Back to Journals » OncoTargets and Therapy » Volume 13
Down-Regulation of circNRIP1 Promotes the Apoptosis and Inhibits the Migration and Invasion of Gastric Cancer Cells by miR-182/ROCK1 Axis
Received 2 July 2019
Accepted for publication 26 February 2020
Published 30 June 2020 Volume 2020:13 Pages 6279—6288
DOI https://doi.org/10.2147/OTT.S221633
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Takuya Aoki
Lu Liang,1 Lu Li2
1Department of Oncology, Shangqiu First People’s Hospital, Shangqiu, Henan Province 476100, People’s Republic of China; 2School of Chemistry and Chemical Engineering, Shangqiu Normal University, Shangqiu, Henan Province 476100, People’s Republic of China
Correspondence: Lu Li
School of Chemistry and Chemical Engineering, Shangqiu Normal University, No. 55 Ping Yuan Road, Shangqiu, Henan Province 476100, People’s Republic of China
Tel +86 0370 3112844
Email [email protected]
Aim: Circular RNAs (circRNAs) play important roles in the progression of human cancers. circRNA nuclear receptor interacting protein 1 (circNRIP1) has been reported to play as an oncogene in gastric cancer. However, the mechanism underlying circNRIP1 in gastric cancer progression is far from understood.
Patients and Methods: Forty-five gastric cancer patients were recruited and overall survival of patients was analyzed. Gastric cancer cell lines MGC-803 and AGS cells were cultured for study in vitro. The expression levels of circNRIP1, microRNA (miR)-182 and rho-associated protein kinase 1 (ROCK1) were detected by quantitative real-time polymerase chain reaction or Western blot. Cell migration, invasion, cell cycle distribution and apoptosis were determined by transwell, flow cytometry and Western blot assays, respectively. The target association between miR-182 and circNRIP1 or ROCK1 was assessed by luciferase reporter assay and RNA immunoprecipitation.
Results: circNRIP1 expression was enhanced in gastric cancer tissues and cells and high expression of circNRIP1 indicated poor survival of patients. Knockdown of circNRIP1 suppressed cell migration and invasion, arrested cell cycle at G0-G1 phase and promoted apoptosis in gastric cancer cells. miR-182 was a target of circNRIP1 and its deficiency reversed the effect of circNRIP1 silence on cell migration, invasion, cell cycle distribution and apoptosis in gastric cancer cells. Moreover, ROCK1 was validated as a target of miR-182 and competitively regulated by circNRIP1.
Conclusion: Silence of circNRIP1 inhibited progression of gastric cancer by increasing miR-182 and decreasing ROCK1, providing a novel target for the treatment of gastric cancer.
Keywords: gastric cancer, circNRIP1, miR-182, ROCK1, apoptosis, migration
Introduction
Gastric cancer is a serious health problem with the leading causes of cancer-related death around the world.1 In recent years, many advances have been gained in the diagnosis and management of gastric cancer.2 However, the overall survival of patients especially those with advanced gastric cancer remains poor in most countries.3 Therefore, it is expected to explore new targets for the treatment of gastric cancer.
Circular RNAs (circRNAs) are a class of novel noncoding RNAs which are associated with the occurrence and development of multiple diseases and cancers.4 Moreover, previous research has indicated that circRNAs could serve as pivotal targets for the diagnosis and development of gastric cancer.5 For example, circRNA proteasome subunit C3 (circPSMC3), hsa_circ_0001368 and protein kinase B3 (circAKT3) have been reported to act as competing endogenous RNAs (ceRNAs) to promote or inhibit gastric cancer progression by regulating proliferation, metastasis and drug resistance.6–8 As for circRNA nuclear receptor interacting protein 1 (circNRIP1), a novel circRNAs exhibits a promoting role in the development of gastric cancer.9 However, the mechanism by which circNRIP1 participates in gastric cancer progression remains elusive.
MicroRNA (miR)-182 has been reported to act as an oncogene or tumor suppressor by regulating cell processes including proliferation, cell cycle, apoptosis, migration and invasion in different cancers because of the varying microenvironment.10,11 Furthermore, previous studies demonstrate that miR-182 displays the suppressive role in gastric cancer development.12–14 Rho-associated protein kinase 1 (ROCK1) is an important oncogene in human cancers, including gastric cancer.15–19 To explore the association among circNRIP1, miR-182 and ROCK1, we used starBase online to predict the putative seed sites of miR-182 and circNRIP1 or ROCK1. Hence, we hypothesized that miR-182 and ROCK1 might be responsible for the regulatory mechanism of circNRIP1 in gastric cancer.
