Back to Journals » OncoTargets and Therapy » Volume 13
Down-regulation of lncRNA LINC00152 Suppresses Gastric Cancer Cell Migration and Invasion Through Inhibition of the ERK/MAPK Signaling Pathway
Received 28 May 2019
Accepted for publication 19 February 2020
Published 9 March 2020 Volume 2020:13 Pages 2115—2124
DOI https://doi.org/10.2147/OTT.S217452
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Takuya Aoki
Yan Shi,1 Huihui Sun2
1Department of Hyperbaric Oxygen, Dongying People’s Hospital, Dongying, Shandong, 257091, People’s Republic of China; 2Department of Gastroenterology, Jinan First People’s Hospital, Jinan, Shandong 250011, People’s Republic of China
Correspondence: Yan Shi
Department of Hyperbaric Oxygen, Dongying People’s Hospital, No. 317, Dongcheng South First Road, Dongying, Shandong 257091, People’s Republic of China
Tel +86-0546-8901770
Email [email protected]
Purpose: The aim of this study was to explore the regulatory role and mechanism of long noncoding RNA LINC00152 in gastric cancer (GC) cells.
Methods: LINC00152 expression in GC tissues and cells was detected by reverse transcription-polymerase chain reaction (qRT-PCR). MKN45 and MGC-803 cells were selected and assigned into different groups after transfection with si-LINC00152, activated ERK/MAPK signaling pathway (SA), or negative control. Cell proliferation, apoptosis, cycle, migration and invasion were assessed by CCK-8, flow cytometry, Transwell assay and Scratch test, respectively. Western blot analysis was conducted to detect the expression of E-cadherin, N-cadherin and ERK/MAPK signaling pathway protein.
Results: Compared with the normal tissues, higher expression of LINC00152 was found in GC tissues and LINC00152 was remarkably correlative with clinical stage and lymphatic metastasis. LINC00152 expression in GC cells was higher than that in GES-1 cells. Compared with the NC group, the cell proliferation rate, cells in G2/M phase, migration and invasion abilities as well as the expression of N-cadherin and p-ERK-1/2 were significantly decreased, and the expression of E-cadherin, cells in G0/G1 phase and cell apoptosis rate were significantly increased in the si-LINC00152-1 group. ERK/MAPK signaling pathway activator SA could reverse the biological role of LINC00152 in GC cells.
Conclusion: These results demonstrated that the interference of LINC00152 expression may inhibit the invasion and migration of GC cells by inhibiting the ERK/MAPK signaling pathway.
Keywords: lncRNA LINC00152, gastric cancer, ERK/MAPK signaling pathway, migration, invasion
Introduction
As one of the most common malignant tumors, gastric cancer (GC) has been considered as the second leading cause of cancer deaths in the world1,2 The incidence of GC varies widely around the world, and it occurs about twice as common in males than females, with the highest incidence in East Asia.3 The pathogenesis of GC is involved in single or multiple gene mutations referring to cell proliferation, invasion and metastasis.4 Local invasion is the most significant process before tumor metastasis and lymph node metastasis is also deemed as an important part of GC metastasis. In the early GC, the rate of lymph node metastasis is more than 20%, which plays a crucial role in the prognosis of patients.5,6 Although efforts in surgical techniques and targeted drug chemotherapy have been made, the 5-year overall survival rate of GC remains unsatisfactory. Thus, a detailed understanding of the mechanisms underlying GC progression is critical for discovering a new way for diagnosis and treatment of GC.
