Back to Journals » International Journal of Nanomedicine » Volume 10 » Special Issue on diverse applications in Nano-Theranostics
Dopamine-functionalized InP/ZnS quantum dots as fluorescence probes for the detection of adenosine in microfluidic chip
Authors Ankireddy SR, Kim J, An SSA
Received 12 May 2015
Accepted for publication 8 July 2015
Published 25 August 2015 Volume 2015:10(Special Issue on diverse applications in Nano-Theranostics) Pages 121—128
DOI https://doi.org/10.2147/IJN.S88465
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Thomas Webster
Seshadri Reddy Ankireddy, Jongsung Kim
Department of Chemical and Biological Engineering, Gachon University, Seongnam, Gyeonggi-Do, South Korea
Abstract: Microbeads are frequently used as solid supports for biomolecules such as proteins and nucleic acids in heterogeneous microfluidic assays. Chip-based, quantum dot (QD)-bead-biomolecule probes have been used for the detection of various types of DNA. In this study, we developed dopamine (DA)-functionalized InP/ZnS QDs (QDs-DA) as fluorescence probes for the detection of adenosine in microfluidic chips. The photoluminescence (PL) intensity of the QDs-DA is quenched by Zn2+ because of the strong coordination interactions. In the presence of adenosine, Zn2+ cations preferentially bind to adenosine, and the PL intensity of the QDs-DA is recovered. A polydimethylsiloxane-based microfluidic chip was fabricated, and adenosine detection was confirmed using QDs-DA probes.
Keywords: quantum dots, photoluminescence, microfluidic chip, adenosine, mercaptopropionic acid, mercaptoundecanoic acid, PDMS
Introduction
Adenosine is an inhibitory neurotransmitter and a signaling molecule that is involved in energy production in the form of adenosine triphosphate (ATP) at the molecular level. Adenosine signaling was first discovered in the beginning of the 20th century, and it is known to regulate innumerable physiological functions within animal cells. Adenosine also plays a key role in chemical neurotransmission1 and in the control of coronary blood flow in human beings. The effects of adenosine at the cellular level are complicated, because its mediation can be either excitatory or inhibitory. Adenosine is also used to treat supraventricular tachycardia, which is cardiac arrhythmia caused by faulty electrical functioning of the heart.2
Over the past two decades, several analytical methods have been developed for quantifying adenosine, including potentiometry, colorimetry,3 electrochemical methods,4 high-performance liquid chromatography with fluorescence detection,5,6 and capillary electrophoresis.7 All these methods have several drawbacks, including poor sensitivity, poor detection limits, difficulties with sample separation, and expensive instrumentation. Photoluminescence (PL) spectroscopy has high sensitivity and a wide linear working range, and the instrumentation is simple and inexpensive; it has therefore become an attractive analytical tool for the analysis of pharmaceutical and biological samples.
Polydimethylsiloxane (PDMS) is a biocompatible amorphous polymer8 and is commonly used as a drug-delivery vehicle in medicine. Recently, reactive plasmas have been developed to enhance the adhesion of PDMS on glass slides and the immobilization of biomolecules. The chemical and topographical properties of the exposed surfaces can be modified. Several studies have been performed on surface modifications of PDMS using plasma treatment.9 Microfluidic techniques are attracting increased attention because of their advantages over macroscopic instrumentation, eg, small amounts of samples and reagents are needed, and the techniques are faster and more accurate. Recently, the integration of efficient sample handling by microfluidics and microbead-based arrays has been reported for multitarget detection, providing an ideal platform for sensitive detection of disease-related biomolecules and neurotransmitters.10 A microfluidic bead-based platform has been developed that requires smaller amounts of samples and gives reaction times of a few minutes rather than several hours.11
