Back to Journals » Cancer Management and Research » Volume 11
Downregulation Of LncRNA PMS2L2 In Endometrial Adenocarcinoma Upon Carboplatin Treatment
Authors Zhang D, Sun X, Zhang Y
Received 29 June 2019
Accepted for publication 5 August 2019
Published 10 October 2019 Volume 2019:11 Pages 8905—8910
DOI https://doi.org/10.2147/CMAR.S221274
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
Dan Zhang,1 Xiuyun Sun,1 Yuyang Zhang2
1Department of Gynaecology, General Hospital of Fushun Mining Bureau of Liaoning Health Industry Group, Fushun City, Liaoning Province 113008, People’s Republic of China; 2The Second Department of Oncology, General Hospital of Fushun Mining Bureau of Liaoning Health Industry Group, Fushun City, Liaoning Province 113008, People’s Republic of China
Correspondence: Yuyang Zhang
The Second Department of Oncology, General Hospital of Fushun Mining Bureau of Liaoning Health Industry Group, No.24 Central Street, Xinfu District, Fushun City, Liaoning Province 113008, People’s Republic of China
Tel +86 413 24-52533037
Email [email protected]
Background: LncRNA PMS2L2 plays critical protective roles in chondrocytes during lipopolysaccharide-induced inflammation. Our preliminary deep sequencing revealed the altered expression of PMS2L2 in endometrial adenocarcinoma (EA) during chemotherapy. This observation triggered our interest to explore the functions of PMS2L2 in EA.
Methods: Levels of PMS2L2 in plasma were measured by qPCR. ROC curve analysis was used for diagnostic analysis. Cell viability was analyzed by cell viability assay.
Results: We showed that plasma PMS2L2 was downregulated in EA patients compared with healthy controls, and downregulation of PMS2L2 distinguished early-stage EA patients from healthy controls. During carboplatin-based chemotherapy, plasma levels of PMS2L2 were significantly downregulated in endometrial cancer patients. Overexpression of PMS2L2 led to decreased viability of EA cells, while PMS2L2 siRNA silencing led to increased viability of EA cells.
Conclusion: LncRNA PMS2L2 in endometrial cancer was downregulated during carboplatin treatment and regulates chemosensitivity.
Keywords: endometrial adenocarcinoma, lncRNA PMS2L2, chemosensitivity, carboplatin
Introduction
The development of chemotherapy has significantly improved the survival of cancer patients, especially for those who were diagnosed at advanced stages.1 Endometrial adenocarcinoma (EA) is the most common type of endometrial cancer, which is the major type of common gynaecological cancer in developed countries.2 EA is characterized by its extreme aggressive nature and low early diagnosis rate.3 A considerable portion of EA patients are initially diagnosed with the existence of tumor invasion, which are not candidates for radical tumor resection.4 Although chemotherapy is helpful for EA patients, chemo-resistance will inevitably develop, leading to poor treatment outcomes.5
It has been reported that chemotherapy modulates the expression of endogenous genes in the body of cancer patients, and the altered expression of certain genes may induce the sensitivity of cancer cells to chemical drugs.6,7 Recent studies also showed that long non-coding RNAs (lncRNAs, longer than 200 nt) were critical regulators of cancer cell behaviors8 and also participate in the development of chemo-resistance during chemotherapy.9 Therefore, regulation of lncRNA expression may assist the use of chemical drugs in cancer treatment. PMS1 Homolog 2, Mismatch Repair System Component Pseudogene 2 (PMS2L2) is a novel lncRNA that has protective effects on chondrocytes during lipopolysaccharide-induced inflammation.10 In this process, PMS2L2 regulates miR-203/MCL-1 axis to increase the viability of chondrocytes. Interestingly, our preliminary deep sequencing data suggested the altered expression of PMS2L2 in plasma of EA patients at 1 and 3 months after carboplatin-based chemotherapy comparing to pre-treatment level. The present study was therefore carried out to study the role of PMS2L2 in EA.
Patients And Methods
Subjects
Patient group in this study were 48 EA patients (46 to 68 years, 56.3 ± 5.2 years) who were enrolled in General Hospital of Fushun Mining Bureau of Liaoning Health Industry Group between June 2016 to June 2018. Inclusion criteria were the following: 1) EA confirmed by histopathological examinations; 2) first time diagnosis; 3) no therapies performed. Exclusion criteria were the following: 1) history of malignancies; 2) family history of malignancies; 3) complicated with any other clinical disorders. Based on AJCC staging, there were 12, 11, 20 and 15 cases at stages I–IV, respectively. Based on cancer grading results, there were 13, 16 and 19 cases at grades I–III, respectively. Control group in this study included 46 healthy females (45 to 68 years, 57.1 ± 5.4 years) with similar age distribution of the patient group. Those healthy females received systemic physiological examinations in General Hospital of Fushun Mining Bureau of Liaoning Health Industry Group during the same time period and all physiological parameters were within the normal range. All patients and healthy controls signed informed consent. Ethics Committee of General Hospital of Fushun Mining Bureau of Liaoning Health Industry Group approved this study (ID: GHFMB201603245646).