Long noncoding RNAs (lncRNAs) (more than 200 nucleotides in length) are being widely reported in various kinds of cancers.7,8 LINC00152, a 828 bp lncRNA (chromosome 2p11.2), has been found differentially hypomethylated during hepatocarcinogenesis.9 In GC, LINC00152 is significantly increased compared to healthy samples.10 Additionally, LINC00152 is reportedly upregulated and is closely related to the progression and prognosis of retinoblastoma and breast cancer.11,12 Mitogen-activated protein kinases (MAPKs), evolutionarily well-conserved serine and threonine protein enzymes, take part in signaling pathways which can make a connection with cell surface receptors to regulate nuclear and intracellular targets.13 Extracellular signal-regulated kinase (ERK) is a well-known member of the mammalian MAPK family.14 It is reported that MAPK pathways are involved in the modulation of some basic cellular processes, including cell growth, survival, apoptosis and migration.15 The involvement of the MAPK signaling pathway is also found in CASC2 mediated proliferation of GC cells.16
The relationship between LncRNA and ERK/MAPK signaling pathway has been reported in a variety of cancers, including hepatocellular carcinoma, bile duct carcinoma and lung cancer.17–19 LncRNA PICART1 inhibits cell proliferation by regulating ERK/MAPK signaling pathways in GC.20 In addition, LncRNA CARLo-5 promotes proliferation and activates the ERK/MAPK pathway in GC.21 However, the effect of LINC00152 on GC with the involvement of the ERK/MAPK signaling pathway remains largely unknown. In this study, we detected the effect of LINC00152 on GC cell proliferation, apoptosis, cell cycle, migration and invasion, and examined the role of LINC00152 on ERK/MAPK pathway in GC.
Materials and Methods
Clinical Samples
A total of 40 pairs of GC tissues and adjacent normal tissues were collected in our hospital from January 2017 to December 2018. No patients were treated with chemotherapy or pre-operative radiotherapy. This study was approved by the Ethics Committee of our hospital and it was satisfied with the Declaration of Helsinki.
Cell Culture
GC cells MGC-803, HGC27, MKN45, SGC-7901, AGS and normal gastric mucosal epithelial cell GES-1 were all purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China) and Oncology Institute (Hospital), Peking Union Medical College (Beijing, China). All cells were cultured in RPMI-1640 culture medium (HyClone) containing 10% fetal bovine serum (FBS) (Gobio, Grand Island, NY, USA) in a 37°C, 5% CO2 constant temperature incubator. Cell passage was conducted at the ratio of 1:3, when cell reached 90% confluency.
Cell Grouping
LINC00152 expression interference plasmid (sequence: CACCGCTCAGAGAAATGACATAACT and GGAAUGCAGCUGAAAGAUUTT) or negative control plasmid (NC, sequence: TTCTCCGAACGTGTCACGT) was transfected into cells via Lipo 2000 transfection kit (Invitrogen, Carlsbad, CA, USA) as si-LINC00152 group and NC group. Besides, ERK/MAPK signaling pathway activator (staurosporine aglycone, SA, 30 mmol/mL) was added to the cells transfected with LINC00152 expression interference plasmid as si-LINC00152 + SA group. Cells without transfection were regarded as blank group. All the plasmids were brought from Promega Corporation (Madison, Wisconsin, USA).
Real-Time Quantitative PCR (qRT-PCR)
Cells were collected and the total RNA was extracted via Trizol. Reverse transcription reaction was conducted in the PCR amplification instrument for synthetizing cDNA template. qRT-PCR was performed using ABI7500 instrument (ABI, Austen, TX, USA) with the reaction conditions of pre-denaturation at 95°C for 10 min and 50 cycles of 95°C for 15 s, 60°C for 1 min and 72°C for 40 s. The primers used in the reaction are shown in Table 1. 18S rRNA was used as the internal reference for LINC00152 detection, and the data were analyzed by 2−ΔΔCt. The experiment was repeated three times.
 |
Table 1 Primer Sequence for RT-PCR |
Cell Counting Kit-8 (CCK-8)
After transfected for 48 hrs, the cells were inoculated into a 96-well plate. When the cells were adhered to the well, the number of cells was measured at a specific time point (0, 24, 48 and 72 hrs). The original medium was discarded, and 100 μL fresh culture medium containing 10 μL CCK-8 reagent (Beyotime Biotechnology Co., Shanghai, China) was added. The plate was placed in the carbon dioxide incubator for 2 hrs, and the OD value was measured by an enzyme labeling apparatus (Bio-Rad, Hercules, CA, USA) under the 450 nm wavelength. Cell proliferation rate was calculated: cell proliferation rate (%) = (OD experiment well – OD control well)/OD control well × 100%.
