Back to Journals » Cancer Management and Research » Volume 10
Downregulation of lncRNA OGFRP1 inhibits hepatocellular carcinoma progression by AKT/mTOR and Wnt/β-catenin signaling pathways
Authors Chen W , You J, Zheng Q, Zhu Y
Received 9 February 2018
Accepted for publication 13 April 2018
Published 2 July 2018 Volume 2018:10 Pages 1817—1826
DOI https://doi.org/10.2147/CMAR.S164911
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Harikrishna Nakshatri
Wei Chen, Jia You, Qi Zheng, Yue-Yong Zhu
Center for Liver Diseases, The First Affiliated Hospital, Fujian Medicine University, Fuzhou 350005, Fujian Province, China
Introduction: Increasing evidence demonstrates that long noncoding RNAs (lncRNAs) play important roles in the progression of hepatocellular carcinoma (HCC) by regulating gene expression. However, the identification of functional lncRNAs in HCC remains insufficient. Our study aimed to investigate the function of lncRNA OGFRP1, which has not been functionally researched before, in Hep3B and HepG2 cells.
Methods: lncRNA OGFRP1 in HCC cells was down-regulated by using RNAi technology. Quantitative real-time polymerase chain reaction was used to determine the mRNA expression of lncRNA OGFRP1. Cell proliferation was examined by CCK8 and clone formation assays. Cell cycle and apoptosis were analyzed by flow cytometry. Cell migration and invasion were assessed by using Scratch assay and transwell assay, respectively. Protein expression of signaling pathways was determined by using Western blot.
Results: Cell proliferation of Hep3B was significantly inhibited by down-regulation of lncRNA OGFRP1 (P<0.05). Moreover, siOGFRP1 transfection induced Hep3B cell cycle arrest and apoptosis by regulating the expression of related proteins. Cell migration and invasion of Hep3B were also significantly inhibited by down-regulation of lncRNA OGFRP1. Wnt/β-catenin signaling pathway, involved in epithelial–mesenchymal transition (EMT), was inactivated by lncRNA OGFRP1 downregulation, including decreased expression of Wnt3a, β-catenin, N-cadherin and vimentin and increased expression of E-cadherin. We also found that the inhibitory effect of lncRNA OGFRP1 knockdown on Hep3B was mediated by the AKT/mTOR signaling pathway and IGF-1, an AKT signaling activator, could rescue the cellular phenotype. However, knockdown of lncRNA OGFRP1 did not influence cell proliferation, migration and invasion in HepG2 cells.
Conclusion: We found that downregulation of lncRNA OGFRP1 suppressed the proliferation and EMT of HCC Hep3B cells through AKT and Wnt/β-catenin signaling pathways. However, lncRNA OGFRP1 exhibited a differentiated function in different HCC cell lines, which required further study in the future.
Keywords: lncRNA OGFRP1, HCC, EMT, AKT/mTOR, Wnt/β-catenin
Introduction
Hepatocellular carcinoma (HCC), with high morbidity and mortality, has become one of the most life-threatening tumors. Among all liver cancer cases, the percentage of HCC has reached ~80%–90% and >600,000 people die of it every year.1–3 Previous reports have shown that HCC morbidity is mainly caused by hepatitis B/C virus infection, liver cirrhosis, alcohol addiction and environmental pollution.4–6 There are some inspiring therapies having been designed to treat HCC, such as liver transplantation, sorafenib and percutaneous ablation.1,7 However, the treatment effect is still unsatisfactory, and the 5-year survival rate of HCC patients is still only 3%–5%.8–10 Therefore, it is urgent to explore new regulating mechanisms for HCC progression to identify more therapeutic targets for HCC treatment.11–13
Long noncoding RNAs (lncRNAs) refer to RNA molecules with more than 200 bp size and lacking the ability of coding protein.14,15 In the past few decades, genetic studies were mainly concentrated on protein-coding genes, which account for only 2% of gene transcripts and noncoding RNAs were once thought as transcribed noise.16,17 However, increasing data have shown that lncRNAs are involved in nearly all biological processes.18 The functions of lncRNAs mainly include regulating gene expression through chromatin remodeling and histone modification, epigenetic modification or sponge effect.19–21 At present, ENCODE and GENCODE projects have predicted the prevalence of ~10,000 lncRNAs.22 However, there are still much more lncRNAs having not been identified, not to say corresponding function annotations.22,23,24 In addition, there are some inherent difficulties in lncRNA studies, such as low expression, high tissue specificity and narrow time frames.25–28 Therefore, identification and functionally annotating for lncRNAs is a very promising study field for exploring the regulating mechanisms of biological processes.
