Back to Journals » OncoTargets and Therapy » Volume 12
Downregulated expression of RACK1 results in pancreatic cancer growth and metastasis
Authors Zhang L, Lv Y , Rong Y , Chen W, Fang Y, Mao W, Lou W , Jin D, Xu X
Received 1 June 2018
Accepted for publication 5 December 2018
Published 1 February 2019 Volume 2019:12 Pages 1007—1020
DOI https://doi.org/10.2147/OTT.S176101
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Takuya Aoki
Lei Zhang,* Yang Lv,* Yefei Rong,* Wenqi Chen, Yuan Fang, Weilin Mao, Wenhui Lou, Dayong Jin, Xuefeng Xu
Department of General Surgery, Zhongshan Hospital, Fudan University, Shanghai, China
*These authors contributed equally to this work
Background: The expression and function of the Receptor for Activated C Kinase 1 (RACK1) in cancer growth and metastasis are confused in different cancers, especially in pancreatic ductal adenocarcinoma (PDAC).
Methods: One-hundred and eighty-two PDAC tissue specimens (95 males and 87 females) including pancreatic cancer tissue and para-carcinoma tissue were collected for analysis between 2005 to 2012. Blood phenotypic parameters using cell count and capillary electrophoresis were investigated. HE staining, real time PCR, Western blot analysis, and soft agar assays were performed to determine the role of RACK1.
Purpose: In this study, we aim to determine the specific role of RACK1 in the untility of PDAC.
Results: We found that RACK1 expression was significantly lower in pancreatic cancer tissue than in para-carcinoma normal pancreatic tissue both in clinic and mice with pancreatic cancer at the early stage. Our results suggested that RACK1 silence could significantly promote cell growth and metastasis of pancreatic cancer cells. But we found that the overexpression of RACK1 has the opposite effect in vitro. In vivo MIAPaca-2 cells overexpressing RACK1, the results demonstrated lower metastatic ability than MIAPaca-2 cells. RACK1 overexpression could decrease the NF-κB transactivation activity of MIAPaca-2 cells, which was consistent with the inhibitory effect of RACK1 overexpression on the pro-migration and pro-invasive target gene of NF-κB, while which could be increased by RACK1 silence. RACK1 silence also enhanced protein expression of pro-migration and pro-invasive NF-κB target genes, which on the contrary, could be reversed by IκBα. Besides, RACK1 expression was significantly associated with lymph node metastasis, vessels metastasis, invasion of nerves as well as TNM staging. The 3-year survival rate of patients with high RACK1 expression was significantly higher than those patients with low RACK1 expression. However, RACK1 expression was not an independent risk factor for of the long-term postoperative survival of patients with pancreatic cancer.
Conclusion: The obtained results in our study suggested that the low expression of RACK1 was associated with cancer cell growth and metastasis in pancreatic cancer through the activation of the NF-κB pathway. RACK1 could be a potential therapeutic drug target to pancreatic cancer and metastasis.
Keywords: receptor for activated C kinase 1, pancreatic cancer, NF-κB, in vivo
Introduction
Pancreatic ductal adenocarcinoma (PDAC) has an extremely poor prognosis and a low 5-year overall survival rate of less than 5% in patients due to cancer metastasis and recurrence.1 Although surgery is the only potential curative therapy for PDAC, only 10%–20% of patients can be treated with surgery because most of them are diagnosed at advanced metastatic stages, which contributes to the restriction of potential therapeutic methods and the loss of the chance for curative therapy for patients.2 Thus, the clarification of the molecular mechanism of distant metastasis in pancreatic cancer is a very critical issue for therapeutics’ development.
RACK1, which belongs to a WD40 superfamily of proteins, is a 36 kDa cytosolic protein with a propeller-like structure of seven WD40 repeats which includes the subunit of G-proteins.3 RACK1 is a multifaceted scaffolding protein known to be involved in the regulation of signaling events required for neuronal protection. Previous research indicated that RACK1 overexpression is involved in growth and metastasis of lung cancer, pulmonary adenocarcinomas, glioma, breast cancer, esophageal squamous cell carcinoma, colorectal carcinoma, epithelial ovarian cancer, prostate cancer, and hepatocellular carcinoma, etc.4 However, Chen et al found that loss of RACK1 promotes metastasis of gastric cancer by inducing an miR-302c/IL-8 signaling loop.5 Thus, we speculated that the function of RACK1 in cancer growth and metastasis may be related to cell-type characteristics, and whether it acts as an oncogene or tumor suppressor gene needs to be further addressed. Up until now, there is still inconclusive evidence of the role of RACK1 in pancreatic cancer growth and metastasis.