Flow Cytometry
After transfected for 48 hrs, cells were washed by pre-cooled PBS for two times, added with Rnase A (20 μL), and filtered via a 400-mesh screen. Subsequently, 400 μL PI dye was added at 4°C for 30 mins-1 hr. At last, the samples were filtered with a yellow nylon net, and the cell cycle of each group was measured by flow cytometry (BD, Franklin lakes, NJ, USA).
For cell apoptosis detection, cells were first added with 500 μL Annexin V Binding Buffer, 5 μL Annexin V-FITC, and 5 μL Propidium Iodide in the dark at room temperature for 10 mins. Then, the samples were filtered with a yellow nylon net, and the cell apoptosis of each group was measured by flow cytometry (BD, Franklin lakes, NJ, USA).
Scratch Test
After transfected for 48 hrs, cells were inoculated into a 6-well plate (5 × 105 cells/well). When cell confluency reached 80%, a straight-line mark was drawn vertically from the center of each well with 1 mL aseptic liquid remover. After washing, cells were added with culture medium, incubated in a 37°C, 5% CO2 incubator for 24 hrs. The change of scratch width of crawling slices in each group was observed under a light microscope. Cell migration distance was measured by Image-Pro Plus Analysis software (Media Cybernetics, Silver Spring, MD, USA).
Transwell Assay
The Matrigel dissolved at 4°C was added to the pre-cooled Transwell chamber. After 48 h of transfection, the cell concentration was adjusted to 1 × 105/100 μL with a serum-free medium. Cell suspension (100 μL) was added into the upper chamber (BD, USA) of Transwell in a 24-well plate, and culture medium containing 10% FBS (500 μL) was added into the lower chamber. After incubation for 48 hrs, the chamber was taken out and the cells in the upper chamber were wiped out via cotton bud. The chamber was then fixed by 4% paraformaldehyde for 15 mins, stained by crystal violet for 10 mins, and washed with PBS.
Western Blot Analysis
After 48 hrs transfection, the cells were collected. The protein was subjected to 10% SDS-PAGE and transferred onto polyvinylidene fluoride (PVDF) membrane (Millipore, Billerica, MA, USA) which was sealed by 0.5% skim milk powder at room temperature for 1 hr. After washing, the membrane was added with the following rabbit anti-human primary antibodies: t-ERK-1/2 (total-ERK-1/2, ab176660, 1:100), p-ERK-1/2 (phospho-ERK-1/2, ab214362, 1:100), E-cadherin (ab1416, 1:50), N-cadherin (ab18203, 1:300), p-MEK1/2 (phosphor-MEK1/2, 2338, CST, 1:1000), t-MEK1/2 (total-MEK1/2, 9126, CST, 1:1000), c-fos (2550, CST, 1:1000) and GAPDH (ab9385, 1:5000) for incubation at 4°C overnight. After washing with phosphate buffer saline with Tween-20, the membrane was incubated with the second antibody (anti-rabbit) at room temperature for 1 hr, and developed with chemiluminescence reagent. Analysis of protein expression was conducted by using Image software with GAPDH as an internal reference.
Statistical Analysis
The statistical analysis was conducted with SPSS 20.0 (SPSS, Inc, Chicago, IL, USA). Measurement data were presented by mean ± standard deviations. A comparison of two groups was conducted by Student’s t-test, and comparisons among multiple groups were conducted using one-way ANOVA followed by Tukey’s multiple comparisons. P < 0.05 was considered statistically different.
Results
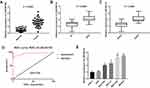
LINC00152 Is Up-Regulated and Correlated with Clinical Features in GC
qRT-PCR results showed that the expression of LINC00152 was markedly increased in GC tissues compared with adjacent normal tissues (Figure 1A). LINC00152 expression was significantly correlated with clinical stage and lymphatic metastasis (Figure 1B and C). In addition, the diagnostic value of LINC00152 on GC was further evaluated by ROC curves. The sensitivity and specificity of LINC00152 in diagnosis of GC were 0.85 and 0.975, respectively (AUC, 0.95; 95% CI, 0.9–1.0) (Figure 1D). In GC cell lines, there was no significant difference in the expression of LINC00152 between GES-1 cell and AGS cell (P > 0.05) (Figure 1E). Compared with the GES cell, higher expression of LINC00152 was found in MGC-803 and MKN45 cells. Thus, MGC-803 and MKN45 cells were selected for the subsequent experiment.