In this study, we expect to investigate the function of a novel lncRNA OGFRP1, which has not been functionally investigated before. We investigated the effect of downregulation of lncRNA OGFRP1 on Hep3B cell functions, including cell proliferation, migration, invasion, cell cycle and apoptosis. Wnt/β-catenin and AKT/mTOR signaling pathways were also detected to explain the action mechanism. In addition, we also explored the function of lncRNA OGFRP1 in another HCC line called HepG2.
Materials and methods
Cell culture and transfection
Hep3B and HepG2 cells were purchased from the Cell Bank of Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Hep3B and HepG2 cells were respectively cultured in the Roswell Park Memorial Institute (RPMI)-1640 and DMEM (Thermo Fisher Scientific, Waltham, MA, USA) media at 37°C with 5% CO2. Both media were supplemented with 10% fetal bovine serum (FBS; Thermo Fisher Scientific), 100 mg/mL streptomycin (Thermo Fisher Scientific) and 100 U/mL penicillin. Hep3B and HepG2 cells were transfected with siOGFRP1 or siNC by using Lipofectamine 2000 (Thermo Fisher Scientific). The siRNAs sequences (Guangzhou RiboBio Co., Ltd, Guangzhou, China) were as follows: 5′-GGTGTTCACATGGCAGTAA-3′ for siRNA1, 5′-GGATACTGAGAGTGCACAA-3′ for siRNA2 and 5′-GCATTGACATGTTTGGCAT-3′ for siRNA3.
Analysis of mRNA expression
After transfection, total RNA of cells was extracted using a Stratagene Absolutely RNA Microprep Kit (Stratagene, La Jolla, CA, USA) according to manufacturer’s instructions. Purified RNA was stored at -80°C. RNA was reverse transcribed with Stratagene’s AffinityScript QPCR cDNA Synthesis Kit. The mRNA expression level was analyzed by quantitative polymerase chain reaction (qPCR).
Cell counting kit-8 (CCK-8) assay
CCK-8 assay was performed to test cell proliferation. After being plated in a 96-well plate, Hep3B and HepG2 cells were transfected with siOGFRP1 or siNC. Every 24 h, cells in each well were incubated with 10 μL CCK-8 solutions for 2 h. Then, OD values at 450 nm were measured using a microplate reader.
Clone formation assay
After transfection for 24 h, HCC cells were seeded in a 6 cm culture dish at a density of 200 cells/well and cultured until visible in naked eyes. Then, the supernatants were removed, and cells were stained with crystal violet for 20 min. Images of the colonies were captured by a digital camera.
Flow cytometry detection for cell cycle and apoptosis
For cell cycle detection, transfected cells were harvested and fixed with 70% ethanol for 24 h at -20°C. Fixed cells were then successively going through PBS washing, staining by propidium iodide (PI) and flow cytometry analysis.
Annexin V-FITC Apoptosis Detection Kit (Thermo Fisher Scientific) was used to detect cell apoptosis following standard protocols. Briefly, transfected cells were harvested, centrifuged and washed with precooled PBS. Then the cells were sequentially stained with Annexin V-FITC for 15 min and PI for 5 min in the dark. After that, flow cytometry and FlowJo software (TreeStar, San Carlos, CA, USA) were used to analyze cell apoptosis.
Scratch assay
Cells were cultured until 100% confluence and then scratched with a 200 μL pipette tip. The detached cells were washed away and then photos were taken; photos after incubation for 24 h were also taken. The Image J software was used to determine the wound areas. Cell migration was expressed as the percentage of wound closure/initial wound area.
Transwell invasion assay
To assess cell invasive ability in vitro, HCC cells were seeded on Matrigel-coated transwell inserts with 500 μL of serum-free medium. The lower chamber was filled with 500 μL of medium containing 10% FBS. After incubation for 24 h, the cells remaining on upper surface of transwell inserts were wiped with cotton wool. The invaded cells were stained with crystal violet for 5 min. Photos were taken, and cell number was counted.