In view of this, we performed this study to investigate the expression of RACK1 in human pancreatic cancer patient samples, and further elucidated the effect of RACK1 knock-in on pancreatic cancer growth and metastasis in vitro and in vivo, finally exploring the underlying molecular mechanism of RACK1 in pancreatic cancer.
Materials and methods
Ethics statement
The use of the tissue specimens in the present study was approved by the Ethics Committee of Zhongshan Hospital, Fudan University, and the patients provided written informed consent (including tissue specimens).
Reagents and antibodies
The recombinant human RACK1 was obtained from the Department of Biochemistry and Molecular Biology, School of Basic Medical Sciences, Fudan University, Shanghai. RACK1 antibody (B-3), GAPDH, and b-actin antibody were purchased from Santa Cruz Biotechnology Inc., Dallas, TX, USA; β-actin antibody was obtained from Cell Signaling Technology Inc, Danvers, MA, USA. RACK1 shRNA (h) lentiviral particles were purchased from Santa Cruz Biotechnology Inc. Puromycin was purchased from EMD Millipore (Billerica, MA, USA). Matrigel™ Basement Membrane Matrix was obtained from BD (Franklin Lakes, NJ, USA). PcDNA3.1-Myc/His vector was purchased from Invitrogen (Thermo Fisher Scientific, Waltham, MA, USA). Luciferin-endotoxin Free Luciferin Na was purchased from Promega Corporation, Fitchburg, WI, USA. NF-κB luciferase reporter plasmid was obtained from Genomeditech, Shanghai, China and TK Renilla luciferase plasmid was purchased from Pierce (Thermo Fisher Scientific). Dual-luciferase reporter assay system was obtained from Promega Corporation.
Cell culture and treatment
The human pancreatic cancer cell lines MIAPaca-2, PANC-2, BXPC-3, and Capan-1 were obtained from the American Type Culture Collection and maintained in DMEM and a RPMI-1640 medium supplemented with 10% FBS.
MIAPaca-2, PANC-2, BXPC-3, and Capan-1 cell lines were treated with stable overexpressed RACK1 gene. Crystal-violet test, soft agar colony test, and Boyden Chamber technique were used to examine pancreatic cancer cell lines after overexpression of RACK1. The lentivirus, which was constructed by shRNA interference technique, was used to transfect pancreatic cancer cells to silence the expression of endogenous RACK1 gene. Crystal-violet test, soft agar colony test, and Boyden Chamber technique were applied to evaluate the changes of cell growth, colony formation, invasion, and migration of the pancreatic cancer cell lines after downregulation of RACK1 gene. IκBα plasmids were constructed and used to transfect RACK1-silent MIAPaca-2 cell lines. Rescue experiment was performed using IκBα, a negative regulator of the NF-κB pathway.
Patients and pancreatic cancer tissues
A total of 182 PDAC tissue specimens (95 males and 87 females) including pancreatic cancer tissue, para-carcinoma tissue, and clinical and pathological data were collected from patients with PDAC who underwent surgical treatment at Zhongshan Hospital, Fudan University (Shanghai, China) between 2005 and 2012, none of these patients had received chemotherapy prior to the surgery. All of the tissue specimens were routinely fixed in 4% phosphate-buffered neutral formalin and were embedded in paraffin. At least one pathologist, who did not know the stage of the disease, examined the H&E-stained sections to confirm the presence of more than 85% tumor cells. Correlation between RACK1 expression and clinical pathological data was also analyzed.
H&E staining
The paraffin slides from patients’ tissue specimens, after being embedded and sectioned, were de-paraffinized in xylene I, II, and III for 15, 10, and 10 minutes, respectively, and dehydrated in 100%, 95%, 85%, and 75% ethanol for 5 minutes, respectively. After that, the sections were stained with H&E, dehydrated in 95%, 85%, and 75% ethanol, cleared in xylene, and finally mounted with Permount Mounting Medium (VWR, Shanghai, China). Morphological changes in the pancreatic cancer and para-carcinoma normal tissues were observed and photographed under a light microscope (200× objective) equipped with a Nikon color digital camera system.