In this work, we measured the expression level of circNRIP1, investigated the effect of circNRIP1 on migration, invasion and apoptosis, and explored the potential association among circNRIP1, miR-182 and ROCK1 in gastric cancer.
Patients and Methods
Patients and Tissues
Tumor tissues and corresponding adjacent samples were harvested from 45 patients with gastric cancer who did not receive any treatment before surgery in Department of Oncology, Shangqiu First people’s Hospital. All patients have provided the written informed consent, and supported the study and agreed to publish a manuscript. And this was conducted in accordance with the Declaration of Helsinki. The samples were maintained at −80°C until used. The overall survival of patients was analyzed according to the median level of circNRIP1. This research was accepted by the ethics committee of Department of Oncology, Shangqiu First people’s Hospital.
Cell Culture and Transfection
The human gastric cancer cell lines (MGC-803, AGS, HGC-27 and SGC-7901) and normal human gastric mucosal epithelial cell GES-1 were purchased from Bena Culture Collection (Beijing, China). DMEM medium (Sigma, St. Louis, MO, USA) plus 10% fetal bovine serum and antibiotics was applied to the culture of gastric cancer cells and normal cells at 37°C with 5% CO2.
The overexpression vector of circNRIP1 (has_circ_0002711) was generated by inserting into pcDNA3.1 empty vector (pcDNA) (Thermo Fisher Scientific, Wilmington, DE, USA). Small interfering RNA (siRNA) against circNRIP1 (si-circNRIP1) (5ʹ-ACGCACAAAGAAAGAAGTGTT-3ʹ), siRNA negative control (si-NC) (5ʹ-UCUCCGAACGUGUCACGUTT-3ʹ), miR-182 mimic (miR-182) (5ʹ-UUUGGCAAUGGUAGAACUCACACU-3ʹ), miRNA negative control (miR-NC) (5ʹ-CCUGGUAAUGGUAGAAUCUACACU-3ʹ), miR-182 inhibitor (anti-miR-182) (5ʹ-AGUGUGAGUUCUACCAUUGCCAAA-3ʹ) and inhibitor negative control (anti-miR-NC) (5ʹ-UAAUUCAAAAGACUAAAGGAAUCA-3ʹ) were generated by GenePharm (Shanghai, China). These constructed oligonucleotides (20 nM) or vectors (1 μg) were transfected into MGC-803 and AGS cells using Lipofectamine 3000 (Thermo Fisher Scientific). The non-transfected cells were regarded as the mock group. After the transfection for 24 h, cells were collected and used for further experiments.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA from gastric cancer tissues and cells was isolated using Trizol reagent (Thermo Fisher Scientific) and 1 μg extracted RNA was reversely transcribed using the special RT-PCR Kit (Thermo Fisher Scientific). For the purity of circRNAs, the RNA was incubated with RNase R (Geneseed, Guangzhou, China). The diluted product was used for qRT-PCR using SYBR Green mix (Vazyme, Nanjing, China) along with specific primers: circNRIP1 (Forward, 5ʹ-ACAGCCAGAAGATGCACACTTG-3ʹ; Reverse, 5ʹ-TGGGGCTACGAGATAAAGGAGA-3ʹ); ROCK1 (Forward, 5ʹ- AACATGCTGCTGGATAAATCTGG-3ʹ; Reverse, 5ʹ- TGTATCACATCGTACCATGCCT-3ʹ); miR-182 (Forward, 5ʹ-ACCTGGATTTGGCAATGGTAG-3ʹ; Reverse, 5ʹ-TATGCTTGTTCTCGTCTCTGTGTC-3ʹ). The relative RNA levels were normalized to GAPDH (Forward, 5ʹ- TGGTGAAGGTCGGTGTGAAC-3ʹ; Reverse, 5ʹ-GCTCCTGGAAGATGGTGATGG-3ʹ) or U6 (Forward, 5ʹ- TGCGGGTGCTCGCTTCGGCAGC-3ʹ; Reverse, 5ʹ- CCAGTGCAGGGTCCGAGGT-3ʹ). The experiments were repeated 3 times. The data were analyzed by 2−ΔΔCt method.20
Transwell Assay
The abilities of invasion and migration were assessed by transwell chambers with or without pro-coat of Matrigel (BD Biosciences, San Jose, CA, USA). Transfected MGC-803 and AGS cells (1 × 104/well) in 200 μL DMEM medium without serum were placed into the upper chambers and lower chambers were filled with 600 μL medium containing 10% fetal bovine serum. The samples were prepared in triplicate. After 24 h, migrated or invasive cells were stained with 0.5% crystal violet and observed under a microscope (Olympus, Tokyo, Japan).
