Back to Journals » Cancer Management and Research » Volume 10
Downregulation of hsa_circ_0011946 suppresses the migration and invasion of the breast cancer cell line MCF-7 by targeting RFC3
Authors Zhou J, Zhang WW , Peng F, Sun JY, He ZY , Wu SG
Received 2 November 2017
Accepted for publication 11 January 2018
Published 19 March 2018 Volume 2018:10 Pages 535—544
DOI https://doi.org/10.2147/CMAR.S155923
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Juan Zhou,1,* Wen-Wen Zhang,2,* Fang Peng,3 Jia-Yuan Sun,2 Zhen-Yu He,2 San-Gang Wu4
1Department of Obstetrics and Gynecology, the First Affiliated Hospital of Xiamen University, Xiamen 361003, People’s Republic of China; 2Department of Radiation Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center of Cancer Medicine, Guangzhou 510060, People’s Republic of China; 3Department of Radiation Oncology, the First Affiliated Hospital of Sun Yat-sen University, Guangzhou 510080, People’s Republic of China; 4Department of Radiation Oncology, Xiamen Cancer Hospital, the First Affiliated Hospital of Xiamen University, Xiamen 361003, People’s Republic of China
*These authors contributed equally to this work
Introduction: Although some circRNAs have been found to regulate the progression of malignancies, their functions and coupled molecular mechanisms are still unclear. In our study, we sought to assess the underlying molecular mechanisms of circRNAs in breast cancer and therefore explored the differentially expressed circRNAs and co-expression networks, followed by in vitro experiments.
Materials and methods: High-throughput RNA sequencing was performed to obtain an unbiased profile of circRNA expression. CircRNA-miRNA-mRNA co-expression networks were predicted, and sequence analyses were carried out. The MTT, transwell migration and invasion assay was conducted in Michigan Cancer Foundation-7 cells that had been transfected with si-circRNA and si-negative control (si-NC).
Results: A total of 152 circRNAs were differentially expressed in breast cancer tissues, among which 85 were upregulated and 67 downregulated. Out of these, hsa_circ_0011946 was selected and the subsequent bioinformatics analysis predicted that hsa_circ_0011946 sponging miR-26a/b directly targeted replication factor C subunit 3 (RFC3) and that its knockdown could inhibit RFC3 mRNA and protein expression. Furthermore, hsa_circ_0011946 loss-of-function significantly suppressed the migration and invasion of Michigan Cancer Foundation-7 cells.
Conclusion: Together, these results indicate that hsa_circ_0011946 and RFC3 comprise a novel pathway involved in the progression of breast cancer.
Keywords: hsa_circ_0011946, MCF-7, RFC, breast cancer, high-throughput sequencing
Introduction
Breast cancer is the most frequently occurring female malignancy and has a gradually increasing incidence all over the world.1 Genetic mutations and epigenetic alterations make the mechanisms of its progression difficult to understand. With the development of gene chip and high-throughput sequencing technology, many studies have been focused on the identification of differentially expressed non-coding RNAs, including microRNAs, long non-coding RNAs, and circular RNAs (circRNAs), which are considered to be promising biomarkers for the early detection of breast cancer, as well as potential therapeutic targets.2–4
CircRNAs are a class of special non-coding RNA and are characterized by a highly stable, covalently closed continuous loop, without any 5′–3′ polarity or a polyadenylated tail, giving them the distinct ability to counteract RNA exonucleolytic digestion.5 In recent years, circRNA research has experienced explosive growth, and more than 20,000 circRNAs have now been identified in eukaryocytes.6 Functional studies indicate that circRNAs are involved in multiple physiological and pathological processes by acting as microRNA sponges to regulate gene and protein expression.7,8 An increasing number of studies have demonstrated that the dysregulated expression of circRNAs is closely related to the development and progression of tumors, including breast cancer.9,10 Intriguingly, blood-based circRNAs can serve as novel non-invasive biomarkers for the diagnosis of cancers.11 Moreover, hsa_circ_006054, hsa_circ_100219, and hsa_ circ_406697 are upregulated in breast cancer tissues, and the combination of these circRNAs has a high diagnostic value for the detection of breast cancer.12 Together, these findings suggest that circRNAs play a major role in tumorigenesis. It is important to highlight that the precise mechanisms behind the involvement of circRNAs in breast cancer progression have not been completely clarified.