Western blot
After 48 h of transfection, proteins of HCC cells were extracted by using radioimmunoprecipitation assay buffer containing 1% protease inhibitors (Hoffman-La Roche Ltd., Basel, Switzerland). Bicinchoninic acid assay was used to determine protein concentration. Proteins were separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) at 20 μg/lane and transferred to a nitrocellulose membrane (EMD Millipore, Billerica, MA, USA). Then, the membrane was blocked with 5% nonfat milk for 2 h and incubated with primary antibodies at 4°C overnight and the secondary antibodies for 1 h at room temperature. After washing with TBST (20 mM Tris–HCl, pH 7.6, 140 mM NaCl, 0.1% Tween-20) for three times, protein bands were developed by ECL (Perkin-Elmer, Waltham, MA, USA). The primary antibodies included anti-P70S6K (Cat no. ab32529; Abcam, Shanghai, China), anti-CyclinD1 (Cat no. ab40754; Abcam), anti-p53 (Cat no. ab26; Abcam), anti-Bax (Cat no. ab32503; Abcam), anti-Bcl2 (Cat no. ab32124; Abcam), anti-Caspase-9 (Cat no. ab32539; Abcam), anti-Active-Casepase3 (Cat no. ab2302; Abcam), anti-Wnt3a (Cat no. ab28472; Abcam), anti-β-catenin (Cat no. ab16051; Abcam), anti-N-cadherin (Cat no. ab18203; Abcam), anti-E-cadherin (Cat no. ab1416; Abcam), anti-vimentin (Cat no. ab92547; Abcam), anti-p-Akt (Cat no. ab81283; Abcam), anti-p-mTOR (Cat no. ab131538; Abcam) and anti-GAPHD (Cat no. ab8245; Abcam). The secondary antibodies were obtained from Proteintech (Wuhan, China). The ImageJ software was utilized to quantify the density of each band.
Statistical analysis
Student’s t-test was used to analyze statistical difference between the two groups. P < 0.05 was considered as statistically significant.
Results
Inhibition of Hep3B proliferation by downregulation of lncRNA OGFRP1
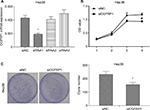
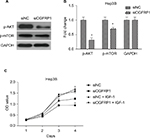
We used loss-of-function experiments to evaluate the biological function of lncRNA OGFRP1 in HCC. The interference efficiencies of three candidate siRNAs are shown in Figure 1A, which suggested that siRNA1 efficiently reduced lncRNA OGFRP1 expression and was applied in the following experiments. The CCK-8 assay revealed that Hep3B proliferation was significantly inhibited when silencing lncRNA OGFRP1 (Figure 1B). To validate the result, we also performed clone formation assay in Hep3B cells. It was suggested that silencing of lncRNA OGFRP1 dramatically reduced clone numbers of Hep3B cells (Figure 1C). These data indicated that lncRNA OGFRP1 displayed a positive role in Hep3B cell proliferation.
Induction of Hep3B cell cycle arrest and apoptosis by downregulation of lncRNA OGFRP1
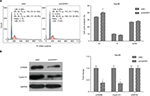
Induction of cell cycle arrest is one of the important mechanisms for inhibition of cell viability; hence, we analyzed cell cycle population in lncRNA OGFRP1 silencing Hep3B cells by using flow cytometry. As shown in Figure 2A, compared to the siNC group, cell cycle of lncRNA OGFRP1-silencing Hep3B cells was arrested at the G1 phase. We further examined the expression of cell cycle proteins p70S6K and Cyclin D1, which were involved in promoting G1–S transition. As shown in Figure 2B, silencing of lncRNA OGFRP1 in Hep3B cells reduced the expression of p70S6K and Cyclin D1 to <40% of that in the siNC group. These data suggested that downregulation of p70S6K and Cyclin D1 by silencing of lncRNA OGFRP1 was responsible for the G1-phase arrest in Hep3B cells.