Flow Cytometry
Cell cycle distribution and apoptosis of MGC-803 and AGS cells were analyzed using flow cytometry. For cell cycle assay, MGC-803 and AGS cells were fixed with 100% ethanol overnight after the indicated transfection for 72 h. The samples were prepared in triplicate. After washing with cold PBS, cells were incubated with RNase A and PI for 20 min in the dark. Cell cycle distribution was measured by a flow cytometer (BD Biosciences) and the percentage of cells in G0-G1, S or G2-M phase was analyzed by Cell Quest software.
For cell apoptosis assay, MGC-803 and AGS cells were collected after the transfection for 72 h. The samples were prepared in triplicate. The cells that incubated in binding buffer were interacted with Annexin V-FITC and PI solution in the Annexin V-FITC Apoptosis Detection kit (Beyotime, Shanghai, China). The stained cells were analyzed with a flow cytometer. The apoptotic rate of MGC-803 and AGS cells at early apoptosis was expressed as the percentage of cells with Annexin V-FITC staining positive and PI staining negative.
Western Blot
MGC-803 and AGS cells were washed with PBS and lysed in RIPA lysis. After the centrifugation and quantification, protein lysates (30 μg) were loaded onto SDS-PAGE and then suffered from the membranes transfer using PVDF membranes purchased from Millipore (Billerica, MA, USA). The membranes were blocked with 5% non-fat milk, incubated with primary antibodies against Bax (ab199677, 1:1000 dilution, Abcam, Cambridge, MA, USA), Bcl-2 (ab196495, 1:5000 dilution, Abcam), ROCK1 (ab97592, 1:2000 dilution, Abcam) at 4°C, and then interacted with HRP- conjugated IgG (ab205718, 1:10,000 dilution, Abcam) for 2 h. The antibody against β-actin (ab227387, 1:10,000 dilution, Abcam) was used as loading control. The protein band signaling was developed by enhanced chemiluminescence solution (Beyotime) and grayscale value was analyzed by Image Lab software (Bio-Rad, Hercules, CA, USA).
Luciferase Reporter Assay and RNA Immunoprecipitation (RIP)
StarBase online (http://starbase.sysu.edu.cn/) predicted the binding sites of miR-182 and circNRIP1 or ROCK1. The sequences of circNRIP1 or 3ʹ UTR fragments of ROCK1 containing wild-type (WT) or mutant (MUT) miR-182 binding sites were inserted into the downstream of luciferase reporter gene in pmirGLO luciferase reporter vector (Promega, Madison, WI, USA), generating the corresponding luciferase reporter vectors circNRIP1-WT, circNRIP1-MUT, ROCK1-WT and ROCK1-MUT, respectively. MGC-803 and AGS cells were co-transfected with miR-182 or miR-NC and these constructed luciferase reporter vectors. At 48 h after the transfection, luciferase activity assay was performed in each group with a luciferase reporter assay kit (Promega).
For RIP assay, MGC-803 and AGS cells transfected with miR-182 or miR-NC were collected and incubated with Magna RNA immunoprecipitation kit (Millipore) following the manufacturer’s protocols. The magnetic beads were conjugated with antibody against Ago2 (ab32381, Abcam) or IgG (AP112, Sigma). The abundances of circNRIP1 and ROCK1 enriched by Ago2 and IgG RIP were determined by qRT-PCR.
Statistical Analysis
The experimental data from three independent repeats were analyzed and presented as mean ± standard deviation (SD), processed by SPSS 17.0 (Chicago, IL, USA). The overall survival of patients was assessed by Kaplan–Meier method and a Log-rank test. The comparisons of two or more groups were performed by Student’s t-test or ANOVA with Tukey’s post hoc test. P<0.05 was defined as statistically significant.