Real time PCR
Total RNA was extracted from the pancreatic cells by using Trizol reagent according to the manufacturer’s instructions. Reverse transcription was conducted with Primescript RT reagent kit. Real time PCR was performed on an ABI 7500 sequence detection system (Applied Biosystems, Thermo Fisher Scientific) using SYBR Premix EX Taq. The amplification conditions were as follows: 95°C, 10 minutes; then followed by 45 cycles (95°C, 10 seconds; 60°C, 10 seconds; 72°C, 15 seconds); finally, 95°C, 15 seconds; 60°C, 1 hour; 95°C, 15 seconds. The relative expression level of related genes was normalized to that of GAPDH in the same sample by using LightCycler Software Version 3.5 (Roche Applied Science, Hoffman-La Roche Ltd., Basel, Switzerland).
Western blot analysis
Proteins from cells or tissues were extracted by sonication in CelLytic™ MT mammalian tissue lysis reagent with protease and phosphatase inhibitor cocktails. After the centrifugation at 12,000 rpm for 10 minutes at 4°C, the supernatant of the lysate was collected and the protein concentration was determined by bicinchoninic acid assay. In total, 30 μg of proteins from each sample were separated by 10% SDS-PAGE and transferred onto polyvinylidene difluoride membranes. The membranes, after being blocked with 5% skim milk, were incubated with respective primary antibodies against RACK1, N-cadherin, E-cadherin, Twist, Snail, Slug, ZEB1, and GAPDH and, sequentially, secondary antibodies conjugated with HRP (Life Technologies, Thermo Fisher Scientific).
The signal was visualized with a chemiluminescence prime kit (GE Healthcare UK Ltd, Little Chalfont, UK). Relative quantification of the bands was performed by using ImageJ 1.46 r (NIH, Bethesda, MD, USA).
Immunohistochemistry (IHC)
After anesthetization, mice were intracardially perfused with PBS followed by 4% paraformaldehyde in PBS. IHC procedure was performed according to the methods described previously.6 Briefly, 5 μm sections were permeabilized and blocked with 10% donkey serum in PBS containing 0.3% Triton X-100 for half an hour. Consequently, they were incubated with primary antibody against RACK1 at 4°C overnight followed by thorough washing with PBS. After incubation with secondary antibody conjugated with HRP, the signal was visualized with diaminobenzidine solution, cell nucleus was stained with hematoxylin and, finally, sealed with neutral resin. The immunohistochemical criteria were in accordance with the composite expression score, and the ROC curve was constructed and calculated. After the process, a score of 4 was considered as the cut-off value. The specific data are shown in Table S1.
Cell growth assay
One thousand pancreatic cancer cells with/without stably overexpressing RACK1 and silenced RACK1 were cultured in 12-well culture plates for 14 days. The DMEM was refreshed every other day, and the medium was removed at day 14, then 0.1% crystal violet dye solution was added and left to stain for 5 minutes at room temperature. Finally, the cells were washed with PBS three times to remove the floating color, after that, the cells stained with crystal violet were dried at 37°C and photographed under a light microscope (200× objective) equipped with a Nikon color digital camera system.
Soft agar clone experiment
An amount of 1.5 mL of mixture of serum-supplemented medium and 0.6% agar at 40°C was added to 6-well plates and allowed to solidify (base agar). Next, a mixture of serum supplemented medium and 0.3% agar (total of 1 mL) containing 104 cells at 40°C was added on top of the base layer and allowed to solidify (top agar). Subsequently, the dishes were kept in a tissue culture incubator maintained at 37°C and 5% CO2 for 2 or 3 weeks to allow colony growth. All assays were done in triplicate. Colonies were counted and photographed under a microscope (40×), and the colony size was determined by ImageJ software.
Cell migration
In total, 1×105 cells suspended in 80 μL medium containing 1% FBS, were seeded onto the upper part of the 12-well Boyden chambers coated with collagen (50 mg/mL in 0.02 N acetic acid). FBS (10%) was used as the chemoattractant in the bottom chambers.
After incubation for 8 hours, the non-adhesive cells were removed and the chambers were fixed and stained with eosin for 5 minutes, cells were finally counted and photographed under the microscope.