The Best Time to Interfere with LINC00152 Expression Is 48 hrs After Transfection
qRT-PCR results showed that the inhibition rate of si-LINC00152 in MGC-803 cells at 24, 48, and 72 hrs were 13.45%, 74.54%, and 49.67%, respectively. Similarly, the inhibition rate of si-LINC00152 in MKN45 cells was also the highest at 48 hrs (Figure 2A and B), suggesting that the best time to interfere with LINC00152 expression is 48 hrs after transfection. Additionally, compared with the blank and NC group, siRNA interference of LINC00152 could significantly decrease the expression of si-LINC00152 in MGC-803 and MKN45 cells (P < 0.05) (Figure 2C).
si-LINC0015 Inhibits GC Cell Proliferation
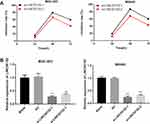
Cell proliferation in each group was detected by CCK-8 assay. The result found that compared with the blank and NC group, the proliferation rate of MGC-803 and MKN45 cells in the si-LINC00152 group was significantly decreased at 48 and 72 hrs (P < 0.05), while no such significant difference was observed between the NC group and the si-LINC00152-1 + SA group (P > 0.05). The proliferation rate in the si-LINC00152-1 + SA group was higher than that in the si-LINC00152-1 group at 72 hrs in MGC-803 and HGC-27 cells (Figure 3). These results revealed that si-LINC00152 could inhibit the proliferation of GC cells, but activation of the ERK/MAPK signaling pathway could block the inhibitory effect of si-LINC00152 on GC cells.
si-LINC0015 Promotes GC Cell Apoptosis
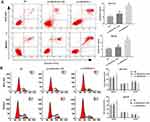
GC cell cycle and apoptosis assays demonstrated that GC cell apoptosis rate and cells in G0/G1 phase were significantly increased, while cells in G2/M phase were significantly decreased in the si-LINC00152-1 group than those in the NC group (P < 0.05). In addition, cell apoptosis rate and cells in G0/G1 phase in the si-LINC00152-1 + SA group were significantly decreased compared with the si-LINC00152-1 group, while GC cells in G2/M phase in the si-LINC00152-1 + SA group were higher than those in the si-LINC00152-1 group (P < 0.05). No significant difference was found between the NC group and the si-LINC00152 + SA group (P > 0.05) (Figure 4). These results show that si-LINC00152 promotes cell apoptosis, inhibits cell cycle, and activation of the ERK/MAPK signaling pathway blocks the effects of si-LINC00152 on apoptosis and cell cycle of GC cells.
si-LINC0015 Inhibits GC Cell Migration and Invasion
The scratch test results are shown in Figure 5A, compared with the NC group, the migration distance of MGC-803 and MKN45 cells in the si-LINC00152-1 group were significantly decreased (P < 0.05). Additionally, the migration distance in the si-LINC00152-1 + SA group was increased than that in the si-LINC00152-1 group (P < 0.05). Consistent with the scratch teat, Transwell assay showed that compared with the NC group, the invasive rate of GC cells in the si-LINC00152-1 group were significantly decreased, and the invasive rate in the si-LINC00152-1 group was obviously decreased than that in si-LINC00152-1 + SA group (P < 0.05) (Figure 5B). Western blot analysis clarified that compared with the NC group, the E-cadherin expression was increased while N-cadherin expression was decreased in the si-LINC00152-1 group (P < 0.05) (Figure 5C). Additionally, the expression of E-cadherin was reduced and N-cadherin was increased in the si-LINC00152-1 + SA group compared with the si-LINC00152-1 group (P < 0.05). No such significant difference was found between the NC group and the si-LINC00152-1 + SA group (P > 0.05). These results indicate that si-LINC00152 can inhibit GC cell migration and invasion, and activation of the can block the effects of si-LINC00152 on migration and invasion of GC cells.