Cells And Plasma
All in vitro cell experiments were used in Human EA cell lines MFE-296 (Sigma-Aldrich, USA) and TOV-112D (ATCC, USA). Cells of these two cell lines were cultivated with MEM (2mM Glutamine, 10% FBS). Cell culture conditions were 37°C and 5% CO2.
Fasting blood (5 mL) was extracted from healthy controls during their systemic physiological examinations. The same amount of fasting blood was also extracted from EA patients before and at 3 and 6 months after carboplatin-based chemotherapy (doses vary according to disease conditions). Blood samples were transferred to EDTA tubes and centrifuged at room temperature for 15 mins at 1200 g to prepare plasma.
Transient Cell Transfection
Full-length PMS2L2 cDNA was inserted into pcDNA3.1 to establish PMS2L2 expression vectors. This vector construction service was provided by Sangon (Shanghai China). PMS2L2 siRNA (5ʹ-TCGAGTCCCTACCTTCGCCG-3ʹ) and negative control siRNA (5ʹ-UUGGUAUCUGGACUGGUACU-3ʹ) were also designed by Sangon (Shanghai China). MFE-296 and TOV-112D cells were cultivated overnight to 70–80% confluence, followed by transient cell transfections performed using lipofectamine 2000 reagent (Invitrogen, USA) with 40 nM siRNA and 10 nM vector. To confirm the successful transfections, un-transfected cells (control) and cells transfected with negative control siRNA or empty vector were included to serve as two controls. Following experiments were carried out at 24hrs after transfections.
RT-qPCR
Ribozol reagent (Thermo Fisher Scientific., Inc.) was used to extract total RNAs from MFE-296 and TOV-112D cells as well as plasma samples. cDNA samples were prepared using AMV Reverse Transcriptase (Promega Corporation, USA) with RNA samples as template. With cDNA as template, SYBR Green Master Mix (Bio-Rad, USA) was used to prepare qPCR reaction systems to analyze the expression of PMS2L2 with 18S rRNA as an endogenous control. Primer sequences were 5ʹ-AGTCTAAGCACTGCGGTGAA-3ʹ (forward) and 5ʹ-GGTAGAAATGGTGACATCAT-3ʹ (reverse) for PMS2L2; 5ʹ-CTACCACATCCAAGGAAGCA-3ʹ (forward) and 5ʹ-TTTTTCGTCACTACCTCCCCG-3ʹ (reverse) for human 18S rRNA. This experiment was performed in triplicate manner All data were processed using 2−ΔΔCT method.
MTT Assay
MFE-296 and TOV-112D cells were harvested at 24hrs after transfections. Cell suspensions were prepared using the cell culture medium mentioned above. Cell density was adjusted to 3×104 cells/mL. Cells were transferred to a 96-well plate with 0.1 mL cell suspension per well. Then, carboplatin was added to varied concentrations (0, 100 and 300 µM). Three replicate wells were set for each concentration. Cells were cultivated for 24hrs. MTT solution (10μL) was then added into each well. After cell culture for additional 4hrs, OD values at 570 nm were measured to reflect cell viability.
Statistical Analysis
All experiments in this study were performed in triplicate manner. Differences between patient and control groups were analyzed by unpaired t test. Differences among different cell transfection groups were analyzed by ANOVA (one-way) and Tukey t test. Differences among different time points were analyzed by repeated-measures ANOVA. Diagnostic values of PMS2L2 were evaluated by performing ROC curve analysis. In this analysis, EA patients at stage I and II were true positive cases and healthy females were true negative cases. All other parameters were defaults. With the median expression level of PMS2L2 in plasma as cutoff values, patients were divided into high- and low-level groups (n=24). Correlations between levels of PMS2L2 expression and patients’ clinical data were analyzed by Chi-square test. Differences were statistically significant when p<0.05.
Results
Plasma PMS2L2 Was Downregulated In EA Patients Compared To Healthy Controls
Plasma PMS2L2 in patient group (n=48) and control group (n=46) was detected by performing RT-qPCR, and expression data were analyzed by unpaired t test. It was observed that plasma PMS2L2 was significantly downregulated in EA patients compared to healthy controls (Figure 1, p<0.05). It was observed that expression levels of PMS2L2 were significantly correlated with patients’ clinical stage (p<0.01) and distant metastasis (p<0.05), but were not significantly correlated with patients’ age (p>0.05) and tumor grades (p>0.05).