Results
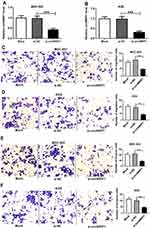
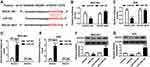
The Expression of circNRIP1 Is Increased in Gastric Cancer
To obtain the expression of circNRIP1 in gastric cancer, 45 cancer tissues and corresponding normal samples were collected. Compared with that in adjacent group, the level of circNRIP1 was significantly increased in tumor tissues (Figure 1A). Moreover, the abundance of circNRIP1 in gastric cancer cell lines (MGC-803, AGS, HGC-27 and SGC-7901) was higher than that in GES-1 cells (Figure 1B). In addition, the patients were divided into high (n=23) or low circNRIP1 group (n=22) according to the median value of circNRIP1 in cancer tissues. As shown in Figure 1C, the patients with high circNRIP1 level displayed lower overall survival (P=0.033).
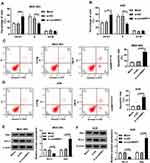
Knockdown of circNRIP1 Inhibits Migration and Invasion and Promotes Apoptosis in Gastric Cancer Cells
To explore the role of circNRIP1 in gastric cancer, MGC-803 and AGS cells with the relatively higher level of circNRIP1 were transfected with si-circNRIP1 or si-NC. As shown in Figure 2A and B, the expression of circNRIP1 was unchanged by si-NC treatment compared with that in mock group but obviously decreased by the transfection of si-circNRIP1 in MGC-803 and AGS cells. Moreover, the data of transwell assay revealed that knockdown of circNRIP1 evidently decreased the abilities of migration and invasion in MGC-803 and AGS cells (Figure 2C–F). Furthermore, silence of circNRIP1 induced cell cycle arrest at G0-G1 stage in the two cell lines, revealed by the increased percentage of G0-G1 phase and reduced percentage of S phase (Figure 3A and B). In addition, analysis of flow cytometry described that circNRIP1 knockdown led to great apoptosis production in MGC-803 and AGS cells (Figure 3C and D). Meanwhile, Western blot assay displayed that interference of circNRIP1 resulted in obvious decrease in Bcl-2 level and increase in Bax level (Figure 3E and F).
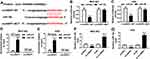
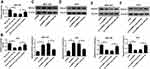
circNRIP1 Is a Decoy of miR-182
StarBase online predicted the potential target of circNRIP1 and provided the binding sites of circNRIP1 and miR-182 (Figure 4A). To validate the association between them, the luciferase reporter vectors circNRIP1-WT and circNRIP1-MUT were generated (Figure 4A) and transfected into MGC-803 and AGS cells. As shown in Figure 4B and C, overexpression of miR-182 led to the great reduction of luciferase activity in circNRIP1-WT group, while it did not affect the activity in circNRIP1-MUT group. Moreover, addition of miR-182 induced higher level of circNRIP1 enriched by Ago2 RIP in MGC-803 and AGS cells, whereas it showed little enrichment in IgG RIP group (Figure 4D and E). Besides, the abundance of miR-182 in MGC-803 and AGS cells was evidently decreased by circNRIP1 overexpression and increased via circNRIP1 knockdown (Figure 4F and G).
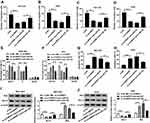
Inhibition of miR-182 Reverses the Effect of circNRIP1 Knockdown on Migration, Invasion and Apoptosis in Gastric Cancer Cells
To explore whether miR-182 is required for circNRIP1-addressed regulation of gastric cancer, MGC-803 and AGS cells were transfected with si-NC, si-circNRIP1, si-circNRIP1 and anti-miR-NC or anti-miR-182. As shown in Figure 5A–D, miR-182 knockdown markedly restored circNRIP1 silence-mediated inhibition of migration and invasion in MGC-803 and AGS cells. Moreover, silence of circNRIP1-induced G0-G1 phase arrest was obviously attenuated via deficiency of miR-182 (Figure 5E and F). Meanwhile, the increased apoptotic rate caused by circNRIP1 knockdown in MGC-803 and AGS cells was weakened by miR-182 exhaustion (Figure 5G and H). Besides, the effect of circNRIP1 deletion on Bcl-2 and Bax protein levels was reversed via miR-182 down-regulation in MGC-803 and AGS cells (Figure 5I and J).