si-LINC0015 May Affect the Biological Function of GC Cells by Inhibiting the ERK/MAPK Signaling Pathway
Western blot analysis showed that compared with the NC group, the expression of p-ERK-1/2, p-MEK1/2 and c-fos were significantly decreased in the si-LINC0015-1 group (P < 0.05), while no change was found in t-ERK-1/2 and t-MEK1/2 expression (P > 0.05). Meanwhile, the expression of p-ERK-1/2, p-MEK1/2 and c-fos were significantly increased in the si-LINC0015-1 + SA group compared with those in the si-LINC00152-1 group (P < 0.05) (Figure 6). No such significant difference was found between the NC group and the si-LINC00152-1 + SA group (P > 0.05). Taken together, these results show that si-LINC00152 can affect the biological function of GC cells by inhibiting the ERK/MAPK signaling pathway.
Discussion
GC is known as one leading cause of cancer-related deaths in the world, and most patients occur with advanced or metastatic disease at the time of diagnosis with the 5-year-survival rate of about 10–15%.22 In this study, we found that LINC00152 expression was notably elevated in GC tissues and cell lines, and was associated with clinical stage and lymphatic metastasis. Additionally, knockdown of LINC00152 restrained GC cell migration and invasion through the inhibition of the ERK/MAPK signaling pathway.
Accumulating evidences have proved that lncRNAs play a crucial role in tumor occurrence, development, invasion, and metastasis and could be regarded as biomarkers in various kinds of human cancers.23,24 Previous evidences have proved that LINC00152 works as an oncogene in GC and cell lines, and the silencing of LINC00152 can suppress the proliferation of GC cells.10,25 Moreover, overexpressed LINC00152 in multiple tumor types is able to enhance tumor metastasis.26 In the present study, we found that knockdown of LINC00152 inhibited the proliferation and induced the apoptosis in GC cells, which were consistent with previous studies in colon cancer,27 gallbladder cancer28 and lung cancer.29 Furthermore, Chen et al30 have reported that LINC00152 is correlated with tumor invasion depth, lymph node metastasis and TNM stage. In our study, we found that LINC00152 promoted GC cell proliferation and was associated with clinical stage and lymphatic metastasis, suggesting that LINC00152 may be an oncogenic lncRNA.
During the progression of epithelial to mesenchymal transition (EMT), downregulation of E-cadherin and upregulation of N-cadherin were found, which could decrease epithelial cell-cell adhesion and facilitate cancer cell invasion and migration.31 It is reported that the positive expression of E-cadherin is correlated with a better survival of patients with GC.32 Additionally, Gao et al have clarified that highly expressed N-cadherin was observed in GC.33 A similar study of Zhang et al have clarified the role of LINC00152 acted in cell cycle arrest, EMT, cell apoptosis, migration as well as invasion in GC.34 In the present study, we found that the silencing of LINC00152 could suppress the EMT process and cell cycle arrest in GC cells, suggesting that LINC00152 could promote GC cell migration, invasion and cell cycle.
LncRNA has been reported as an enhancer factor or suppression factor with specific protein through direct binding. For example, the previous study noted that lncRNA Malat1 could promote neurite outgrowth via the activation of the ERK/MAPK signaling pathway in N2a cells.35 The MAPKs, regulated by Ras/Raf expression, have three main serine/threonine-related protein kinases, among which the ERK signaling pathway is especially correlated with cell survival, proliferation and differentiation and protecting cell against apoptosis.36 It is reported that MAPK signaling can regulate many kinds of biological processes in cells, such as cell proliferation, apoptosis, differentiation, migration and invasion.37,38 Abnormally expressed MAPK is proved to be related to tumorigenesis and metastatic potential for GC.39 Furthermore, the activation of the MAPK/ERK signaling pathway is regarded as one of the mechanisms to promote the apoptosis of GC cells.22 The previous study has found that MAGI1 acts as a tumor suppressor via suppression of the MAPK/ERK signaling pathway in GC.40 Linc-RoR could promote cell growth of breast cancer via the MAPK/ERK signaling pathway.41 Our present study demonstrated that LINC00152 could suppress ERK/MAPK signaling pathway, and activation of the ERK/MAPK signaling pathway obviously reversed the suppressive effect of LINC00152 knockdown on the biological effect in GC cells.