Replication factor C (RFC) is composed of 5 subunits and plays an important role in eukaryotes in deoxyribonucleic acid (DNA) replication, DNA damage repair, and checkpoint control during cell cycle progression.13,14 RFC subunit 3 (RFC3) is one of the small subunits on the RFC complex and was originally purified from HeLa cells and shown to be essential for the in vitro replication of Simian virus 40.14 In recent years, the various roles of RFC3 have been increasingly illuminated in studies of malignancies, including hepatocellular carcinoma,15 ovarian carcinoma,16 esophageal adenocarcinoma,17 colorectal cancer,18 and breast cancer,19,20 suggesting that RFC3 may be a potential oncogenic gene involved in tumorigenesis. However, it is not clear how RFC3 regulates the progression of breast cancer when combined with circRNAs.
In the present study, we utilized high-throughput sequencing and experimental validation in vitro to uncover the differentially expressed circRNAs in breast cancer tissues and cell lines and found that hsa_circ_0011946 was significantly upregulated. Moreover, a bioinformatics analysis showed RFC3 to be a target gene of hsa_circ_0011946. Furthermore, we downregulated hsa_circ_0011946 in the Michigan Cancer Foundation-7 (MCF-7) cell line and performed cell proliferation, migration, and invasion experiments, in order to elaborate on its function.
Materials and methods
Patients and specimens
Three pairs of breast cancer and corresponding adjacent non-cancerous tissues were acquired from patients who underwent surgical operations in Sun Yat-sen University Cancer Center in 2016. The commonly used clinicopathologic features of breast cancer are given in Table S1. All patients were diagnosed with invasive ductal breast cancer and the tumor-node-metastasis (TNM) classification was in accordance with the American Joint Committee on Cancer TNM Staging. Samples were rapidly stored in liquid nitrogen for high-throughput RNA sequencing. Written informed consent was obtained from all participants prior to sample collection. The study was approved by the Ethics Committee of the First Affiliated Hospital of Xiamen University and Sun Yat-sen University Cancer Center.
Cell culture
Six breast cancer cell lines (HS-578T, T47D, MCF-7, BT549, MDA-MB-231, and SKBR-3) were purchased from the Cell Bank of China Academy of Sciences, Shanghai, China. All were cultured in Roswell Park Memorial Institute medium 1640 (Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS; Gibco, Grand Island, NY, USA) and 1% penicillin–streptomycin at 37°C in 5% CO2 and were plated in 6-well plates at a density of 2×105 well. After 2 days of incubation, the cells were collected via RNA isolation, and used in proliferation, migration, and invasion assays.
High-throughput RNA sequencing of circRNA
In order to build a library, high-throughput RNA sequencing was performed as previously described.21 The clean reads were aligned to the reference genome with Bowtie2 (http://bowtie-bio.sourceforge.net/bowtie2/manual.shtml).22 The junctions of the unmapped reads were then chosen using a back splice algorithm. Finally, circRNAs were verified using circbase (http://www.circbase.org). “Mapped backsplicing junction reads per million mapped reads” (RPM) was used to measure the expression level of each circRNA.
Kyoto encyclopedia of genes and genomes (KEGG) biological pathway and co-expression analysis
The KEGG pathway analysis was performed to determine the involvement of linear transcripts in different biological pathways, as previously described.21 Differentially expressed circRNAs were used as an alignment to enrich the circRNA-miRNA-mRNA network according to the miRNA target prediction, which was based on TargetScan and miRanda, as previously described.23,24 Cytoscape software (V. 3.2.1) was employed to build a circRNA-miRNA-mRNA network.
