by pcDNA-SDPR-AS compared to pcDNA control (Figure 4C, *P<0.05).
Knockdown of SDPR-AS promoted the migration and invasion of 769-P cell line but showed no evident association with proliferation
To further validate the functional role of SDPR-AS in RCC cells, we performed knockdown experiments by transfecting the 769-P cell line with SDPR-AS-si or NC si and examined their effects on cellular activity. There was no evident difference in proliferation of 769-P cells transfected with either SDPR-AS-si or NC si (Figure 5A). The wound healing and matrigel invasion assays, however, revealed significantly enhanced migration and invasion abilities of 769-P cells treated with SDPR-AS-si compared with those treated with NC si (Figure 5B and C, *P<0.05).
Correlation analyses of SDPR-AS expression and clinical variables of RCC patients
Studies were carried out to assess the correlation between SDPR-AS and clinical characteristics of RCC patients. The statistical analysis revealed an evident association of SDPR-AS expression levels with differentiation grade, tumor node metastasis (TNM) stage, and lymph node metastasis (Table 2). However, no statistically significant associations were found in other clinicopathological features, including age, tumor size, and tumor location.
The association between SDPR-AS expression and prognosis of RCC patients
Kaplan–Meier survival and log-rank tests were performed to determine the association between SDPR-AS expression and prognosis. The overall expression levels of SDPR-AS were classified based on the mean relative expression ratio in RCC tissue specimens as high (SDPR-AS expression ratio ≥ median ratio, n=21) and low (SDPR-AS expression ratio ≤ median ratio, n=35). The data suggested that high expression of SDPR-AS was significantly associated with increased overall survival, whereas low expression indicated poor overall survival outcomes. Thus, SDPR-AS expression may be associated with prognosis of RCC patients (Figure 6, *P<0.05).
Discussion
Dozens of lncRNAs have recently been discovered through RNA sequencing and are annotated by the GENCODE project.24 Interest has been generated as a result of the fact that numerous lncRNAs are strongly linked with various cancers. For example, Chen et al9 reported that long intergenic non-coding RNA ROR (lincRNA-ROR) was involved in breast cancer invasion through participating in the epithelial–mesenchymal transition process. Huang et al25 demonstrated that low expression of DGCR5 was an important negative prognostic factor for hepatocellular carcinoma. In RCC, a number of lncRNAs, including CRNDE,26 TCL6,27 and MALAT-128 have been reported to be aberrantly expressed. Although alterations of lncRNAs in RCC have already been recognized, the functional roles of many remain to be understood. In the present study, we analyzed the functional roles of the lncRNA SDPR-AS and its prognostic relevance in RCC.
Recently, a number of studies have demonstrated that AS lncRNAs can regulate their sense mRNA partners in a discordant or concordant fashion.29,30 Numerous AS lncRNAs have been identified to be associated with a variety of malignant tumors.31,32 Generally, they modulate their sense partners by epigenetic regulation at the promoter regions or through other mechanisms.33,34 lncRNA SDPR-AS is the AS partner of the mRNA of SDPR. It has also been demonstrated that SDPR is an important tumor suppressor in certain cancers, including prostate cancer,20,35 breast cancer,21,36 and hepatocellular carcinoma.37 In particular, a previous study reported that SDPR was remarkably downregulated in RCC cells,22 which sparked our interest in conducting this study to investigate the functional and prognostic roles of its AS lncRNA in RCC.
In the present study, we first demonstrated the significant downregulation of SDPR-AS in RCC tissues in comparison to their matched nontumorous tissues. By loss-of-function and gain-of-function approaches, we confirmed that SDPR-AS played a critical role in cell migration and invasion, but had no evident effect on cell proliferation. In addition, the results also demonstrated a positive correlation between SDPR and SDPR-AS expression. Based on clinical data analysis, we determined that low expression of SDPR-AS was associated with tumor differentiation grade, TNM stage and lymph node metastasis. However, no statistically significant associations were found in other clinicopathological features, including age, tumor size, and tumor location. Furthermore, a multivariate analysis suggested that SDPR-AS expression level could serve as an independent prognostic predictor for RCC. Taken together, these results revealed a key role of SDPR-AS in RCC metastasis, possibly through modulation of the levels and functions of its sense partner. To our knowledge, our data may provide the first evidence for clarifying the functional and prognostic roles of SDPR-AS in RCC.