Conclusions
In conclusion, we demonstrate that LINC00152 is highly expressed in GC tissues and cell lines. Silencing LINC00152 could inhibit the proliferation, migration and invasion, and promote the apoptosis of GC cells through the suppression of the ERK/MAPK pathway. LINC00152 may serve as potential targets for GC therapy in the future.
Ethics Approval and Consent to Participate
This study was approved by the ethics committee of Dongying People’s Hospital. Written informed consent was obtained from all subjects as well as parental consent for subjects aged less than 18 years.
Author Contributions
Both authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
There is no funding in the research.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Zhu X, Tian X, Yu C, et al. A long non-coding RNA signature to improve prognosis prediction of gastric cancer. Mol Cancer. 2016;15(1):60. doi:10.1186/s12943-016-0544-0
2. Xie J, Chen M, Zhou J, et al. miR-7 inhibits the invasion and metastasis of gastric cancer cells by suppressing epidermal growth factor receptor expression. Oncol Rep. 2014;31(4):1715–1722. doi:10.3892/or.2014.3052
3. Cover TL. Helicobacter pylori diversity and gastric cancer risk. mBio. 2016;7(1):e01869–e01815. doi:10.1128/mBio.01869-15
4. Wei B, Huang QY, Huang SR, Mai W, Zhong XG. MicroRNA34a attenuates the proliferation, invasion and metastasis of gastric cancer cells via downregulation of MET. Mol Med Rep. 2015;12(4):5255–5261. doi:10.3892/mmr.2015.4110
5. Guo HM, Zhang XQ, Xu CH, Zou XP. Inhibition of invasion and metastasis of gastric cancer cells through snail targeting artificial microRNA interference. Asian Pac J Cancer Prev. 2011;12(12):3433–3438.
6. Zhao G, Zhu G, Huang Y, et al. IL-6 mediates the signal pathway of JAK-STAT3-VEGF-C promoting growth, invasion and lymphangiogenesis in gastric cancer. Oncol Rep. 2016;35(3):1787–1795. doi:10.3892/or.2016.4544
7. Wu Y, Tan C, Weng WW, et al. Long non-coding RNA Linc00152 is a positive prognostic factor for and demonstrates malignant biological behavior in clear cell renal cell carcinoma. Am J Cancer Res. 2016;6(2):285–299.
8. Liu Y, Wei G, Ma Q, Han Y. Knockdown of long noncoding RNA TP73-AS1 suppresses the malignant progression of breast cancer cells in vitro through targeting miRNA-125a-3p/metadherin axis. Thorac Cancer. 2020;4(10):1759–7714.
9. Ji J, Tang J, Deng L, et al. LINC00152 promotes proliferation in hepatocellular carcinoma by targeting EpCAM via the mTOR signaling pathway. Oncotarget. 2015;6(40):42813–42824. doi:10.18632/oncotarget.v6i40
10. Pang Q, Ge J, Shao Y, et al. Increased expression of long intergenic non-coding RNA LINC00152 in gastric cancer and its clinical significance. Tumour Biol. 2014;35(6):5441–5447. doi:10.1007/s13277-014-1709-3
11. Li S, Wen D, Che S, et al. Knockdown of long noncoding RNA 00152 (LINC00152) inhibits human retinoblastoma progression. Onco Targets Ther. 2018;11:3215–3223. doi:10.2147/OTT
12. Hu XL, Wang J, He W, Zhao P, Wu WQ. Down-regulation of lncRNA Linc00152 suppressed cell viability, invasion, migration, and epithelial to mesenchymal transition, and reversed chemo-resistance in breast cancer cells. Eur Rev Med Pharmacol Sci. 2018;22(10):3074–3084. doi:10.26355/eurrev_201805_15067
13. Sun X, Liu C, Qian M, Zhao Z, Guo J. Ceramide from sphingomyelin hydrolysis differentially mediates mitogen-activated protein kinases (MAPKs) activation following cerebral ischemia in rat hippocampal CA1 subregion. J Biomed Res. 2010;24(2):132–137. doi:10.1016/S1674-8301(10)60021-8
14. Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. 2010;1802(4):396–405. doi:10.1016/j.bbadis.2009.12.009
15. Wang Q, Du H, Li M, et al. MAPK signal transduction pathway regulation: a novel mechanism of rat HSC-T6 cell apoptosis induced by FUZHENGHUAYU tablet. Evid Based Complementary Alternat Med. 2013;2013:368103.
