Back to Journals » Cancer Management and Research » Volume 11
Downregulation of lncRNA NCK1-AS1 Inhibits Cancer Cell Migration and Invasion in Nasopharyngeal Carcinoma by Upregulating miR-135a
Received 29 June 2019
Accepted for publication 6 November 2019
Published 17 December 2019 Volume 2019:11 Pages 10531—10537
DOI https://doi.org/10.2147/CMAR.S221326
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Haili Hu,* Haixia Li,* Xiao Feng
Department of Otolaryngology, Huaihe Hospital, Henan University, Kaifeng City, Henan Province 475000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Haixia Li
Department of Otolaryngology, Huaihe Hospital, Henan University, Kaifeng City, Henan Province 475000, People’s Republic of China
Tel +86 13148493290
Email [email protected]
Introduction: The present study was carried out to explore the functionality of lncRNA NCK1-AS1 in nasopharyngeal carcinoma (NPC).
Methods: Levels of NCK1-AS1 were measured by performing qPCR and were compared by ANOVA (one-way) performed in combination with Tukey’s test. Expression levels of miR-135a in plasma of NPC patients were measured by performing qPCR. The effects of transfections on the invasion and migration of C666-1 cells were analyzed by Transwell assays.
Results and discussion: In the present study, we found that the plasma levels of NCK1-AS1 were significantly higher in NPC patients than the levels in patients with arthritis of the temporomandibular joint (TMJ), as well as healthy participants. No significant difference in plasma levels of NCK1-AS1 was found between TMJ patients and healthy participants. Upregulation of NCK1-AS1 distinguished NPC patients from TMJ patients and healthy participants. A significant and inverse correlation between NCK1-AS1 and miR-135a was found in NPC patients. NCK1-AS1 siRNA silencing led to the upregulation of miR-135a. NCK1-AS1 siRNA silencing and miR-135a overexpression resulted in inhibited cell migration and invasion, and miR-135a inhibition attenuated the effects of NCK1-AS1 siRNA silencing.
Conclusion: The downregulation of lncRNA NCK1-AS1 inhibited cancer cell migration and invasion in NPC by upregulating miR-135a.
Keywords: NCK1-AS1, nasopharyngeal carcinoma, arthritis of the temporomandibular joint, miR-135a
Introduction
Nasopharyngeal carcinoma (NPC) originates from the nasopharynx epithelium, which is a rare type of head and neck cancer that only accounts for 2% of all cases.1 The incidence of NPC varies a lot in different countries over the world.2 In China, NPC affects about 30 of 100,000 people.2 The prognosis of NPC is generally poor, especially for metastatic NPC, which is common among NPC patients.3–5 In addition, in clinical practice, NPC can be easily be misdiagnosed as other clinical disorders, such as arthritis of the temporomandibular joint (TMJ), owing to their similar symptoms.6 Therefore, early diagnosis and diagnostic markers are urgently needed to improve the survival rate of NPC.
Histopathological examination is the gold standard for cancer diagnosis.7 Due to the invasiveness, histopathological examination is not appropriate for cancer screening.7 Recent studies have shown that cancer development is usually accompanied by changes in certain substances in blood.8 Therefore, monitoring the changes in levels of certain blood substances may provide information to predict disease conditions.8 Long non-coding RNAs (lncRNAs, >200 nt) are critical players in cancer biology through their roles in regulating gene expression.9,10 LncRNAs are usually spatially or temporally expressed; however, they can be released into the blood to systemically regulate gene expression.11 NCK1-AS1 is a recently identified oncogenic lncRNA in cervical cancer.12,13 Our preliminary RNA-seq analysis revealed the altered expression of NCK1-AS1 in NPC and its inverse correlation with miR-135a, which is a tumor-suppressive miRNA in NPC14]. This study aimed to investigate the involvement of NCK1-AS1 in NPC and explore its early diagnostic values as well as its potential interaction with miR-135a.