Conclusion
This study revealed the molecular mechanistic insights of SDPR-AS in RCC. In addition, this study provides some evidence that SDPR-AS may act as an independent prognostic predictor of RCC patients.
Acknowledgments
This work was supported by Medical Scientific Research Foundation of Heilongjiang Province, People’s Republic of China (2016–214) and China Postdoctoral Science Foundation.
Disclosure
The authors report no conflicts of interest in this work.
References
Ljungberg B, Cowan NC, Hanbury DC, et al. EAU guidelines on renal cell carcinoma: the 2010 update. Eur Urol. 2010;58(3):398–406. | ||
Ljungberg B, Campbell SC, Choi HY, et al. The epidemiology of renal cell carcinoma. Eur Urol. 2011;60(4):615–621. | ||
Zheng T, Yang CG. Targeting SPOP with small molecules provides a novel strategy for kidney cancer therapy. Sci China Life Sci. 2017;60(1):91–93. | ||
Pantuck AJ, Zisman A, Belldegrun AS. The changing natural history of renal cell carcinoma. J Urol. 2001;166(5):1611–1623. | ||
Guttman M, Amit I, Garber M, et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458(7235):223–227. | ||
Washietl S, Kellis M, Garber M. Evolutionary dynamics and tissue specificity of human long noncoding RNAs in six mammals. Genome Res. 2014;24(4):616–628. | ||
Guttman M, Rinn JL. Modular regulatory principles of large non-coding RNAs. Nature. 2012;482(7385):339–346. | ||
Ren X, Lan T, Chen Y, Shao Z, Yang C, Peng J. LncRNA uc009yby.1 promotes renal cell proliferation and is associated with poor survival in patients with clear cell renal cell carcinomas. Oncol Lett. 2016;12(3):1929–1934. | ||
Chen YM, Liu Y, Wei HY, Lv KZ, Fu P. Linc-ROR induces epithelial-mesenchymal transition and contributes to drug resistance and invasion of breast cancer cells. Tumour Biol. 2016;37(8):10861–10870. | ||
Iguchi T, Uchi R, Nambara S, et al. A long noncoding RNA, lncRNA-ATB, is involved in the progression and prognosis of colorectal cancer. Anticancer Res. 2015;35(3):1385–1388. | ||
Xiong J, Liu Y, Jiang L, Zeng Y, Tang W. High expression of long non-coding RNA lncRNA-ATB is correlated with metastases and promotes cell migration and invasion in renal cell carcinoma. Jpn J Clin Oncol. 2016;46(4):378–384. | ||
Xue S, Li QW, Che JP, Guo Y, Yang FQ, Zheng JH. Decreased expression of long non-coding RNA NBAT-1 is associated with poor prognosis in patients with clear cell renal cell carcinoma. Int J Clin Exp Pathol. 2015;8(4):3765–3774. | ||
Zhang M, Lu W, Huang Y, et al. Downregulation of the long noncoding RNA TUG1 inhibits the proliferation, migration, invasion and promotes apoptosis of renal cell carcinoma. J Mol Histol. 2016;47(4):421–428. | ||
Liu Y, Qian J, Li X, et al. Long noncoding RNA BX357664 regulates cell proliferation and epithelial-to-mesenchymal transition via inhibition of TGF-beta1/p38/HSP27 signaling in renal cell carcinoma. Oncotarget. 2016;7(49):81410–81422. | ||
Wang M, Huang T, Luo G, et al. Long non-coding RNA MEG3 induces renal cell carcinoma cells apoptosis by activating the mitochondrial pathway. J Huazhong Univ Sci Technolog Med Sci. 2015;35(4):541–545. | ||
Burgener R, Wolf M, Ganz T, Baggiolini M. Purification and characterization of a major phosphatidylserine-binding phosphoprotein from human platelets. Biochem J. 1990;269(3):729–734. | ||
Hansen CG, Bright NA, Howard G, Nichols BJ. SDPR induces membrane curvature and functions in the formation of caveolae. Nat Cell Biol. 2009;11(7):807–814. | ||