Invasion assay
Briefly, 200 μL of pancreatic cancer cells (1×106 cells in serum-free medium) were seeded onto the upper part of the 24-well transwell chambers coated with Matrigel (BD). FBS (10%) was used as the chemoattractant in the bottom chambers. After incubation at 37°C for 24 hours, the non-invaded cells were removed from the top of the transwell membrane with a cotton swab. The invaded cells were fixed with 4% 1-phosphofructaldolase for 10 minutes, followed by incubation with 2% crystal violet staining solution for 15 minutes and finally observed under a microscope.
Conditioned K-RasG 12D knock-in mice
K-RasG 12D knock-in mice were hybridized with Pdx-Cre to activate RasG 12D gene, thereby inducing tumor-like lesions in pancreatic endothelial cells in K-RasG12D knock-in mice. RACK1 expression in tumor-like lesions and in control normal tissue in mice pancreas was compared at different time stages by real time qPCR, Western blot, and immunohistochemical staining. There were three mice in each of MIAPaca-2/pcDNA3.1 and MIAPaca-2/RACK1 cohorts, and metastatic lesion formation was observed 4 weeks after the infusion.
Experimental metastasis model
Male BALB/C nude mice (5–6 weeks old) used in this study were maintained under specific pathogen-free conditions, and were manipulated in accordance with the ethical guidelines provided under the protocols approved by the Experimental Animal Care Commission at Fudan University. In addition, the committee approved the experiment. The number of mice was six – three for each cohort. The mice were randomly assigned to two groups and were injected in the left ventricle with 3×106 fluorescent-labeled cells (MIAPaca-2/pcDNA3.1 or MIAPaca-2/RACK1 cells) to induce cancer metastasis.
Optical in vivo imaging
MIAPaca-2/pcDNA3.1 and MIAPaca-2/RACK1 cells were labeled with luciferase and injected into the left ventricles of the mice. The cells diffused through the whole body of the mice through blood circulation. Optical in vivo imaging was used to determine the influence of RACK1 on the metastasis of the pancreatic cancer cells.
Luciferase activity assay
Pancreatic cancer cells were seeded in a 12-well plate at 1×105 cells/well and cultured overnight. The cells were transiently co-transfected with NF-κB reporter (Genomeditech, Shanghai, China, [cat#GM021001]) and TK Renilla luciferase plasmids (Pierce, Thermo Fisher Scientific [cat#85270]) using Lipofectamine™ 2000. Six hours after transfection, the cells were cultured in fresh culture medium for an additional 24 hours. Finally, cells were washed once with PBS and lysed in 300 μL of 1× passive lysis buffer (Promega Corporation). The lysates were centrifuged at 10,000 rpm for 5 minutes at 4°C. The luciferase activity of the supernatant was detected using a Dual-luciferase reporter assay system (Promega Corporation [cat#E1910]). To avoid the bias of transfection efficiency, final activities were designated as the activity of firefly luciferase normalized to that of Renilla luciferase.
Statistical analysis
Difference among groups was analyzed by one-way ANOVA using GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, CA, USA) with Dunnett’s multiple comparison test. Meanwhile, Student’s unpaired t-test was employed to compare the difference between two groups. Difference was considered significant when P<0.05. All data were presented as mean ± SEM.
Results
RACK1 was downregulated in pancreatic cancer tissue
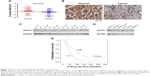
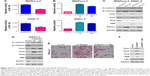
As shown in Figure 1, qPCR, IHC, and Western blot results revealed that RACK1 was expressed at low levels in both pancreatic cancer tissues and para-carcinoma normal pancreatic tissue (Figure 1A, Table S2). The IHC results of RACK1 in pancreatic cancer specimens showed low expression in 54.4% (99/182) and high expression in 45.6% (83/182) cases (Figure 1B). Protein expression of RACK1 in different pancreatic cancer cell lines by Western blot assay was demonstrated in Figure 1C and D. The Kaplan–Meier analysis also demonstrated that patients with higher expression of RACK had significant worse survival outcome than RACK1 low expression group (Figure 1E).