To determine whether cell apoptosis contributed to the inhibitory effect of silencing lncRNA OGFRP1, we analyzed cell apoptosis of Hep3B cells using flow cytometry. It was indicated that silencing of lncRNA OGFRP1 significantly increased the percentage of total apoptotic cells from 3.663% ± 0.555% to 10.457% ± 0.765% in the siNC group (P<0.05, Figure 3A). To further investigate the molecular mechanisms through which lncRNA OGFRP1 regulated cell apoptosis, we detected the expression of apoptosis-associated proteins. As shown in Figure 3B, silencing of lncRNA OGFRP1 significantly increased the expression of proapoptotic proteins p53, Bax, Caspase-9 and Active-Caspase-3 and decreased the expression of antiapoptotic protein Bcl2. These data suggested that downregulation of lncRNA OGFRP1 promoted cell apoptosis through regulating apoptosis-associated protein.
Inhibition of Hep3B cell migration and invasion by downregulation of lncRNA OGFRP1
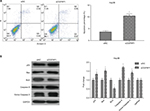
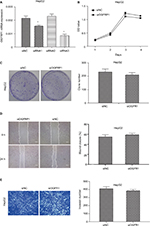
In addition to almost permanent proliferation, migration and invasion are also important features of cancer cells, which often trigger tumor metastasis.29,30 Here, we performed scratch assay and transwell assay to analyze the effect of lncRNA OGFRP1 downregulation on cell migration and invasion of Hep3B. As shown in Figure 4A, silencing of lncRNA OGFRP1 significantly increased the velocity of cell movements. The percentage of wound closure at 24 h was decreased from 61% in the siNC group to 11.6% in the siOGFRP1 group (P < 0.05). The transwell invasion assay suggested that cell invasion of Hep3B cells was also inhibited by silencing of lncRNA OGFRP1 (Figure 4B). The invasion cell number per field in the siOGFRP1 group was 127 ± 17, which was significantly less than 291 ± 23 in the siNC group (P < 0.05).
In order to explain the mechanism underlying anti-mobility of lncRNA OGFRP1 downregulation, we examined the expression of Wnt/β-catenin signaling pathway components. As shown in Figure 4C, downregulation of lncRNA OGFRP1 led to decreased expression of Wnt3a, β-catenin, N-cadherin and Vimentin and increased expression of E-cadherin, suggesting an inactivated status of Wnt/β-catenin signaling pathway was triggered by downregulation of lncRNA OGFRP1 in HCC cells.
Downregulation of lncRNA OGFRP1 inhibits Hep3B by AKT signaling pathway
In order to determine the mechanism underlying the inhibitory effect of lncRNA OGFRP1 downregulation on Hep3B cells, we investigated AKT/mTOR signaling pathway. The AKT/mTOR signaling pathway is reported to play a central role in regulating tumor cell proliferation, cell cycle, apoptosis and movements.31 Activation of AKT and mTOR is dependent on protein phosphorylation. As shown in Figure 5A and B, the phosphorylated levels of AKT and mTOR in Hep3B cells were significantly downregulated when silencing lncRNA OGFRP1 (P < 0.05). These data suggested that downregulation of lncRNA OGFRP1 inactivated AKT/mTOR signaling pathway in Hep3B HCC cells. In addition, we also applied IGF-1, an activator of AKT, to evaluate the role of AKT signaling pathway in lncRNA OGFRP1 knockdown-mediated cell phenotype changes. It was suggested that IGF-1 could markedly increase cell vitality of lncRNA OGFRP1-silenced Hep3B cells, which proved that functional effects of lncRNA OGFRP1 were mediated by inhibition of AKT signaling pathway (Figure 5C).
Effects of downregulation of lncRNA OGFRP1 on HepG2 cells
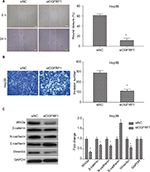
In order to investigate the physiological role of OGFRP1 in HCC cells more comprehensively, we also knocked down lncRNA OGFRP1 in another HCC cell line called HepG2. As shown in Figure 6A, siRNA3 was the most potent siRNA to knock down lncRNA OGFRP1. However, cell proliferation analysis including CCK-8 assay and clone formation assay indicated no significant difference between the siOGFRP1 group and siNC group (Figure 6B and C). Likewise, lncRNA OGFRP1 knockdown also had no significant influence on HepG2 cell migration and invasion (Figure 6D and E). These results indicated that lncRNA OGFRP1 might exert different functions in different HCC cell lines.