16. Li P, Xue WJ, Feng Y, Mao QS. Long non-coding RNA CASC2 suppresses the proliferation of gastric cancer cells by regulating the MAPK signaling pathway. Am J Transl Res. 2016;8(8):3522–3529.
17. Bao H, Guo CG, Qiu PC, Zhang XL, Dong Q, Wang YK. Long non-coding RNA Igf2as controls hepatocellular carcinoma progression through the ERK/MAPK signaling pathway. Oncol Lett. 2017;14(3):2831–2837. doi:10.3892/ol.2017.6492
18. Kong L, Wu Q, Zhao L, Ye J, Li N, Yang H. Upregulated lncRNA-UCA1 contributes to metastasis of bile duct carcinoma through regulation of miR-122/CLIC1 and activation of the ERK/MAPK signaling pathway. Cell Cycle. 2019;18(11):1212–1228. doi:10.1080/15384101.2019.1593647
19. Liao Y, Cheng S, Xiang J, Luo C. lncRNA CCHE1 increased proliferation, metastasis and invasion of non-small lung cancer cells and predicted poor survival in non-small lung cancer patients. Eur Rev Med Pharmacol Sci. 2018;22(6):1686–1692. doi:10.26355/eurrev_201803_14581
20. Li JF, Li WH, Xue LL, Zhang Y. Long non-coding RNA PICART1 inhibits cell proliferation by regulating the PI3K/AKT and MAPK/ERK signaling pathways in gastric cancer. Eur Rev Med Pharmacol Sci. 2019;23(2):588–597. doi:10.26355/eurrev_201901_16871
21. Zhang Y, Ma M, Liu W, Ding W, Yu H. Enhanced expression of long noncoding RNA CARLo-5 is associated with the development of gastric cancer. Int J Clin Exp Pathol. 2014;7(12):8471–8479.
22. Qu J, Zhao M, Teng Y, et al. Interferon-alpha sensitizes human gastric cancer cells to TRAIL-induced apoptosis via activation of the c-CBL-dependent MAPK/ERK pathway. Cancer Biol Ther. 2011;12(6):494–502. doi:10.4161/cbt.12.6.15973
23. Yang G, Lu X, Yuan L. LncRNA: a link between RNA and cancer. Biochim Biophys Acta. 2014;1839(11):1097–1109. doi:10.1016/j.bbagrm.2014.08.012
24. Li J, Wang X, Tang J, et al. HULC and Linc00152 Act as Novel Biomarkers in Predicting Diagnosis of Hepatocellular Carcinoma. Cel Physiol Biochem. 2015;37(2):687–696. doi:10.1159/000430387
25. Zhao J, Liu Y, Zhang W, et al. Long non-coding RNA Linc00152 is involved in cell cycle arrest, apoptosis, epithelial to mesenchymal transition, cell migration and invasion in gastric cancer. Cell Cycle. 2015;14(19):3112–3123. doi:10.1080/15384101.2015.1078034
26. Zhang J, Yin M, Huang J, et al. Long noncoding RNA LINC00152 as a novel predictor of lymph node metastasis and survival in human cancer: a systematic review and meta-analysis. Clin chim acta. 2018;483:25–32. doi:10.1016/j.cca.2018.03.034
27. Yue B, Cai D, Liu C, Fang C, Yan D. Linc00152 functions as a competing endogenous RNA to confer oxaliplatin resistance and holds prognostic values in colon cancer. Mol Ther. 2016;24(12):2064–2077. doi:10.1038/mt.2016.180
28. Cai Q, Wang Z, Wang S, et al. Long non-coding RNA LINC00152 promotes gallbladder cancer metastasis and epithelial-mesenchymal transition by regulating HIF-1alpha via miR-138. Open Biol. 2017;7(1):160247. doi:10.1098/rsob.160247