Validation of circRNA and head-to-tail splicing
The complementary DNA (cDNA) was acquired as described below and genomic DNA (gDNA) was isolated using the DNeasy Blood & Tissue Kit (Qiagen, Inc., Valencia, CA, USA). Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) was performed with the SYBR Select Master Mix (Applied Biosystems) using ABI7300 System (Applied Biosystems, Foster City, CA, USA). We designed divergent primers that bound to the circRNA transcript (chr1: 41578954-41618413) formed in a 3′–5′ fashion. After gel purification using the QIAquick Gel Extraction Kit (Qiagen), the RT-qPCR product was sequenced using the Sanger method, in order to confirm the head-to-tail splicing, as previously described.21,25
MTT assay
The proliferation of each breast cancer cell line was monitored using an MTT Cell Proliferation/Viability Assay kit (R&D Systems, Inc., Minneapolis, MN, USA), according to the manufacturer’s protocol. Briefly, following transfection with si-hsa_circ_0011946 or si-NC, 15 µL of MTT was added to each well (5×104) and incubated for 4 h at 37°C. Then, the stop solution was added and the solution was kept overnight at room temperature. The optical density value was measured at 492 nm using a SpectraMax M5 ELISA plate reader (Molecular Devices, LLC, Sunnyvale, CA, USA).
Cell transfection
The small interfering RNAs (siRNA) utilized for cell transfection were synthesized by GenePharma Co., Ltd. (Shanghai, China) and the sequence of the functional si-hsa_circ_0011946 was ACCAAAGCATCTAGTGCTTTT. MCF-7 cells were transfected with si-hsa_circ_0011946 for 48 h at 37°C using Lipofectamine 2000 (Invitrogen), according to the manufacturer’s protocol.
RT-qPCR
The RNA was extracted using TRIzol (Invitrogen; Thermo Fisher Scientifc, Inc., Waltham, MA, USA). Moloney Murine Leukemia Virus reverse transcriptase (Promega Corporation, Madison, WI, USA) and oligo dT 15 primers (Thermo Fisher Scientific, Inc.) were used to synthesize cDNA. Divergent primers were designed to ensure the amplification of the head-to-tail splicing of circRNA using the ABI7300 System (Applied Biosystems) with the SYBR Select Master Mix (Applied Biosystems), as previously described.26 Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and U6 levels were utilized to normalize the expressions of the circRNAs and RFC3 mRNA, respectively. The PCR primers used in this study were as follows: hsa_circ_0011946, forward 5′-GCTGGTGTTCCTTGACTGGA-3′ and reverse 5′-CACTGTAGCAAACCAGCATTTCT-3′; hsa_circ_0007534, forward 5′-GTGACGGAAATCCAATTGCACC-3′ and forward 5′-ATGGAATTGCTGGCGAGTTG-3′; hsa_circ_0093859, forward 5′-ACCCAATGGAGTTCTCAGCAG-3′ and forward 5′-TGGAAGGTCTGACAGGAATGTG-3′; RFC3, forward 5′-TCCCTGCTTCTGATTTCCTTTACC-3′ and forward 5′-GGCTTCCCTGACCACCCTATTTA-3′; GAPDH, forward 5′-GCACCGTCAAGCTGAGAAC-3′ and reverse 5′-TGGTGAAGACGCCAGTGGA-3′; U6, forward 5′-AGCCCGCACTCAGAACATC-3′ and reverse 5′-GCCACCAAGACAATCATCC-3′. The reaction conditions were 95°C for 2 min, 36 cycles of denaturation at 95°C for 15 s, annealing at 60°C for 30 s, and extension at 60°C for 30 s. The relative expression levels of circRNAs and RFC3 were calculated using the 2−ΔΔCq method.27
Western blotting
The protein was extracted using NP-40 buffer (Beyotime Institute of Biotechnology, Haimen, China), and the concentration was determined using the Bicinchoninic Acid Kit for Protein Determination ( Sigma-Aldrich, St. Louis, MO, USA). Samples containing 50 μg of protein were separated on a 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The primary antibody anti-RFC3 was purchased from Santa Cruz Biotechnology, Dallas, TX, USA (Cat. no: sc-390293; dilution: 1:1,000). The membranes were incubated with the appropriate horseradish peroxidase-conjugated secondary antibody (Cat. no: sc-516102; dilution: 1:10,000; Santa Cruz Biotechnology), following visualization with chemiluminescence (Thermo Fisher Scientific, Inc.). GAPDH primary antibody (Cat. no: 2118; dilution: 1:2,000; Cell Signaling Technology, Inc., Danvers, MA, USA) was used as the control antibody.