Materials and Methods
Study Subjects
This study passed the review board of the Ethics Committee of Huaihe Hospital. Study subjects included 50 cases of NPC patients (squamous cell carcinoma, gender: 29 males and 21 females; age: 47 to 72 years’ old; mean age: 57.4±6.3 years’ old), 50 cases of TMJ patients (gender: 29 males and 21 females; age: 46 to 71 years’ old; mean age: 57.0±6.9 years’ old), and 50 cases of healthy volunteers (gender: 29 males and 21 females; age: 48 to 73 years’ old; mean age: 57.7±5.4 years’ old). All the participants were selected in Huaihe Hospital between March 2015 and March 2019. Patients’ inclusion criteria: 1) disease confirmed by histopathological exams; 2) newly diagnosed cases. Exclusion criteria: 1) therapies were initiated; 2) other clinical disorders were diagnosed; 3) history of previous malignancies. All participants were informed of experimental details and all of them signed informed consent. According to the AJCC stage, there were 22 and 28 cases at stage I and II, respectively.
Plasma and NPC Cells
Before the initiation of therapies, 5ml blood was extracted from patients under fasting conditions. To separate plasma, blood was transferred to EDTA tubes, which were subsequently centrifuged at 1200g for 20 min.
Human NPC cell lines C666-1 and 13-9B were purchased from SHUNRAN (Shanghai, China). Cells were cultivated in the mixture composed of 90% RPMI medium 1640 and 10% FBS. Cell culture conditions were 37°C, 5% CO2, and 95% humidity.
Cell Transfections
Negative control (NC) siRNA (5ʹ-UUCUCCGAACGUGUCACGUTT-3ʹ) and NCK1-AS1 (NCBI Reference Sequence: NR_110175.1) siRNA (5ʹ-GAAUGUCAUCCCAGCCGAAT-3ʹ), NC miRNA (5′−GGCAGGUAACGUGCGUUUCGA−3ʹ) and miR-135a mimic (5ʹ-UAUGGCUUUUUAUUCCUAUGUGA-3ʹ), as well as NC inhibitor (5ʹ−GGCAGGUAACGUGCGUUUCGA−3) and miR-135a inhibitor (antisense) were all from Sangon (Shanghai, China). C666-1 cells were harvested at the confluence of 70–80%. Cells were counted and transient transfections were performed to transfect 10 nM siRNA (NC siRNA as NC group), or 10 nM miRNA (NC miRNA as NC group) or 10 nM inhibitor (NC inhibitor as NC group) into 3×106 cells. Transient transfections were mediated by lipofectamine 2000 (Sangon). Besides the NC group, control (C, untransfected cells) group was also included for all transfections. All the following experiments were performed using cells harvested at 24h post-transfection.
RNA Extraction and qPCR
At 24h post-transfection, C666-1 cells were harvested and counted. Total RNAs in 3×105 cells or 0.25 mL plasma were extracted using RNAzol (Sigma-Aldrich, USA) with all steps performed following the manufacturer’s instruction. MiRNAs were harvested by precipitating RNAs using 85% ethanol.
To remove genomic DNA, RNA samples were incubated with DNase I at 37°C for 1h. Tetro Reverse Transcriptase (Bioline) was used to perform all reverse transcriptions and QuantiTect SYBR Green PCR Kit (Qiagen) was used to prepare qPCR mixtures with 18S rRNA as an endogenous control to measure the expression levels of NCK1-AS1.
All-in-One™ miRNA qRT-PCR Detection Kit (Genecopoeia) was used to perform poly (A) addition of mature miRNAs, miRNA reverse transcription and qPCR assay. U6 was used as the endogenous control to measure the expression levels of miR-135a.