Discussion
Owing to difficult early diagnosis and poor prognosis, HCC has become the rapidest growing cause of cancer death worldwide.32,33 Emerging evidence indicates that a number of lncRNAs are vital for the HCC progression. Therefore, the present research aimed to investigate the function and regulating mechanism of a new tumor promoting lncRNA OGFRP1 in HCC cells.
Although lncRNAs have no ability to encode protein, they can participate in numerous cancer-related biological processes, such as cell proliferation, survival, migration and invasion, through the regulation of target gene expression.18 At present, a variety of lncRNAs have been found to be aberrantly expressed in HCC and involved in modulating HCC malignant phenotype.34 For instance, NEAT1 promotes HCC tumor growth and metastasis through downregulating miR-613,35 and lncRNA AOC4P promotes the degradation of vimentin and suppresses epithelial–mesenchymal transition (EMT) in HCC.36 In addition, Xu et al37 report that lncRNA URHC regulates cell proliferation and apoptosis via ZAK through the ERK/MAPK signaling pathway in HCC progression.
However, as far as we know, there is no research reporting the function of lncRNA OGFRP1 in HCC. Our study showed that downregulation of lncRNA OGFRP1 significantly inhibited proliferation of Hep3B cells by using CCK-8 and clone formation assays. Cell cycle analysis indicated that cell cycle was arrested at the G1 phase and the expression of p70S6K and Cyclin D1 were downregulated to <40% of that in the negative group. Cyclin D1 is a cell cycle protein promoting G1–S transition by regulating CDK4,38 and the expression of Cyclin D1 depends on p70S6K.39 In addition, cell apoptosis percentage also increased from 3.663% ± 0.555% to 10.457% ± 0.765% when lncRNA OGFRP1 was downregulated. p53 signaling pathway was also investigated by using Western blot, which indicated that p53 and its downstream proapoptotic proteins Bax, Caspase-9 and Active-Caspase-3 were upregulated, whereas Bcl2, an antiapoptotic protein antagonizing Bax, was downregulated. Hep3B cell migration and invasion were found to be significantly inhibited by downregulation of lncRNA OGFRP1 using scratch and transwell assays. Wnt/β-catenin pathway is reported to play an important role in EMT, which is the main cause of tumor metastasis.40–44 Western blot showed that downregulation of lncRNA OGFRP1 significantly inhibited the expression of Wnt3a, β-catenin, N-cadherin and Vimentin but increased the expression of E-cadherin, suggesting that Wnt/β-catenin pathway was inactivated. We also investigated the impact of lncRNA OGFRP1 downregulation on AKT/mTOR signaling pathway, which was reported to regulate numerous biological processes in tumor, such as cell proliferation and protein synthesis.45 It was suggested that the phosphorylation levels of AKT and mTOR were significantly decreased in the siOGFRP1 group, which confirmed an inactivation status of AKT/mTOR signaling pathway. However, we could not confirm the protumor function of lncRNA OGFRP1 in another HCC cell line called HepG2, which demonstrated the high specificity of lncRNA functions and the complexity of tumor intracellular environment.
Conclusion
For the first time, our study found that the downregulation of lncRNA OGFRP1 inhibited cell proliferation and EMT in HCC Hep3B cells through AKT/mTOR and Wnt/β-catenin pathways. We believed that this study will extend the understanding of the function of lncRNAs in liver tumorigenesis, and these findings may be important for developing a potential therapeutic target for HCC treatment. However, lncRNA OGFRP1 does not influence cell proliferation, migration and invasion in HepG2 cells, suggesting the functional complexity of lncRNA OGFRP1 in HCC and the need for further in-depth study.
Acknowledgment
This work was funded by the Natural Science Foundation of Fujian Province (2016J0105).
Disclosure
The authors report no conflicts of interest in this work.