Migration and invasion assays
The MCF-7 cells were transfected with si-circRNA and si-NC for 48 h and cells were resuspended in 5% FBS medium to achieve a density of 1×106 cells/mL. A total of 100 μL cell suspension medium with 5% FBS was added to the upper chamber, whereupon 600 μL complete medium was added to the bottom chamber and incubated at 37°C with 5% CO2. For the transwell migration assays, the cells on the upper surface of the membrane were removed with cotton swabs, and those on the lower surface were the migrated cells. After being fixed with 4% paraformaldehyde and stained with 0.1% crystal violet solution, the cells that passed through the filter were photographed by inverted fluorescence microscope (Leica Microsystems GmbH, Wetzlar, Germany). The transwell invasion assay was carried out as described above and as previously described.28
Statistical analysis
SPSS (version 15.0; SPSS, Inc., Chicago, IL, USA) and GraphPad Prism (version 6.0; GraphPad Software, Inc., La Jolla, CA, USA) were used for the general statistical analysis. The student’s t-test was used to analyze 2-group differences. Inter-group differences were analyzed by one-way analysis of variance, followed by a post hoc Tukey test for multiple comparisons. P<0.05 was considered statistically significant.
Results
Differential expression of circRNAs in breast tumor and normal tissues
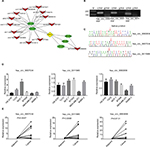
Differentially expressed circRNAs were selected by false discovery rate ≤0.001 and fold change ≥2 or fold change ≤0.5. We found that 152 circRNAs were differentially expressed between the breast cancer tissues and the corresponding adjacent non-cancerous tissues, among which 85 were upregulated and 67 downregulated (Figure 1A). In order to analyze the features and function of differentially expressed circRNAs and their host genes, KEGG signaling pathway analysis was performed using the host genes, which showed a strong correlation with PI3K/AKT, focal adhesion, and protein digestion, and the absorption signaling pathway. The PI3K/AKT signaling pathway was the top pathway for the host genes (Figure 1B). Our findings suggest that these pathways might contribute to the pathogenesis and development of breast cancer.
Construction of the circRNA-miRNA-mRNA interaction network
We found a network consisting of 13 circRNAs and its target genes. All the circRNAs are capable of indirectly regulating RFC3 by interacting with miR-26a, miR-26b, miR-200b, miR-200c, or miR-429 (Figure 2A). This indicates the tight correlation between RFC3 and breast cancer progression.
Verification of circRNA expression by RT-qPCR
We selected 3 circRNAs (hsa_circ_0093859, hsa_circ_ 0007534, and hsa_circ_0011946) on which to focus in our study. Divergent primers were designed for use in detecting the circRNAs. Our results indicated that the circRNAs were only amplified using the divergent primers and cDNA as templates; however, the amplified bands showed no signs of gDNA (Figure 2B). This confirmed that a head-to-tail splicing existed in these circRNAs. In order to further investigate the head-to-tail back-spliced site, Sanger sequencing was performed and subsequently validated the back-spliced junction sites labeled by red arrows, as shown in Figure 2C. Based on the above observations, the hsa_circ_0093859, hsa_circ_0007534, and hsa_circ_0011946 expression levels were measured in 6 different breast cancer cell lines (HS-578T, T47D, MCF-7, BT549, MDA-MB-231, and SKBR-3). We noted that hsa_circ_0093859, hsa_circ_0007534, and hsa_circ_0011946 had an obviously higher expression in the MCF-7 cell line than in other cell lines. More importantly, hsa_circ_0011946 was stably expressed in the majority of cell lines, except for MDA-MB-231 (Figure 2D). Therefore, we finally focused on hsa_circ_0011946 and the MCF-7 cell line in our study. In addition, we also measured the relative expressions of hsa_circ_0093859, hsa_circ_0007534, and hsa_circ_0011946 circRNAs in 12 pairs of breast cancer patient specimens (Figure 2E). The results showed that these 3 circRNAs were upregulated in breast cancer tissues.