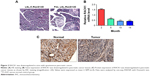
As demonstrated in Figure 2, spontaneous pancreatic cancer in mice was induced by breeding K-RasG12D knock-in mice with Pdx-Cre mice. In mice with spontaneous pancreatic cancer, the results of qPCR and IHC indicated that the expression of RACK1 in mice was downregulated in pancreatic cancer tissue, which was consistent with the findings in human pancreatic cancer tissue samples.
Loss of RACK1 in pancreatic cancer cell lines promoted growth, colony formation, invasion, and migration of pancreatic cancer cells
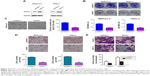
To confirm the effect of RACK1 on the pathological process of pancreatic cancer, we silenced RACK1 to observe the changes of cell growth, colony formation, migration, and invasion of cancer cells. As illustrated in Figure 3, we found that downregulation of RACK1 in pancreatic cancer cell lines significantly promoted cell growth (Figure 3B), colony formation (Figure 3C), migration (Figure 3D), and invasion (Figure 3E).
Overexpression of RACK1 in pancreatic cancer cell lines inhibited growth, colony formation, invasion, and migration
To further confirm the effect of RACK1 expression status in pancreatic cancer, we overexpressed RACK1 to observe the changes in cell growth, colony formation, migration, and invasion of cancer cells. As illustrated in Figure 4, we found that downregulation of RACK1 in pancreatic cancer cell lines significantly promoted cell growth, colony formation, migration, and invasion.
Overexpression of RACK1 significantly inhibited the metastasis of MIAPaca-2 pancreatic cancer cell lines in nude mice
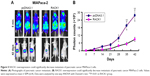
As shown in Figure 5, the overexpression of RACK1 in MIAPaca-2 and PANC-2 cells could inhibit the cell growth, colony formation, migration, and invasion of pancreatic cancer cells. Meanwhile, overexpression of RACK1 significantly inhibited metastasis in MIAPaca-2 pancreatic cancer cell lines in nude mice.
In order to further confirm the inhibitory effect of RACK1 on pancreatic cancer metastasis, we detected the metastatic ability of overexpression of RACK1 in MIAPaca-2 cells in vivo. Results from optical in vivo imaging indicated that the overexpression of RACK1 in MIAPaca-2 cells showed lower metastatic ability.
RACK1 regulated NF-κB activity and NF-κB pathway
We also found that the overexpression of RACK1 significantly inhibited the reporter activity of NF-κB in both MIAPaca-2 and PANC-2 cells (Figure 6A). In RACK1-silent MIAPaca-2 and BXPC-3 cells, the reporter activity of NF-κB was significantly activated (Figure 6B).
Both in MIAPaca-2 and PANC-2 cells, overexpression of RACK1 downregulated the protein expression of N-cadherin, Twist, Snail, Slug, and ZEB1, and upregulated expression of E-cadherin (Figure 6C). However, in BXPC-3 cells, downregulation of RACK1 conspicuously enhanced the expression of N-cadherin, Twist, Snail, Slug, and ZEB1, and inhibited E-cadherin expression (Figure 6D).
IκBα is an inhibitory molecule for NF-κB activity. To clarify whether RACK1 inhibit tumor through NF-κB pathway on pancreatic cancer, we transfected IκBα overexpressing plasmid, after RACK1 silencing in MIAPaca-2 cells. The Boyden chambers results revealed that silencing RACK1 significantly promoted migration of MIAPaca-2 cells, which could be reversed by IκBα overexpression (Figure 6E). Also, the increased expression of Twist, Snail, Slug, and ZEB induced by silencing RACK1 could be reversed by the overexpression of IκBα (Figure 6F).
Low expression of RACK1 in pancreatic cancer was associated with lymph node metastasis, invasion of nervous system, vessel metastasis, and TNM stage
The IHC results of RACK1 in pancreatic cancer specimens showed low expression in 54.4% (99/182) and high expression in 45.6% (83/182) cases. RACK1 expression was significantly associated with lymph node metastasis, vessel metastasis, invasion of nerves as well as TNM stage (all P<0.05; Table 1).