No-template reaction was used as a negative control. The slope of the standard curve was used to calculate the amplification efficiency (all between 95.6 and 98.1%). Primer sequences were: 5ʹ−TTCCCATTTCTCCCAGGTCC−3ʹ and 5ʹ−TGGTTACTTTGAGCCTGCCT−3ʹ for NCK1-AS1; 5ʹ−GTAACCCGTTGAACCCCATT−3ʹ and 5ʹ−CCATCCAATCGGTAGTAGCG−3ʹ for 18S rRNA. The forward primer of miR-135a was 5ʹ-UAUGGCUUUUUAUUCC-3ʹ. MiR-135a reverse primer and U6 primers were included in the kit.
The 2−ΔΔCT method was used to process all data, and each experiment was performed in 3 replicates.
Transwell Assays
At 24h post-transfection, C666-1 and 13-9B cells were harvested and counted. A cell pellet containing 3×103 cells were resuspended in 1 mL mixture of 99% RPMI-1640 medium and 1% FBS to make cell suspensions. Transwell assays were performed to analyze the effects of transfections on the invasion and migration of C666-1 and 13-9B cells. Before invasion assay, Transwell chambers were coated with Matrigel (Millipore) to mimic the conditions of in vivo invasion. Upper Transwell chamber was filled with cell suspension and a mixture of 80% RPMI-1640 medium and 20% FBS was used to fill the lower chamber. Under conditions of 37°C, 5% CO2, and 95% humidity, cells were cultivated for 12h, followed by 0.5% crystal violet (Sigma-Aldrich, USA) staining for 25 min at 24 °C. A light microscope was used to count invading and migrating cells.
Data Process
Experiments were performed in 3 biological replicates and mean values were counted and were used for all data analyses. Linear regression was used for correlation analyses. Differences were explored among 3 groups of participants or among different cell groups by ANOVA (one-way) in combination with the Tukey’s test. Diagnostic analyses were performed by performing a ROC curve analysis. In the ROC curve, true positive cases were NPC patients and true negative cases were TMJ patients or healthy participants. p<0.05 was statistically significant.
Results
Levels of NCK1-AS1 in Plasma Were Specifically Increased in NPC Patients
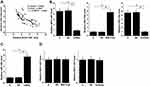
Analysis of the TCGA dataset revealed the upregulation of NCK1-AS1 in head and neck cancer compared to non-tumor tissues (HNSC, 4.99 vs. 1.85, http://gepia.cancer-pku.cn/detail.php?gene=NCK1-AS1). Levels of NCK1-AS1 in plasma samples derived from 50 NPC patients, 50 TMJ patients and 50 healthy participants (Control) were measured by performing qPCR and were compared by performed ANOVA (one-way) in combination with Tukey’s test. Comparing to the TMJ and Control group, significantly higher levels of NCK1-AS1 in plasma were observed in NPC patients (Figure 1, p<0.05). However, plasma levels of NCK1-AS1 were not significantly different between TMJ and Control groups.
Increase Plasma Levels of NCK1-AS1 Showed Diagnostic Values for NPC
The potentials of plasma levels of NCK1-AS1 in the diagnosis of NPC were analyzed by performed ROC curve analysis through the aforementioned methods. The area under the curve (AUC) >0.05 indicates potential diagnostic values. With TMJ as true negative cases, AUC was 0.96 (95% confidence interval 0.93–0.95; standard error: 0.017) (Figure 2A). With healthy participants as true negative cases, AUC was 0.97 (95% confidence interval 0.94–1.08; standard error: 0.015) (Figure 2B).
NCK1-AS1 Regulated miR-135a Expression in C666-1 Cells
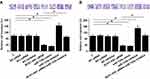
Expression levels of miR-135a in plasma of NPC patients were measured by performing qPCR. Correlations between miR-135a and NCK1-AS1 were analyzed by performing linear regression. It can be observed that plasma levels of miR-135a were significantly and inversely correlated with plasma levels of NCK1-AS1 (Figure 3A). To further investigate the interactions between NCK1-AS1 and miR-135a, C666-1 cells were transfected with NCK1-AS1 siRNA, miR-135a mimic or miR-135a inhibitor. Expression levels of NCK1-AS1 and miR-135a were measured by qPCR at 24h post-transfection. Comparing to C and NC groups, expression levels of NCK1-AS1 and miR-135a were significantly altered (Figure 3B, p<0.05). Moreover, NCK1-AS1 siRNA silencing led to the upregulation of miR-135a (Figure 3C, p<0.05). However, NCK1-AS1 expression was not significantly affected by miR-135 overexpression and inhibition (Figure 3D).