Downregulated hsa_circ_0011946 inhibited RFC3 expression and migration, and invasion in MCF-7 cells
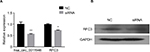
To distinguish the effects of hsa_circ_0011946 on RFC3 expression, we examined the effects of its downregulation on RFC3 expression in MCF-7 cells transfected with hsa_circ_0011946 inhibitors. As expected, the downregulation of hsa_circ_0011946 significantly inhibited the mRNA expression of RFC3 in MCF-7 cells compared with the NC group (Figure 3A). In addition, the protein expression of RFC3 was also suppressed in MCF-7 cells transfected with hsa_circ_0011946 inhibitors, when compared with the control group (Figure 3B).
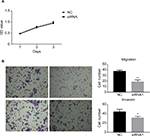
In order to assess the potential effects of hsa_circ_0011946 on cell proliferation, an MTT assay was performed after si-hsa_circ_0011946 transfection at 24, 48, or 72 h. Nevertheless, no obvious differences were found in proliferation between the siRNA-NC and si-hsa_circ_0011946 groups at any time point (Figure 4A). To investigate whether the migration and invasion of MCF-7 cells was regulated by hsa_circ_0011946, si-hsa_circ_0011946, or siRNA-NC, transfected MCF-7 cells were cultured in a transwell plate. After 24-h incubation, the number of migrated cells in the si-hsa_circ_0011946 group was significantly lower than that in the siRNA-NC group. By using a Boyden chamber coated with matrigel, we then measured the effect of si-hsa_circ_0011946 on MCF-7 cell invasion after 24-h incubation. Compared with the siRNA-NC group, the si-hsa_circ_0011946 transfected cells showed a significant reduction in the number of invasive cells (Figure 4B). These findings suggest that hsa_circ_0011946 may be an oncogene and that its loss-of-function can inhibit the migration and invasion of MCF-7 cells.
Discussion
CircRNAs perform a wide variety of biological functions in eukaryotic cells by competing with endogenous RNAs (ceRNAs) or acting as miRNA sponges, interacting with RNA binding proteins, modulating the stability of mRNAs, regulating gene transcription, or translating proteins.8 As a novel gene regulator, circRNAs are dysregulated in multiple pathological processes, including malignancies.11,12 Currently, the clinical diagnostic value of circRNAs has begun to emerge for breast cancer.12,25 Moreover, the overexpression of circ-ABCB10 and circDENND4C has been shown to promote the proliferation of breast cancer cells in vitro.9,10 In the present study, we performed the circRNA expression profiling analysis in breast cancer tissues and in the corresponding adjacent non-cancerous tissues using high-throughput sequencing and found that 85 of 152 differentially expressed circRNAs were significantly upregulated and 67 significantly downregulated in breast cancer tissues. Specifically, the bioinformatics-based circRNA-miRNA-mRNA interaction network revealed that the hsa_circ_0011946/RFC3 signaling pathway might be associated with the progression of breast cancer. Downregulated hsa_circ_0011946 could inhibit the migration and invasion of breast cancer cells, but the underlying mechanism was mediated, at least partially, through the suppression of RFC3 mRNA and protein expression.