ROCK1 Is a Target of miR-182
StarBase exhibited the seeding sites of miR-182 and ROCK1, predicting that ROCK1 might be a target of miR-182 (Figure 6A). To validate this prediction, we constructed the luciferase reporter vectors ROCK1-WT and ROCK1-MUT. Moreover, luciferase activity in MGC-803 and AGS cells transfected with ROCK1-WT was markedly reduced by miR-182 overexpression, while the activity in ROCK1-MUT group was not affected (Figure 6B and C). In addition, the level of ROCK1 enriched by Ago2 RIP was increased by 4.6-fold and 5.4-fold by miR-182 overexpression in the two cell lines (Figure 6D and E). Besides, the protein level of ROCK1 was specially reduced via miR-182 overexpression but enhanced by miR-182 knockdown in MGC-803 and AGS cells (Figure 6F and G).
circNRIP1 Promotes ROCK1 Expression by Competitively Sponging miR-182 in Gastric Cancer Cells
To further confirm the ceRNA network, the effect of circNRIP1 on ROCK1 was investigated. As displayed in Figure 7A and B, the luciferase activity of MGC-803 and AGS cells transfected with ROCK1-WT was significantly inhibited by miR-182 overexpression, but this event was alleviated by the introduction of circNRIP1. Moreover, the protein level of ROCK1 in MGC-803 and AGS cells was notably decreased by circNRIP1 overexpression, which was weakened by miR-182 addition (Figure 7C and D). Meanwhile, the protein level of ROCK1 reduced via silence of circNRIP1 was restored by exhaustion of miR-182 (Figure 7E and F).
Discussion
circRNAs could play as the promising targets for the diagnosis, therapeutics and prevention of gastric cancer.21 However, little is known about the underlying mechanism of circNRIP1 in gastric cancer progression. By comparing with adjacent tissues and normal epithelial cells, the expression of circNRIP1 was enhanced in gastric cancer, indicating the potential carcinogenic role of circNRIP1 in gastric cancer. In the present study, we investigated the inhibitive role of circNRIP1 knockdown in gastric cancer development and this study was the first time to confirm the regulatory mechanism of circNRIP1/miR-182/ROCK1 in vitro.
The abilities of migration and invasion are the major malignancies in gastric cancer.22 Our study showed that circNRIP1 knockdown blocked migration and invasion of gastric cancer cells, which was also in agreement with the data in former report.14 Moreover, cell cycle is associated with cell death in cancer development.23 Bcl-2 family is known as key regulators associated with apoptosis process of cells, in which Bcl-2 is an oncogene-derived protein and Bax is a promoting death protein by competing with Bcl-2.24 Through flow cytometry and Western blot assays, we found that silence of circNRIP1 led to apoptosis production, revealed by cell cycle arrest at G0-G1 phase, the decreased Bcl-2 level and increased Bax abundance. These data uncovered the therapeutic effect of circNRIP1 inhibition on gastric cancer.
The ceRNA network is a key mechanism underlying the function of circRNA in human cancers.25,26 Former work indicated that circNRIP1 could function as a ceRNA to mediate Akt1 by competitively sponging miR-149-5p.9 To figure out a novel mechanism for circNRIP1, we found that miR-182 might act as a target of circNRIP1, which was validated in gastric cancer cells by luciferase reporter assay and RIP. Tang et al reported that miR-182 could suppress cell proliferation via regulating cell cycle process by targeting Zinc finger AN1-type containing 4 in gastric cancer.27 Moreover, Qin et al revealed that miR-182 could repress cell viability, migration and invasion and induce apoptosis in gastric cancer.14 These reports suggested that miR-182 might play an anti-cancer role in gastric cancer development. In the present study, results displayed that inhibition of miR-182 abrogated the suppressive effect of circNRIP1 knockdown on gastric cancer progression, indicating that circNRIP1 was involved in gastric cancer development by sponging miR-182.
ROCK1 has been regarded as an oncogene by promoting proliferation, migration and invasion and decreasing apoptosis in gastric cancer.19,28 To further explore the ceRNA network of circNRIP1, here we first confirmed ROCK1 as a functional target of miR-182 in gastric cancer cells. Furthermore, introduction of circNRIP1 derepressed ROCK1 expression from miR-182, indicating that circNRIP1 could act as a ceRNA for miR-182 to increasing ROCK1 expression in gastric cancer in vitro. Previous studies suggested that nuclear factor-κB (NF-κB) signaling is an important pathway for the development of gastric cancer and ROCK1 is a key mediator of this spathway.29–32 Hence, we hypothesized that NF-κB signaling might be involved in the regulatory mechanism addressed by circNRIP1/miR-182/ROCK1 axis in gastric cancer, which is needed to be explored in the future.