High expression of RACK1 was associated with a better 3-year postoperative survival rate in pancreatic cancer patients
Our analytic results demonstrated that the 1-year, 2-year, and 3-year survival rates of low RACK1 expression group (n=88) were 59.1%, 20.5%, and 11.4%, respectively, and the mean survival time was (78.05±6.98) weeks. Additionally, the 1-year, 2-year, and 3-year survival rates of the high RACK1 expression group (n=81) were 71.3%, 31.3%, and 16.3%, separately, and the mean survival time was (103.71±8.18) weeks. The 3-year survival rate of patients with high RACK1 expression was significantly higher than those patients with low RACK1 expression (P=0.020). Furthermore, the 3-year survival rate of T3 and IIA patients with high RACK1 expression was also significantly higher than those patients with low RACK1 expression (P<0.05). The results (Table 2) of the univariate analysis showed that degree of differentiation of the tumor, TNM stage, invasion of nerve tissue, and RACK1 expression were factors associated with long-term postoperative survival in pancreatic cancer patients.
  | Table 2 The univariate and multivariate analysis of the pancreatic ductal adenocarcinoma patients |
RACK1 was not an independent risk factor for postoperative survival of patients with pancreatic cancer
According to the results of the multivariate analysis, we found that TNM stage was an independent risk factor for postoperative survival of patients with pancreatic carcinoma, while RACK1 expression was not.
Discussion
Previous studies have found that RACK1 overexpression promoted cancer growth and metastasis in many cancers, while in the present study, we found that RACK1 was downregulated in pancreatic cancer tissues, and the low expression of RACK1 in pancreatic cancer enhanced cancer growth and metastasis via regulating the NF-κB pathway.
PDAC is the most common type of pancreatic cancer and has the worst prognosis.7 Distant metastasis and recurrence are commonly regarded as the leading causes of tumor-specific death in patients with PDAC.8 It is reported that the transition from precancerous lesions to pancreatic cancer would take about 17 years, and most of the patients with pancreatic cancer would die in 2–3 years.9 The potential molecular mechanism of the pathogenesis of PDAC has not been well-clarified, which has a great impact on the diagnosis and treatment of PDAC in a clinical setting. Through the profiling of gene expression, human protein interaction network, as well as analysis of topological index, we found that RACK1 was described as one of seven critical network nodes with specific properties, which play an important role in the invasion and distant metastasis of pancreatic cancer. RACK1, a framework protein with a special structure, has great importance in pancreatic tumor development, while its expression and function is still contradictory in other kinds of tumors according to different research.10 On the one hand, Chen et al11 found that loss of RACK1 enhanced gastric cancer metastasis. Additionally, Deng et al proved that RACK1 suppressed gastric tumorigenesis.12 On the other hand, there were other kinds of research which demonstrated that RACK1 was found to promote lung cancer cell growth.13 Meanwhile, Li et al suggested that the overexpression of RACK1 was associated with tumor growth and poor prognosis of PDAC.14 Chauffert et al15 conducted a systematic review, which revealed that RACK1 played important roles in nucleating cell signaling hubs, anchoring proteins at specific subcelular locations, as well as regulating protein activity. In view of this, there is still a lot of work to be done to clarify the function of RACK1 expression in tumor development, invasion, and metastasis. In our study, we demonstrated that the expression of RACK1 was lower in both gene and protein levels in pancreatic cancer tissues in comparison with normal para-carcinoma tissues. In addition, our results are probably contradictory to some current findings,8,16,17 however, this is a discussion-worthy problem – how RACK really affects the prognosis of PDAC patients. Thus, in the future we could consider a larger sample size to elucidate this issue.
Several studies have elucidated that the function of RACK1 was very complex and different in different cancers.18 In our study, we used a K-RasG12D knock-in mouse model to simulate pancreatic cancer-like symptoms in order to further confirm the function of RACK1 in metastasis of PDAC, and the results in the mouse model were consistent with the tissues samples from PDAC patients, with lower gene and protein expression of RACK1 in tumors in mice with pancreatic cancer. Moreover, in in vitro assay, we found that RACK1 overexpression conspicuously suppressed cell growth, colony number, migration, and invasion both in MIAPaca-2 and PANC-2 RACK1 overexpressing cells. However, RACK1 silencing significantly promoted cell growth, colony growth, migration, and invasion both in BXPC-3 and MIAPaca-2 RACK1 silenced cells with siRNA RACK1 transfection. Finally, in in vivo assays, our results demonstrated that the overexpression of RACK1 also inhibited the metastasis of pancreatic cancer. These results prove that the downregulation of RACK1 in tumors was involved in pancreatic cancer growth and metastasis.