Hsa_circ_0011946 is located in chr1: 41578954-41618413, the spliced sequence length is 782 ribonucleotides, and its associated-gene symbol is scm polycomb group protein homolog 1 (SCMH1; circBase, http://www.circbase.org/). SCMH1 encodes a protein with several characteristic domains, including the malignant brain tumor domains, which directly interact with histone methylation, H3K4, H3K9, H3K27, H3K36, and H4K20.29,30 Previous research has confirmed that H3K4 methylation is increased in breast tumors and is driven by an activated PI3K/AKT signaling pathway and inactivated demethylase KDM5A.31 Surprisingly, the KEGG signaling pathway analysis suggested that PI3K/AKT signaling was most closely linked to the abnormal expression of circRNAs in breast cancer tissues. All these findings support the hypothesis that SCMH1-originated hsa_circ_0011946 regulates the development of breast cancer by PI3K/AKT-mediated histone methylation.
Accumulating evidence has demonstrated that the main function of circRNA is regulating miRNA expression by acting as a ceRNA.32–34 For example, hsa_circ_0006528 can inhibit miR-7-5p expression in adriamycin-resistant MCF-7 breast cancer cells.35 Moreover, circ-ABCB10 acts as a sponge for miR-1271 in breast cancer cells.10 Although the fact that circRNA harbors miRNA-binding sites has not been experimentally verified in our study, we did establish circRNA-miRNA-mRNA co-expression networks and found that the top 3 circRNAs (hsa_circ_0011946, hsa_circ_0007534, and hsa_circ_0093859) were related to 5 miRNAs (miR-26a, miR-26b, miR-200b, miR-200c, and miR-429). In MDA-MB-231 cells, the overexpression of miR-26a/b led to a marked decrease in the proliferative, migratory, and invasive capacity of cells.36 MiR-200b/c was also shown to inhibit tumor growth and metastasis in MCF-7 and MDA-MB-231 breast cancer cells.37,38 MiR-429 is a member of the miR-200 family, which is associated with lymph node metastasis in breast cancer patients and the inhibition of migration and invasion in breast cancer cells.39,40 From these studies, we deduced that hsa_circ_0011946, as a miRNA sponge, might regulate the progression of breast cancer by binding up miR-26a/b, miR-200b/c, and miR-429, which would otherwise suppress their expression and reverse their post-translational regulatory mechanism. Unexpectedly, all these miRNAs had a mutual target gene, RFC3, as confirmed by the online prediction software miRanda (http://www.microRNA.org) and TargetScan (http://www. targetscan.org).
Previous studies indicated that the overexpression of RFC3 promotes breast cancer metastasis and MCF-7 cell proliferation, and is associated with poor prognosis via the epithelial–mesenchymal transition, while the knockdown of RFC3 expression can inhibit MCF-7 cell proliferation and invasion.19,20 Consistent with these conclusions, our findings showed that the downregulation of RFC3 levels with small interfering hsa_circ_0011946 resulted in the inhibition of migration and invasion in MCF-7 cells. Breast cancer patients with high RFC3 levels have a higher risk of distant metastases,19 thus RFC3 might be an oncogene and might serve as a therapeutic target for breast cancer.
Conclusion
Taken together, these circRNA expression profile findings from high-throughput RNA sequencing technology showed 152 circRNAs changing remarkably in breast cancer tissues and the preliminarily determined hsa_circ_0011946 being a key regulator of breast cancer. The outcome of the biomathematical prediction and in vitro experiments manifested that the inactivation of the hsa_circ_0011946/RFC3 signaling pathway could inhibit the migration and invasion capacities of MCF-7 cells.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 81402527), the Natural Science Foundation of Fujian Province (No. 2015J01550, 2016J01635), the Science and Technology Planning Projects of Xiamen Science and Technology Bureau (No. 3502Z20174070), and the Guangdong Medical Research Foundation (No. A2017023).
Disclosure
The authors report no conflicts of interest in this work.