References
Roberts LR. Sorafenib in liver cancer – just the beginning. N Engl J Med. 2008;359(4):420–422. | ||
Kuo TY, Hsi E, Yang IP, Tsai PC, Wang JY, Juo SH. Computational analysis of mRNA expression profiles identifies microRNA-29a/c as predictor of colorectal cancer early recurrence. PLoS One. 2012;7(2):e31587. | ||
Khushman M, Morris MI, Diaz L, et al. Syndrome of inappropriate anti-diuretic hormone secretion secondary to strongyloides stercoralis infection in an allogeneic stem cell transplant patient: a case report and literature review. Transplant Proc. 2017;49(2):373–377. | ||
Elkhoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–2502. | ||
Chan SL, Wong VW, Qin S, Chan HL. Infection and cancer: the case of Hepatitis B. J Clin Oncol. 2016;34(1):83–90. | ||
Liu Y, Chai Y, Zhang J, Tang J. A function variant at miR-501 alters susceptibility to hepatocellular carcinoma in a Chinese Han population. Cell Physiol Biochem. 2016;38(6):2500–2508. | ||
Chuang SC, La Vecchia C, Boffetta P. Liver cancer: descriptive epidemiology and risk factors other than HBV and HCV infection. Cancer Lett. 2009;286(1):9–14. | ||
Liao Y, Zheng Y, He W, et al. Sorafenib therapy following resection prolongs disease-free survival in patients with advanced hepatocellular carcinoma at a high risk of recurrence. Oncol Lett. 2017;13(2):984–992. | ||
Elzouki AN, Elkhider H, Yacout K, Al Muzrakchi A, Al-Thani S, Ismail O. Metastatic hepatocellular carcinoma to parotid glands. Am J Case Rep. 2014;15:343–347. | ||
Gokcan H, Savas N, Oztuna D, Moray G, Boyvat F, Haberal M. Predictors of survival in hepatocellular carcinoma patients. Ann Transplant. 2015;20:596–603. | ||
Yu LH, Yu WL, Zhao T, Wu MC, Fu XH, Zhang YJ. Post-operative delayed elevation of ALT correlates with early death in patients with HBV-related hepatocellular carcinoma and post-hepatectomy liver failure. HPB (Oxford). 2018;20(4):321–326. | ||
Cui H, Zhang Y, Zhang Q, Chen W, Zhao H, Liang J. A comprehensive genome-wide analysis of long noncoding RNA expression profile in hepatocellular carcinoma. Cancer Med. 2017;6(12):2932–2941. | ||
Ye B, Hu B, Zheng Z, Zheng R, Shi Y. The long non-coding RNA AK023948 enhances tumor progression in hepatocellular carcinoma. Exp Ther Med. 2017;14(4):3658–3664. | ||
Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10(3):155–159. | ||
Fang J, Sun CC, Gong C. Long noncoding RNA XIST acts as an oncogene in non-small cell lung cancer by epigenetically repressing KLF2 expression. Biochem Biophys Res Commun. 2016;478(2):811–817. | ||
Yang X, Xie X, Xiao YF, et al. The emergence of long non-coding RNAs in the tumorigenesis of hepatocellular carcinoma. Cancer Lett. 2015;360(2):119–124. | ||
Sun L, Xue H, Jiang C, et al. LncRNA DQ786243 contributes to proliferation and metastasis of colorectal cancer both in vitro and in vivo. Biosci Rep. 2016;36(3):e00328. | ||
Zhang H, Zhou D, Ying M, et al. Expression of long non-coding RNA (lncRNA) small nucleolar RNA host gene 1 (SNHG1) exacerbates hepatocellular carcinoma through suppressing miR-195. Med Sci Monit. 2016;22:4820–4829. | ||
Wilusz JE. Long noncoding RNAs: re-writing dogmas of RNA processing and stability. Biochim Biophys Acta. 2016;1859(1):128. | ||
Rinn JL. lncRNAs: linking RNA to chromatin. Cold Spring Harb Perspect Biol. 2014;6(8):a018614. | ||
Ulitsky I, Bartel D. lincRNAs: genomics, evolution, and mechanisms. Cell. 2013;154(1):26–46. | ||
Harrow J, Frankish A, Gonzalez JM, et al. GENCODE: the reference human genome annotation for The ENCODE project. Genome Res. 2012;22(9):1760–1774. | ||
ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489(7414):57–74. | ||