It is reported that RACK1 could promote angiogenesis via the activation of the PI3K/Akt/Rac1/HIF-1α pathway.19 RACK1 regulates integrin-mediated adhesion, protrusion, and chemotactic cell migration via its Src binding site.20 Also, it was proved that RACK1 has an association with NHE5 in focal adhesions and also positively regulated transporter activity,21 which indicates the important role of RACK1 in cancer metastasis. RACK1 recruits ribosomes and paroxysmal kinesigenic choreoathetosis to be the center components of MAPK and PI3K pathways,22 which regulates NF-κB activity. Epithelial-to-mesenchymal transition is generally regarded as the first step of cancer metastasis.23 E-cadherin, as a calcium channel transmembrane protein, is often expressed in epithelial cells, and is also the main protein of tight junctions. Low expression of E-cadherin is suggested to reduce the adhesion of malignant tumor cells, and further contributes to increased ability of malignant tumor cells. Slug, Snail, ZEB, and Twist are functionally equivalent as E-cadherin repressors.24 Moreover, RACK1 silencing could obviously increase protein expression of E-cadherin, and decrease protein expression of Twist, Snail, Slug, ZEB1, and N-cadherin. It has been shown that N-cadherin, Twist, Snail, Slug, and ZEB are the target genes of NF-κB. In our study, we continued to clarify the molecular mechanism of RACK1 in its involvement in pancreatic cancer growth and metastasis. The results of luciferase reporter gene assay indicated that RACK1 overexpression suppressed NF-κB transactivation activity. On the contrary, RACK1 silencing significantly enhanced NF-κB transactivation activity in PDAC cells. IκBα is a critical inhibitory molecule which regulates NF-κB activity.25 When IκBα was transfected into pancreatic cancer cells with RACK1 silencing, the increased migration of pancreatic cancer induced by RACK1 silencing was counteracted, indicating that NF-κB/IκBα pathway was involved in RACK1-mediated suppression of pancreatic cancer growth and metastasis.
Finally, we also analyzed the relation between RACK1 expression and clinical characteristics, such as number of metastatic lymph nodes, vessel metastasis, invasion of nerves, as well as TNM stage and survival rate, our results suggested that low expression of RACK1 in pancreatic cancer was significantly associated with lymph node metastasis, invasion of nervous system, vessel metastasis, and TNM stage (P<0.05). Additionally, low expression of RACK1 was also associated with low 3-year postoperative survival rate in pancreatic cancer patients. However, RACK1 was not an independent risk factor for long-term postoperative survival of patients with pancreatic cancer.
In conclusion, our study provided evidence that the low expression of RACK1 was significantly associated with pancreatic cancer growth and metastasis through activation of the NF-κB/IκBα pathway. Additionally, the low expression of RACK1 was proven to be associated with the clinical characteristics of lymph node metastasis, invasion of nervous system, vessel metastasis, TNM stage, as well as lower 3-year postoperative survival rate. Furthermore, RACK1 was not an independent risk factor for long-term postoperative survival of patients with pancreatic cancer.
Acknowledgments
This study was funded by Project of Clinical Medicine and Key Discipline Construction of Shanghai (2017ZZ02007) and Wu Jieping Medical Foundation (320.2710.1806). We thank all the doctors and laboratory staff for their assistance during the process of diagnosis and treatment as well as the basic experiment.