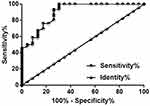
Downregulation Of PMS2L2 Distinguished Early Stage EA Patients From Healthy Controls
Diagnostic values of PMS2L2 were evaluated by performing ROC curve analysis. In this analysis, true positive cases were EA patients at stages I and II (n=23) and true negative cases were healthy females (n=46) as true negative cases. The area under the curve was 0.90 (Figure 2, standard error: 0.040; 95% confidence interval: 0.82–0.98; p<0.001).
Carboplatin Treatment Downregulated PMS2L2 In EA Patients And Cell Lines
Expression levels of plasma PMS2L2 in EA patients at 3 time points (before, and at 3 and 6 months after carboplatin-based chemotherapy) were measured by performing RT-qPCR. Expression data were analyzed by repeated-measures ANOVA. It was observed that levels of plasma PMS2L2 decreased with prolonged treatment (Figure 3A, p<0.05). MFE-296 and TOV-112D cells were treated with carboplatin (0, 100 and 300 µM) for 24hrs, followed by the detection of PMS2L2 expression by RT-qPCR. Expression data were analyzed by one-way ANOVA and Tukey’s test. It was observed that 100 and 300 µM carboplatin significantly downregulated PMS2L2 in MFE-296 and TOV-112D cells (Figure 3B, p<0.05)
PMS2L2 Reduced The Viability Of EA Cells Under 300 µM Carboplatin Treatment
PMS2L2 expression vectors and siRNAs were transfected into MFE-296 and TOV-112D cells. Compared to control (C) and negative control (NC) cells, PMS2L2 expression was significantly altered at 24hrs after transfection (Figure 4A, p<0.05), indicating the successful transfections. After transfection, MFE-296 and TOV-112D cells were treated with carboplatin (0 and 300 µM) for 24hrs, followed by the detection of cell viability by MTT assay. Compared to 0 µM carboplatin, 300 µM carboplatin significantly decreased the viability of both MFE-296 and TOV-112D cells (Figure 4B, p<0.05). In addition, overexpression of PMS2L2 led to decreased viability of EA cells, while PMS2L2 siRNA silencing led to increased viability of EA cells only at 300 µM carboplatin (Figure 4B, p<0.05).
Discussion
Our study mainly investigated the involvement of PMS2L2 in EA. We showed that plasma circulating PMS2L2 may assist in the early diagnosis of EA. We also provided evidence that overexpression of PMS2L2 may assist the application of carboplatin-based chemotherapy in the treatment of EA.
Although lncRNAs are usually expressed in specific types of cells,11,12 they may be released from the site of synthesis into the blood circulation system to achieve systemic trafficking.13 In effect, the development and progression of cancers are usually accompanied by changes in levels of circulating lncRNAs, and the altered levels of circulating lncRNAs may provide diagnostic and prognostic values for cancers. We detected PMS2L2 in all EA patients and healthy controls. However, the site of the synthesis of PMS2L2 is unknown due to the lack of tissue specimen experiments in this study. Treatment of endometrial cancer is still challenged by the low rate of early diagnosis.14,15 Therefore, identification of novel biomarkers is always critical to improve the early diagnosis. In the present study, we showed that altered plasma levels of PMS2L2 distinguished early-stage EA patients from healthy controls, indicating that PMS2L2 may serve as a potential diagnostic biomarker for EA.
Carboplatin has been widely used as a chemical drug to treat different types of cancer, including EA.16,17 However, development of chemo-resistance is always observed after long-term application. It has been reported that carboplatin may interact with certain lncRNAs to affect cancer cell survival.18 We showed that treatment with carboplatin induced the downregulation of PMS2L2 in both EA patients and cells of two EA cell lines. In addition, PMS2L2 negatively regulated the survival of EA cells under carboplatin treatment. Therefore, downregulation of PMS2L2 is likely related to the development of chemo-resistance in EA patients during carboplatin-based chemotherapy. However, the mechanism is still unknown. Our study will focus on the potential interactions between PMS2L2 and the downstream effector of carboplatin.
In conclusion, PMS2L2 is downregulated in EA and has early diagnostic values. PMS2L2 overexpression may improve the outcomes of carboplatin-based therapy by reducing cell viability.