In conclusion, this research demonstrated that circNRIP1 inhibited progression of gastric cancer through decreasing the abilities of migration and invasion and increasing apoptosis production, possibly by miR-182/ROCK1 axis. This study highlighted a novel mechanism for understanding the pathogenesis of gastric cancer.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388(10060):2654–2664. doi:10.1016/S0140-6736(16)30354-3
2. Thrumurthy SG, Chaudry MA, Hochhauser D, Mughal M. The diagnosis and management of gastric cancer. BMJ. 2013;347:f6367. doi:10.1136/bmj.f6367
3. Necula L, Matei L, Dragu D, et al. Recent advances in gastric cancer early diagnosis. World J Gastroenterol. 2019;25(17):2029–2044. doi:10.3748/wjg.v25.i17.2029
4. Lei B, Tian Z, Fan W, Ni B. Circular RNA: a novel biomarker and therapeutic target for human cancers. Int J Med Sci. 2019;16(2):292–301. doi:10.7150/ijms.28047
5. Jiang F, Hong F, Shah MW, Shen X. Circular RNAs as diagnostic biomarkers in gastric cancer: a meta-analysis review. Pathol Res Pract. 2019;215(6):152419. doi:10.1016/j.prp.2019.04.011
6. Rong D, Lu C, Zhang B, et al. CircPSMC3 suppresses the proliferation and metastasis of gastric cancer by acting as a competitive endogenous RNA through sponging miR-296-5p. Mol Cancer. 2019;18(1):25. doi:10.1186/s12943-019-0958-6
7. Lu J, Zhang PY, Li P, et al. Circular RNA hsa_circ_0001368 suppresses the progression of gastric cancer by regulating miR-6506-5p/FOXO3 axis. Biochem Biophys Res Commun. 2019;512(1):29–33. doi:10.1016/j.bbrc.2019.02.111
8. Huang X, Li Z, Zhang Q, et al. Circular RNA AKT3 upregulates PIK3R1 to enhance cisplatin resistance in gastric cancer via miR-198 suppression. Mol Cancer. 2019;18(1):71. doi:10.1186/s12943-019-0969-3
9. Zhang X, Wang S, Wang H, et al. Circular RNA circNRIP1 acts as a microRNA-149-5p sponge to promote gastric cancer progression via the AKT1/mTOR pathway. Mol Cancer. 2019;18(1):20. doi:10.1186/s12943-018-0935-5
10. Feng YA, Liu TE, Wu Y. microRNA-182 inhibits the proliferation and migration of glioma cells through the induction of neuritin expression. Oncol Lett. 2015;10(2):1197–1203. doi:10.3892/ol.2015.3365
11. Li X, Zhang X, Zhang Q, Lin R. miR-182 contributes to cell proliferation, invasion and tumor growth in colorectal cancer by targeting DAB2IP. Int J Biochem Cell Biol. 2019;111:27–36. doi:10.1016/j.biocel.2019.04.002
12. Yu J, Tian X, Chang J, Liu P, Zhang R. RUNX3 inhibits the proliferation and metastasis of gastric cancer through regulating miR-182/HOXA9. Biomed Pharmacother. 2017;96:782–791. doi:10.1016/j.biopha.2017.08.144
13. Kong WQ, Bai R, Liu T, et al. MicroRNA-182 targets cAMP-responsive element-binding protein 1 and suppresses cell growth in human gastric adenocarcinoma. FEBS J. 2012;279(7):1252–1260. doi:10.1111/j.1742-4658.2012.08519.x
14. Qin L, Jia Z, Xie D, Liu Z. Knockdown of long noncoding RNA urothelial carcinoma-associated 1 inhibits cell viability, migration, and invasion by regulating microRNA-182 in gastric carcinoma. J Cell Biochem. 2018;119(12):10075–10086. doi:10.1002/jcb.27344
15. Xu F, Li H, Hu C. MiR-202 inhibits cell proliferation, invasion, and migration in breast cancer by targeting ROCK1 gene. J Cell Biochem. 2019;120(9):16008–16018. doi:10.1002/jcb.28879