NCK1-AS1 Knockdown Resulted in the Suppressed Invasion of Migration of C666-1 and 13-9B Cells Through miR-135a
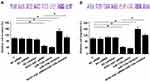
The effects of transfections on the invasion (Figure 4A) and migration (Figure 4B) of C666-1 cells were analyzed by Transwell assays. Comparing to C and NC two groups, NCK1-AS1 siRNA silencing and miR-135a overexpression resulted in inhibited cell migration and invasion (p<0.05). Moreover, miR-135a inhibition played the opposite role and attenuated the effects of NCK1-AS1 siRNA silencing. To further explore the roles of NCK1-AS1 in regulating NPC cell invasion and migration, 13-9B cells were used to repeat Transwell invasion (Figure 5A) and migration (Figure 5B) assay. Similar results were obtained (p<0.05).
Discussion
This study mainly investigated the roles of NCK1-AS1 in NPC. We found that NCK1-AS1 was upregulated in NPC, and NCK1-AS1 knockdown resulted in suppressed invasion and migration of NPC cells. The actions of NCK1-AS1 in this process are likely mediated by miR-135a, which is a tumor-suppressive miRNA in NPC.14
The functions of NCK1-AS1 have only been investigated in cervical cancer.12,13 NCK1-AS1 is overexpressed in cervical cancer and promotes cell cycle progression and cell proliferation of cancer cells through the interactions with CDK1 and miR-6857.13 Inhibition of NCK1-AS1 inhibits the development of chemoresistance during chemotherapies, which in turn assists the clinical treatment of cervical cancer.12 This study is the first to report the upregulation of NCK1-AS1 in NPC and NCK1-AS1 siRNA silencing resulted in the suppressed NPC cell invasion and migration. Therefore, NCK1-AS1 is also an oncogenic lncRNA in NPC.
NPC has similar clinical symptoms to TMJ.15 Therefore, novel molecular biomarkers are needed to distinguish these two clinical disorders. We showed that NCK1-AS1 was specifically upregulated in NPC patients but not in TMJ patients. To evaluate the early diagnostic values of plasma of NCK1-AS1, our study only enrolled NPC patients at clinical stages I and II. We found that increased levels of NCK1-AS1 in plasma could be used to effectively distinguish NPC patients from TMJ patients and healthy participants. Therefore, measuring the levels of NCK1-AS1 in plasma may assist in the early diagnosis of NPC. However, the accuracy should be further tested with a bigger sample size.
MiR-135a is a tumor suppressor in NPC.14 In the development of NPC, miR-135a targets interleukin-17 to inhibit cancer cell proliferation.14 Our study also reported the downregulation of miR-135a in NPC. In addition, miR-135a negatively regulated the invasion and migration of NPC cells, indicating the potential roles of miR-135a in the metastasis of NPC. We showed that NCK1-AS1 siRNA silencing resulted in the upregulation of miR-135a. Our future study will explore the mechanism that mediates the interaction between miR-135a and NCK1-AS1. It is known that non-coding RNAs may also perform its functions through its structure.16 Future studies may focus on the analysis of secondary and tertiary structures of NCK1-AS1.
In conclusion, NCK1-AS1 is upregulated in NPC and NCK1-AS1 siRNA silencing may upregulate miR-135a to suppress cancer cell invasion and migration.
Ethical Statement
This study was approved by the Ethics Committee of Huaihe Hospital and was conducted in accordance with the Declaration of Helsinki.