29. Zhang Y, Xiang C, Wang Y, Duan Y, Liu C, Jin Y. lncRNA LINC00152 knockdown had effects to suppress biological activity of lung cancer via EGFR/PI3K/AKT pathway. Biomed Pharmacother. 2017;94:644–651. doi:10.1016/j.biopha.2017.07.120
30. Chen WM, Huang MD, Sun DP, et al. Long intergenic non-coding RNA 00152 promotes tumor cell cycle progression by binding to EZH2 and repressing p15 and p21 in gastric cancer. Oncotarget. 2016;7(9):9773–9787. doi:10.18632/oncotarget.6949
31. Jiang SB, He XJ, Xia YJ, et al. MicroRNA-145-5p inhibits gastric cancer invasiveness through targeting N-cadherin and ZEB2 to suppress epithelial-mesenchymal transition. Onco Targets Ther. 2016;9:2305–2315. doi:10.2147/OTT.S101853
32. Zhou Y, Li G, Wu J, et al. Clinicopathological significance of E-cadherin, VEGF, and MMPs in gastric cancer. Tumour Biol. 2010;31(6):549–558. doi:10.1007/s13277-010-0068-y
33. Gao P, Xing AY, Zhou GY, et al. The molecular mechanism of microRNA-145 to suppress invasion-metastasis cascade in gastric cancer. Oncogene. 2013;32(4):491–501. doi:10.1038/onc.2012.61
34. Zhang YH, Fu J, Zhang ZJ, Ge CC, Yi Y. LncRNA-LINC00152 down-regulated by miR-376c-3p restricts viability and promotes apoptosis of colorectal cancer cells. Am J Transl Res. 2016;8(12):5286–5297.
35. Liu Y, Zhang X, Sun T, et al. Knockdown of Golgi phosphoprotein 2 inhibits hepatocellular carcinoma cell proliferation and motility. Oncotarget. 2016;7(16):21404–21415. doi:10.18632/oncotarget.7271
36. Zhang Z, Miao L, Xin X, et al. Underexpressed CNDP2 participates in gastric cancer growth inhibition through activating the MAPK signaling pathway. Mol Med. 2014;20:17–28. doi:10.2119/molmed.2013.00102
37. Dong Y, Han Q, Zou Y, et al. Long-term exposure to imatinib reduced cancer stem cell ability through induction of cell differentiation via activation of MAPK signaling in glioblastoma cells. Mol Cell Biochem. 2012;370(1–2):89–102. doi:10.1007/s11010-012-1401-0
38. Xu WH, Zhang JB, Dang Z, et al. Long non-coding RNA URHC regulates cell proliferation and apoptosis via ZAK through the ERK/MAPK signaling pathway in hepatocellular carcinoma. Int J Biol Sci. 2014;10(7):664–676. doi:10.7150/ijbs.8232
39. Liang B, Wang S, Zhu XG, Yu YX, Cui ZR, Yu YZ. Increased expression of mitogen-activated protein kinase and its upstream regulating signal in human gastric cancer. World J Gastroenterol. 2005;11(5):623–628. doi:10.3748/wjg.v11.i5.623
40. Jia S, Lu J, Qu T, et al. MAGI1 inhibits migration and invasion via blocking MAPK/ERK signaling pathway in gastric cancer. Chin J Cancer Res. 2017;29(1):25–35. doi:10.21147/j.issn.1000-9604.2017.01.04
41. Peng WX, Huang JG, Yang L, Gong AH, Mo YY. Linc-RoR promotes MAPK/ERK signaling and confers estrogen-independent growth of breast cancer. Mol Cancer. 2017;16(1):161. doi:10.1186/s12943-017-0727-3
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.