Disclosure
The authors report no conflicts of interest in this work.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. | ||
Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378(9791):607–620. | ||
Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362(17):1605–1617. | ||
Adams DR, Ron D, Kiely PA. RACK1, A multifaceted scaffolding protein: Structure and function. Cell Commun Signal. 2011;9(1):22. | ||
del Vecchio I, Zuccotti A, Pisano F, et al. Functional mapping of the promoter region of the GNB2L1 human gene coding for RACK1 scaffold protein. Gene. 2009;430(1–2):17–29. | ||
Deng YZ, Yao F, Li JJ, et al. RACK1 Suppresses Gastric Tumorigenesis by Stabilizing the β-Catenin Destruction Complex. Gastroenterology. 2012;142(4):812–823. | ||
Fei L, Ma Y, Zhang M, et al. RACK1 promotes lung cancer cell growth via an MCM7/RACK1/Akt signaling complex. Oncotarget. 2017;8(25):40501–40513. | ||
Li X, Xiao Y, Fan S, et al. RACK1 overexpression associates with pancreatic ductal adenocarcinoma growth and poor prognosis. Exp Mol Pathol. 2016;101(2):176–186. | ||
Lin Y, Cui M, Teng H, Wang F, Yu W, Xu T. Silencing the receptor of activated C-kinase 1 (RACK1) suppresses tumorigenicity in epithelial ovarian cancer in vitro and in vivo. Int J Oncol. 2014;44(4):1252–1258. | ||
Lv QL, Huang YT, Wang GH, et al. Overexpression of RACK1 Promotes Metastasis by Enhancing Epithelial-Mesenchymal Transition and Predicts Poor Prognosis in Human Glioma. Int J Env Res Pub He. 2016;13(10):1021. | ||
Mamidipudi V, Cartwright CA. A novel pro-apoptotic function of RACK1: suppression of Src activity in the intrinsic and Akt pathways. Oncogene. 2009;28(50):4421–4433. | ||
Zhou S, Cao H, Zhao Y, et al. RACK1 promotes hepatocellular carcinoma cell survival via CBR1 by suppressing TNF-α-induced ROS generation. Oncol Lett. 2016;12(6):5303–5308. | ||
Chen L, Min L, Wang X, et al. Loss of RACK1 Promotes Metastasis of Gastric Cancer by Inducing a miR-302c/IL8 Signaling Loop. Cancer Res. 2015;75(18):3832–3841. | ||
Shen K, Xi Z, Xie J, et al. Guttiferone K suppresses cell motility and metastasis of hepatocellular carcinoma by restoring aberrantly reduced profilin 1. Oncotarget. 2016;7(35):56650–56663. | ||
Chauffert B, Mornex F, Bonnetain F, et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000-01 FFCD/SFRO study. Ann Oncol. 2008;19(9):1592–1599. | ||
Han H, Wang D, Yang M, Wang S. High expression of RACK1 is associated with poor prognosis in patients with pancreatic ductal adenocarcinoma. Oncol Lett. 2018;15(2):2073–2078. | ||
Wang F, Osawa T, Tsuchida R, Yuasa Y, Shibuya M. Downregulation of receptor for activated C-kinase 1 (RACK1) suppresses tumor growth by inhibiting tumor cell proliferation and tumor-associated angiogenesis. Cancer Sci. 2011;102(11):2007–2013. | ||
Lim KH, Chung E, Khan A, et al. Neoadjuvant therapy of pancreatic cancer: the emerging paradigm? Oncologist. 2012;17(2):192–200. | ||
Sultana A, Smith CT, Cunningham D, Starling N, Neoptolemos JP, Ghaneh P. Meta-analyses of chemotherapy for locally advanced and metastatic pancreatic cancer. J Clin Oncol. 2007;25(18):2607–2615. | ||
Berns H, Humar R, Hengerer B, Kiefer FN, Battegay EJ. RACK1 is up-regulated in angiogenesis and human carcinomas. FASEB J. 2000;14(15):2549–2558. | ||
Cox EA, Bennin D, Doan AT, O’Toole T, Huttenlocher A. RACK1 regulates integrin-mediated adhesion, protrusion, and chemotactic cell migration via its Src-binding site. Mol Biol Cell. 2003;14(2):658–669. | ||
Onishi I, Lin PJ, Diering GH, Williams WP, Numata M. RACK1 associates with NHE5 in focal adhesions and positively regulates the transporter activity. Cell Signal. 2007;19(1):194–203. | ||
Duff D, Long A. Roles for RACK1 in cancer cell migration and invasion. Cell Signal. 2017;35:250–255. | ||
Vomastek T, Iwanicki MP, Schaeffer HJ, Tarcsafalvi A, Parsons JT, Weber MJ. RACK1 targets the extracellular signal-regulated kinase/mitogen-activated protein kinase pathway to link integrin engagement with focal adhesion disassembly and cell motility. Mol Cell Biol. 2007;27(23):8296–8305. | ||
Beuran M, Negoi I, Paun S, et al. The epithelial to mesenchymal transition in pancreatic cancer: a systematic review. Pancreatology. 2015;15(3):217–225. |
Supplementary materials
  | Table S1 Analysis results from ROC curve |
  | Table S2 Composite expression score |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.