Author Contributions
Haixia Li, Haili Hu: study design, experiments, data analysis, manuscript drafting and revising, final approval of the version to be published, and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Xiao Feng: experiments, data analysis, manuscript revising, final approval of the version to be published, and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chua MLK, Wee JTS, Hui EP, et al. Nasopharyngeal carcinoma. Lancet. 2016;387(10022):1012–1024. doi:10.1016/S0140-6736(15)00055-0
2. Lee AW, Ma BB, Ng WT, et al. Management of nasopharyngeal carcinoma: current practice and future perspective. J Clin Oncol. 2015;33(29):3356–3364. doi:10.1200/JCO.2015.60.9347
3. Tang LQ, Li CF, Li J, et al. Establishment and validation of prognostic nomograms for endemic nasopharyngeal carcinoma. J Natl Cancer Inst. 2016;108:1. doi:10.1093/jnci/djv291
4. Zou X, You R, Liu H, et al. Establishment and validation of M1 stage subdivisions for de novo metastatic nasopharyngeal carcinoma to better predict prognosis and guide treatment. Eur J Cancer. 2017;77:117–126. doi:10.1016/j.ejca.2017.02.029
5. Yang X, Dai W, Kwong DL, et al. Epigenetic markers for noninvasive early detection of nasopharyngeal carcinoma by methylation‐sensitive high resolution melting. Int J Cancer. 2015;136(4):E127–E135. doi:10.1002/ijc.29192
6. Jensen A, Nolet PS, Diwan MA. Oral squamous cell carcinoma: an atypical presentation mimicking temporomandibular joint disorder. J Can Chiropr Assoc. 2004;48(4):266–272.
7. Demir C, Yener B. Automated cancer diagnosis based on histopathological images: a systematic survey. Micron. 2012;43(2–3):352–364. doi:10.1016/j.micron.2011.09.016
8. Schwarzenbach H, Hoon DSB, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer. 2011;11(6):426–437. doi:10.1038/nrc3066
9. Gutschner T, Diederichs S. The hallmarks of cancer: a long non-coding RNA point of view. RNA Biol. 2012;9(6):703–719. doi:10.4161/rna.20481
10. Spizzo R, Almeida MI, Colombatti A, et al. Long non-coding RNAs and cancer: a new frontier of translational research? Oncogene. 2012;31(43):4577–4877. doi:10.1038/onc.2011.621
11. Qi P, Zhou X, Du X. Circulating long non-coding RNAs in cancer: current status and future perspectives. Mol Cancer. 2016;15(1):39. doi:10.1186/s12943-016-0524-4
12. Zhang WY, Liu YJ, He Y, et al. Suppression of long noncoding RNA NCK1-AS1 increases chemosensitivity to cisplatin in cervical cancer. J Cell Physiol. 2019;234(4):4302–4313. doi:10.1002/jcp.27198
13. Li H, Jia Y, Cheng J, et al. LncRNA NCK1-AS1 promotes proliferation and induces cell cycle progression by crosstalk NCK1-AS1/miR-6857/CDK1 pathway. Cell Death Dis. 2018;9(2):198. doi:10.1038/s41419-017-0249-3
14. Wang LX, Kang ZP, Yang ZC, et al. MicroRNA-135a inhibits nasopharyngeal carcinoma cell proliferation through targeting interleukin-17. Cell Physiol Biochem. 2018;46(6):2232–2238. doi:10.1159/000489591
15. Mayne JG, Hatch GS. Arthritis of the temporomandibular joint. J Am Dent Assoc. 1969;79(1):125–130. doi:10.14219/jada.archive.1969.0225
16. Wu J, Leontis NB, Zirbel CL, Bisaro DM, Ding B. A three-dimensional RNA motif mediates directional trafficking of potato spindle tuber viroid from epidermal to palisade mesophyll cells in nicotiana benthamiana. PLoS Pathog. 2019;15(10):e1008147. doi:10.1371/journal.ppat.1008147
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.