References
DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64(1):52–62. | ||
Luan T, Zhang X, Wang S, et al. Long non-coding RNA MIAT promotes breast cancer progression and functions as ceRNA to regulate DUSP7 expression by sponging miR-155-5p. Oncotarget. 2017;8(44):76153–76164. | ||
Muluhngwi P, Alizadeh-Rad N, Vittitow SL, Kalbfleisch TS, Klinge CM. The miR-29 transcriptome in endocrine-sensitive and resistant breast cancer cells. Sci Rep. 2017;7(1):5205. | ||
Zhang HD, Jiang LH, Sun DW, Hou JC, Ji ZL. CircRNA: a novel type of biomarker for cancer. Breast Cancer. 2018;25(1):1–7. | ||
Hentze MW, Preiss T. Circular RNAs: splicing’s enigma variations. EMBO J. 2013;32(7):923–925. | ||
Glažar P, Papavasileiou P, Rajewsky N. circBase: a database for circular RNAs. RNA. 2014;20(11):1666–1670. | ||
Hansen TB, Jensen TI, Clausen BH, et al. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495(7441):384–388. | ||
Meng S, Zhou H, Feng Z, et al. CircRNA: functions and properties of a novel potential biomarker for cancer. Mol Cancer. 2017;16(1):94. | ||
Liang G, Liu Z, Tan L, Su AN, Jiang WG, Gong C. HIF1α-associated circDENND4C promotes proliferation of breast cancer cells in hypoxic environment. Anticancer Res. 2017;37(8):4337–4343. | ||
Liang HF, Zhang XZ, Liu BG, Jia GT, Li WL. Circular RNA circ-ABCB10 promotes breast cancer proliferation and progression through sponging miR-1271. Am J Cancer Res. 2017;7(7):1566–1576. | ||
Chen S, Li T, Zhao Q, Xiao B, Guo J. Using circular RNA hsa_circ_0000190 as a new biomarker in the diagnosis of gastric cancer. Clin Chim Acta. 2017;466:167–171. | ||
Lü L, Sun J, Shi P, et al. Identification of circular RNAs as a promising new class of diagnostic biomarkers for human breast cancer. Oncotarget. 2017;8(27):44096–44107. | ||
Shimada M, Okuzaki D, Tanaka S, et al. Replication factor C3 of Schizosaccharomyces pombe, a small subunit of replication factor C complex, plays a role in both replication and damage checkpoints. Mol Biol Cell. 1999;10(12):3991–4003. | ||
Xia S, Xiao L, Gannon P, Li X. RFC3 regulates cell proliferation and pathogen resistance in Arabidopsis. Plant Signal Behav. 2010;5(2):168–170. | ||
Yao Z, Hu K, Huang H, et al. ShRNA-mediated silencing of the RFC3 gene suppresses hepatocellular carcinoma cell proliferation. Int J Mol Med. 2015;36(5):1393–1399. | ||
Shen H, Xu J, Zhao S, Shi H, Yao S, Jiang N. ShRNA-mediated silencing of the RFC3 gene suppress ovarian tumor cells proliferation. Int J Clin Exp Pathol. 2015;8(8):8968–8975. | ||
Lockwood WW, Thu KL, Lin L, et al. Integrative genomics identified RFC3 as an amplified candidate oncogene in esophageal adenocarcinoma. Clin Cancer Res. 2012;18(7):1936–1946. | ||
Kim YR, Song SY, Kim SS, An CH, Lee SH, Yoo NJ. Mutational and expressional analysis of RFC3, a clamp loader in DNA replication, in gastric and colorectal cancers. Hum Pathol. 2010;41(10):1431–1437. | ||
He ZY, Wu SG, Peng F, et al. Up-regulation of RFC3 promotes triple negative breast cancer metastasis and is associated with poor prognosis Via EMT. Transl Oncol. 2017;10(1):1–9. | ||
Maeng S, Kim GJ, Choi EJ, Yang HO, Lee DS, Sohn YC. 9-Cis-retinoic acid induces growth inhibition in retinoid-sensitive breast cancer and sea urchinembryonic cells via retinoid X receptor α and replication factor C3. Mol Endocrinol. 2012;26(11):1821–1835. | ||