Elliott MH, Ashpole NE, Gu X, et al. Caveolin-1 modulates intraocular pressure: implications for caveolae mechanoprotection in glaucoma. Sci Rep. 2016;6:37127. | ||
Drab M, Verkade P, Elger M, et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293(5539):2449–2452. | ||
Li X, Jia Z, Shen Y, et al. Coordinate suppression of Sdpr and Fhl1 expression in tumors of the breast, kidney, and prostate. Cancer Sci. 2008;99(7):1326–1333. | ||
Ozturk S, Papageorgis P, Wong CK, et al. SDPR functions as a metastasis suppressor in breast cancer by promoting apoptosis. Proc Natl Acad Sci U S A. 2016;113(3):638–643. | ||
Gianazza E, Chinello C, Mainini V, et al. Alterations of the serum peptidome in renal cell carcinoma discriminating benign and malignant kidney tumors. J Proteomics. 2012;76:125–140. | ||
Preker P, Almvig K, Christensen MS, et al. PROMoter uPstream Transcripts share characteristics with mRNAs and are produced upstream of all three major types of mammalian promoters. Nucleic Acids Res. 2011;39(16):7179–7193. | ||
Harrow J, Frankish A, Gonzalez JM, et al. GENCODE: the reference human genome annotation for The ENCODE Project. Genome Res. 2012;22(9):1760–1774. | ||
Huang R, Wang X, Zhang W, et al. Down-regulation of LncRNA DGCR5 correlates with poor prognosis in hepatocellular carcinoma. Cell Physiol Biochem. 2016;40(3–4):707–715. | ||
Shao K, Shi T, Yang Y, Wang X, Xu D, Zhou P. Highly expressed lncRNA CRNDE promotes cell proliferation through Wnt/beta-catenin signaling in renal cell carcinoma. Tumour Biol. Epub 2016 Oct 6. | ||
Su H, Sun T, Wang H, et al. Decreased TCL6 expression is associated with poor prognosis in patients with clear cell renal cell carcinoma. Oncotarget. 2017;8(4):5789–5799. | ||
Chen S, Ma P, Zhao Y, et al. Biological function and mechanism of MALAT-1 in renal cell carcinoma proliferation and apoptosis: role of the MALAT-1-Livin protein interaction. J Physiol Sci. Epub 2016 Sep 21. | ||
Faghihi MA, Wahlestedt C. Regulatory roles of natural antisense transcripts. Nat Rev Mol Cell Biol. 2009;10(9):637–643. | ||
Wahlestedt C. Natural antisense and noncoding RNA transcripts as potential drug targets. Drug Discov Today. 2006;11(11–12):503–508. | ||
Yang X, Song JH, Cheng Y, et al. Long non-coding RNA HNF1A-AS1 regulates proliferation and migration in oesophageal adenocarcinoma cells. Gut. 2014;63(6):881–890. | ||
Takayama K, Horie-Inoue K, Katayama S, et al. Androgen-responsive long noncoding RNA CTBP1-AS promotes prostate cancer. EMBO J. 2013;32(12):1665–1680. | ||
Mussotter T, Kluwe L, Hogel J, et al. Non-coding RNA ANRIL and the number of plexiform neurofibromas in patients with NF1 microdeletions. BMC Med Genet. 2012;13:98. | ||
Pasmant E, Sabbagh A, Vidaud M, Bieche I. ANRIL, a long, noncoding RNA, is an unexpected major hotspot in GWAS. FASEB J. 2011;25(2):444–448. | ||
Tenta R, Katopodis H, Chatziioannou A, et al. Microarray analysis of survival pathways in human PC-3 prostate cancer cells. Cancer Genomics Proteomics. 2007;4(4):309–318. | ||
Tian Y, Yu Y, Hou LK, et al. Serum deprivation response inhibits breast cancer progression by blocking transforming growth factor-beta signaling. Cancer Sci. 2016;107(3):274–280. | ||
Jing W, Luo P, Zhu M, Ai Q, Chai H, Tu J. Prognostic and diagnostic significance of SDPR-Cavin-2 in hepatocellular carcinoma. Cell Physiol Biochem. 2016;39(3):950–960. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.