Min L, Garbutt C, Tu C, Hornicek F, Duan Z. Potentials of long noncoding RNAs (LncRNAs) in Sarcoma: from biomarkers to therapeutic targets. Int J Mol Sci. 2017;18(4):E731. | ||
Bartonicek N, Maag JL, Dinger ME. Long noncoding RNAs in cancer: mechanisms of action and technological advancements. Mol Cancer. 2016;15(1):43. | ||
Mercer TR, Dinger ME, Sunkin SM, Mehler MF, Mattick JS. Specific expression of long noncoding RNAs in the mouse brain. Proc Natl Acad Sci U S A. 2008;105(2):716–721. | ||
Cabili MN, Trapnell C, Goff L, et al. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 2011;25(18):1915. | ||
Gloss BS, Dinger ME. The specificity of long noncoding RNA expression. Biochim Biophys Acta. 2015;1859(1):16–22. | ||
Polacheck WJ, Zervantonakis IK, Kamm RD. Tumor cell migration in complex microenvironments. Cell Mol Life Sci. 2013;70(8):1335–1356. | ||
Zhao J, Liu Y, Zhang W, et al. Long non-coding RNA Linc00152 is involved in cell cycle arrest, apoptosis, epithelial to mesenchymal transition, cell migration and invasion in gastric cancer. Cell Cycle. 2015;14(19):3112–3123. | ||
Liu T, Sun Q, Li Q, et al. Dual PI3K/mTOR inhibitors, GSK2126458 and PKI-587, suppress tumor progression and increase radiosensitivity in nasopharyngeal carcinoma. Mol Cancer Ther. 2015;14(2):429–439. | ||
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. | ||
Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27(9):1485–1491. | ||
Ghidini M, Braconi C. Non-coding RNAs in primary liver cancer. Front Med (Lausanne). 2015;2:36. | ||
Wang Z, Zou Q, Song M, Chen J. NEAT1 promotes cell proliferation and invasion in hepatocellular carcinoma by negative regulating miR-613 expression. Biomed Pharmacother. 2017;94:612–618. | ||
Wang TH, Lin YS, Chen Y, et al. Long non-coding RNA AOC4P suppresses hepatocellular carcinoma metastasis by enhancing vimentin degradation and inhibiting epithelial-mesenchymal transition. Oncotarget. 2015;6(27):23342–23357. | ||
Xu WH, Zhang JB, Dang Z, et al. Long non-coding RNA URHC regulates cell proliferation and apoptosis via ZAK through the ERK/MAPK signaling pathway in hepatocellular carcinoma. Int J Biol Sci. 2014;10(7):664–676. | ||
Fernando R, Foster JS, Bible A, et al. Breast cancer cell proliferation is inhibited by BAD: regulation of cyclin D1. J Biol Chem. 2007;282(39):28864–28873. | ||
Huang XM, Dai CB, Mou ZL, et al. Overproduction of cyclin D1 is dependent on activated mTORC1 signal in nasopharyngeal carcinoma: implication for therapy. Cancer Lett. 2009;279(1):47–56. | ||
Bao XL, Song H, Chen Z, Tang X. Wnt3a promotes epithelial-mesenchymal transition, migration, and proliferation of lens epithelial cells. Mol Vis. 2012;18:1983–1990. | ||
Bastos LG, de Marcondes PG, de-Freitas-Junior JC, et al. Progeny from irradiated colorectal cancer cells acquire an EMT-like phenotype and activate Wnt/beta-catenin pathway. J Cell Biochem. 2014;115(12):2175–2187. | ||
Guo YH, Wang LQ, Li B, et al. Wnt/beta-catenin pathway transactivates microRNA-150 that promotes EMT of colorectal cancer cells by suppressing CREB signaling. Oncotarget. 2016;7(27):42513–42526. | ||
Liang J, Liang L, Ouyang K, Li Z, Yi X. MALAT1 induces tongue cancer cells’ EMT and inhibits apoptosis through Wnt/beta-catenin signaling pathway. J Oral Pathol Med. 2017;46(2):98–105. | ||
Xiao C, Wu CH, Hu HZ. LncRNA UCA1 promotes epithelial-mesenchymal transition (EMT) of breast cancer cells via enhancing Wnt/beta-catenin signaling pathway. Eur Rev Med Pharmacol Sci. 2016;20(13):2819–2824. | ||
Arcaro A. Targeting PI3K/mTOR signaling in cancer. Front Oncol. 2014;4:84. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.