Availability of data and materials
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
Ethics Approval And Consent To Participate
The present study was approved by the Ethics Committee of General Hospital of Fushun Mining Bureau of Liaoning Health Industry Group. The research has been carried out in accordance with the World Medical Association Declaration of Helsinki. All patients and healthy volunteers provided written informed consent prior to their inclusion within the study.
Consent For Publication
All authors have read and approved the final manuscript.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare they have no conflicts of interest in this work.
References
1. Davidson BA, Foote J, Clark LH, et al. Tumor grade and chemotherapy response in endometrioid endometrial cancer. Gynecol Oncol Rep. 2016;17:3–6. doi:10.1016/j.gore.2016.04.006
2. Morice P, Leary A, Creutzberg C, et al. Endometrial cancer. Lancet. 2016;387(10023):1094–1108. doi:10.1016/S0140-6736(15)00130-0
3. Valenzano M, Podesta M, Giannesi A, et al. The role of transvaginal ultrasound and sonohysterography in the diagnosis and staging of endometrial adenocarcinoma. Radiol Med. 2001;101(5):365–370.
4. Boes AS, Tousseyn T, Vandenput I, et al. Pitfall in the diagnosis of endometrial cancer: case report of an endometrioid adenocarcinoma arising from uterine adenomyosis. Eur J Gynaecol Oncol. 2011;32(4):431–434.
5. Gentilin E, Minoia M, Bondanelli M, et al. Growth hormone differentially modulates chemoresistance in human endometrial adenocarcinoma cell lines. Endocrine. 2017;56(3):621–632. doi:10.1007/s12020-016-1085-4
6. Kanamori Y, Kigawa J, Itamochi H, et al. PTEN expression is associated with prognosis for patients with advanced endometrial carcinoma undergoing postoperative chemotherapy. Int J Cancer. 2002;100(6):686–689. doi:10.1002/ijc.10542
7. Dong L, Zhou Q, Zhang Z, et al. Metformin sensitizes endometrial cancer cells to chemotherapy by repressing glyoxalase I expression. J Obstet Gynaecol Res. 2012;38(8):1077–1085. doi:10.1111/j.1447-0756.2011.01839.x
8. Gutschner T, Diederichs S. The hallmarks of cancer: a long non-coding RNA point of view. RNA Biol. 2012;9(6):703–719. doi:10.4161/rna.20481
9. Shang C, Lang B, Ao CN, et al. Long non-coding RNA tumor suppressor candidate 7 advances chemotherapy sensitivity of endometrial carcinoma through targeted silencing of miR-23b. Tumuor Biol. 2017;39(6):1010428317707883.
10. Li X, Yu M, Chen L, et al. LncRNA PMS2L2 protects ATDC5 chondrocytes against lipopolysaccharide-induced inflammatory injury by sponging miR-203. Life Sci. 2019;217:283–292. doi:10.1016/j.lfs.2018.12.020
11. Necsulea A, Soumillon M, Warnefors M, et al. The evolution of lncRNA repertoires and expression patterns in tetrapods. Nature. 2014;505(7485):635–640. doi:10.1038/nature12943
12. Engreitz JM, Ollikainen N, Guttman M. Long non-coding RNAs: spatial amplifiers that control nuclear structure and gene expression. Nat Rev Mol Cell Biol. 2016;17(12):756–770. doi:10.1038/nrm.2016.126
13. Qi P, Zhou X, Du X. Circulating long non-coding RNAs in cancer: current status and future perspectives. Mol Cancer. 2016;15(1):39. doi:10.1186/s12943-016-0524-4
14. Muggia, Franco, Santin, Alessandro D., Oliva, Esther (Eds.) Uterine Cancer: Screening, Diagnosis, and Treatment. Springer; 2018.
15. Burke WM, Orr J, Leitao M, et al. Endometrial cancer: a review and current management strategies: part II. Gynecol Oncol. 2014;134(2):393–402. doi:10.1016/j.ygyno.2014.06.003
16. Choi S, Hsu ICJ. Endometrial cancer[M]//Handbook of Evidence-Based Radiation Oncology. Cham: Springer; 2018:645–669.
17. Randall M, Filiaci V, McMeekin D, et al. A phase 3 trial of pelvic radiation therapy versus vaginal cuff brachytherapy followed by paclitaxel/carboplatin chemotherapy in patients with high-risk, early-stage endometrial cancer: a Gynecology Oncology Group study. Int J Radiat Oncol Biol Phys. 2017;99(5):1313. doi:10.1016/j.ijrobp.2017.09.008
18. Liu E, Liu Z, Zhou Y. Carboplatin-docetaxel-induced activity against ovarian cancer is dependent on up-regulated lncRNA PVT1. Int J Clin Exp Pathol. 2015;8(4):3803–3810.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.