16. Tang H, Du W, Jiang Y, et al. Upregulated expression of ROCK1 promotes cell proliferation by functioning as a target of miR-335-5p in non-small cell lung cancer. J Cell Physiol. 2019. doi:10.1002/jcp.28886
17. Zheng B, Liang L, Wang C, et al. MicroRNA-148a suppresses tumor cell invasion and metastasis by downregulating ROCK1 in gastric cancer. Clin Cancer Res. 2011;17(24):7574–7583. doi:10.1158/1078-0432.CCR-11-1714
18. Wang H, Zhang M, Sun G. Long non-coding RNA NEAT1 regulates the proliferation, migration and invasion of gastric cancer cells via targeting miR-335-5p/ROCK1 axis. Pharmazie. 2018;73(3):150–155. doi:10.1691/ph.2018.7877
19. Yu SY, Peng H, Zhu Q, et al. Silencing the long noncoding RNA NORAD inhibits gastric cancer cell proliferation and invasion by the RhoA/ROCK1 pathway. Eur Rev Med Pharmacol Sci. 2019;23(9):3760–3770. doi:10.26355/eurrev_201905_17802
20. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–408. doi:10.1006/meth.2001.1262
21. Shi P, Wan J, Song H, Ding X. The emerging role of circular RNAs in gastric cancer. Am J Cancer Res. 2018;8(10):1919–1932.
22. Yang M, Huang CZ. Mitogen-activated protein kinase signaling pathway and invasion and metastasis of gastric cancer. World J Gastroenterol. 2015;21(41):11673–11679. doi:10.3748/wjg.v21.i41.11673
23. Maddika S, Ande SR, Panigrahi S, et al. Cell survival, cell death and cell cycle pathways are interconnected: implications for cancer therapy. Drug Resist Updat. 2007;10(1–2):13–29. doi:10.1016/j.drup.2007.01.003
24. Basu A, Haldar S. The relationship between BcI2, Bax and p53: consequences for cell cycle progression and cell death. Mol Hum Reprod. 1998;4(12):1099–1109. doi:10.1093/molehr/4.12.1099
25. Chan JJ, Tay Y. Noncoding RNA: RNA regulatory networks in cancer. Int J Mol Sci. 2018;19(5):E1310. doi:10.3390/ijms19051310
26. Zhong Y, Du Y, Yang X, et al. Circular RNAs function as ceRNAs to regulate and control human cancer progression. Mol Cancer. 2018;17(1):79. doi:10.1186/s12943-018-0827-8
27. Tang L, Chen F, Pang EJ, et al. MicroRNA-182 inhibits proliferation through targeting oncogenic ANUBL1 in gastric cancer. Oncol Rep. 2015;33(4):1707–1716. doi:10.3892/or.2015.3798
28. Yu X, Ma C, Fu L, Dong J, Ying J. MicroRNA-139 inhibits the proliferation, migration and invasion of gastric cancer cells by directly targeting ρ-associated protein kinase 1. Oncol Lett. 2018;15(4):5977–5982. doi:10.3892/ol.2018.8038
29. Liu F, Cheng L, Xu J, Guo F, Chen W. miR-17-92 functions as an oncogene and modulates NF-κB signaling by targeting TRAF3 in MGC-803 human gastric cancer cells. Int J Oncol. 2018;53(5):2241–2257. doi:10.3892/ijo.2018.4543
30. Jiao Y, Yang H, Qian J, et al. miR-3664-5P suppresses the proliferation and metastasis of gastric cancer by attenuating the NF‑κB signaling pathway through targeting MTDH. Int J Oncol. 2019;54(3):845–858. doi:10.3892/ijo.2019.4680
31. Gong J, Guan L, Tian P, Li C, Zhang Y. Rho kinase type 1 (ROCK1) promotes lipopolysaccharide-induced inflammation in corneal epithelial cells by activating toll-like receptor 4 (TLR4)-mediated signaling. Med Sci Monit. 2018;24:3514–3523. doi:10.12659/MSM.907277
32. Wang RK, Shao XM, Yang JP, et al. MicroRNA-145 inhibits proliferation and promotes apoptosis of HepG2 cells by targeting ROCK1 through the ROCK1/NF-κB signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23(7):2777–2785. doi:10.26355/eurrev_201904_17551
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.