Li L, Guo J, Chen Y, Chang C, Xu C. Comprehensive CircRNA expression profile and selection of key CircRNAs during priming phase of rat liver regeneration. BMC Genomics. 2017;18(1):80. | ||
Memczak S, Jens M, Elefsinioti A, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495(7441):333–338. | ||
Enright AJ, John B, Gaul U, Tuschl T, Sander C, Marks DS. MicroRNA targets in Drosophila. Genome Biol. 2003;5(1):R1. | ||
Pasquinelli AE. MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nat Rev Genet. 2012;13(4):271–282. | ||
Nair AA, Niu N, Tang X, et al. Circular RNAs and their associations with breast cancer subtypes. Oncotarget. 2016;7(49):80967–80979. | ||
Xia W, Qiu M, Chen R, et al. Circular RNA has_circ_0067934 is upregulated in esophageal squamous cell carcinoma and promoted proliferation. Sci Rep. 2016;6:35576. | ||
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408. | ||
Li J, Guo Y, Duan L, et al. AKR1B10 promotes breast cancer cell migration and invasion via activation of ERK signaling. Oncotarget. 2017;8(20):33694–33703. | ||
Yasunaga S, Ohtsubo M, Ohno Y, et al. Scmh1 has E3 ubiquitin ligase activity for geminin and histone H2A and regulates gemininstability directly or indirectly via transcriptional repression of Hoxa9 and Hoxb4. Mol Cell Biol. 2013;33(4):644–660. | ||
Grimm C, de Ayala Alonso AG, Rybin V, et al. Structural and functional analyses of methyl-lysine binding by the malignant brain tumour repeatprotein sex comb on midleg. EMBO Rep. 2007;8(11):1031–1037. | ||
Spangle JM, Dreijerink KM, Groner AC, et al. PI3K/AKT signaling regulates H3K4 methylation in breast cancer. Cell Rep. 2016;15(12):2692–2704. | ||
Huang XY, Huang ZL, Xu YH, et al. Comprehensive circular RNA profiling reveals the regulatory role of the circRNA-100338/miR-141-3p pathway in hepatitis B-related hepatocellular carcinoma. Sci Rep. 2017;7(1):5428. | ||
Yang ZG, Awan FM, Du WW, et al. The circular RNA interacts with STAT3, increasing its nuclear translocation and wound repair by modulating Dnmt3a and miR-17 function. Mol Ther. 2017;25(9):2062–2074. | ||
Han D, Li J, Wang H, et al. Circular RNA circMTO1 acts as the sponge of microRNA-9 to suppress hepatocellular carcinoma progression. Hepatology. 2017;66(4):1151–1164. | ||
Gao D, Zhang X, Liu B, et al. Screening circular RNA related to chemotherapeutic resistance in breast cancer. Epigenomics. 2017;9(9):1175–1188. | ||
Ma X, Dong W, Su Z, et al. Functional roles of sialylation in breast cancer progression through miR-26a/26b targetingST8SIA4. Cell Death Dis. 2016;7(12):e2561. | ||
Zheng Q, Cui X, Zhang D, et al. miR-200b inhibits proliferation and metastasis of breast cancer by targeting fucosyltransferaseIV and α1, 3-fucosylated glycans. Oncogenesis. 2017;6(7):e358. | ||
Song C, Liu LZ, Pei XQ, et al. miR-200c inhibits breast cancer proliferation by targeting KRAS. Oncotarget. 2015;6(33):34968–34978. | ||
Xu F, He H, Huang W, et al. Decreased expression of MicroRNA-200 family in human breast cancer is associated with lymph node metastasis. Clin Transl Oncol. 2016;18(3):283–288. | ||
Ye ZB, Ma G, Zhao YH, et al. miR-429 inhibits migration and invasion of breast cancer cells in vitro. Int J Oncol. 2015;46(2):531–538. |
Supplementary material
  | Table S1 Clinical characteristics of the study population Abbreviations: ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; PGR, progesterone receptor; TNM, tumor-node-metastasis. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.