Dopamine (DA), which is neurotransmitter belonging to the catecholamine family, plays an important role in the human brain and body. DA is released by nerve cells as a chemical messenger to send signals between neurons. The brain includes several distinct DA systems, one of which plays a major role in reward-motivated behavior, and these DA systems mediate the enhancement of DA neuronal activity in nerve cells.12,13 CdSe, CdS, and CdTe quantum dots (QDs) have several advantages, such as high photoluminescence quantum efficiency and size-tunable absorption and photoluminescence in the visible to Near-infrared (NIR) region, yet concerns about their toxicity due to the presence of cadmium are to be resolved.14 Compared with Cd-based QDs, InP QDs are nontoxic and promising for in vivo imaging and clinical application in medicine field and photodynamic cancer therapy. Although QDs based on Pb and Hg core or shell show NIR absorption and photoluminescence characteristics, those are less promising for biological applications due to the presence of toxic heavy metals. Thus, we excluded Pb-, Cd-, and Hg-based QDs and focused on the synthesis of InP/ZnS core and shell QDs that are being widely applied in clinical applications.15–17
Recently, Ni2+-imidazole moieties in histidine affinity systems have been widely used for the detection of histidine-containing peptides and biomolecules. The specific interaction between Ni2+ and l-histidine was accomplished through coordination of Ni2+ and the imidazole residue of l-histidine.18–21 Here, we used the interaction between Zn2+ and adenosine to develop a novel fluorescence probe for adenosine detection based on beads-QDs-DA. We prepared water-soluble mercaptopropionic acid (MPA)- and mercaptoundecanoic acid (MUA)-capped InP/ZnS QDs. DA was linked to the QD surfaces using a 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide/N-hydroxysuccinimide (EDC/NHS) coupling reaction. The PL intensity of QDs-DA is quenched by Zn2+ via strong coordination interactions. In the presence of adenosine, the Zn2+ cations preferentially bind to adenosine, and the QDs-DA PL intensity is recovered. We constructed a microfluidic chip using a flexible PDMS polymer. The detection of adenosine in the presence of Zn2+ cations was confirmed by fluorescence quenching and recovery of beads-QDs-DA in the microfluidic chip.
Materials and methods
Chemicals
InP/ZnS QDs in toluene (5 mg/mL, emission wavelength 610 nm) were purchased from Mesolight (People’s Republic of China). Polystyrene/divinyl benzene beads (diameter 30 μm) were purchased from GE Healthcare (Seoul, South Korea). SU-8 2050 and SU-8 developers were purchased from Micro Chem (Newton, MA, USA). PDMS (Sylgard 184) and a curing agent were purchased from Dow Corning (Midland, MI, USA). MPA, MUA, L-dopamine hydrochloride, adenosine, EDC, NHS, ZnSO4, and n-hexane were purchased from Sigma-Aldrich (St Louis, MO, USA). Ethanol was purchased from Duksan Pure Chemicals.
Fluorescence emission spectra of the QDs were obtained using a fluoroluminescence spectrometer (Quanta Master, Photon Technology International, NJ, USA) equipped with a xenon lamp (Arc Lamp Housing, A-1010B™), monochromator, and power supply (Brytexbox). Fourier-transform infrared (FTIR) spectra were recorded using a Bruker Vortex 70 FTIR spectrometer. Sonication was performed using a Branson 8510 sonicator. Fluorescence images were obtained using a fluorescence microscope (Nikon, Melville, NY, USA). O2 plasma treatment was performed using plasma cleaner (Harrick Scientific Co., Germany).
Surface modification of InP/ZnS QDs
An InP/ZnS QD solution (100 μL) was added to 2 mL of n-hexane and ethanol, and the mixture was centrifuged at 3,000 rpm for 15 minutes. The precipitate was washed with ethanol, and the centrifugation was repeated twice. The precipitate was collected and redissolved in n-hexane (2 mL). An excess of thiol-terminated MPA and MUA was added to the QD solution. The mixture was sonicated for 30 minutes and kept at room temperature for overnight. The mixture was then centrifuged at 5,000 rpm for 5 minutes and washed with ethanol. The supernatant was removed, and the residue was dried under vacuum for 1 hour to yield water-soluble MPA- and MUA-capped InP/ZnS QDs. The surface-modified QDs (100 μL) were treated with EDC (3.8 mg/mL, 50 μL), NHS (4.6 mg/mL, 50 μL), and DA (3.5 mg/mL, 50 μL) in phosphate buffer for 3 hours continuous stirring at room temperature to produce QDs-DA. The obtained QDs-DA was purified by precipitation with addition of excess phosphate buffer to the reaction system, and the purified QDs-DA powder was redissolved in aqueous solution and stored in a darkroom. These coupling reagents caused reaction of the activated carboxylic acid groups of MPA and MUA with the amine functional group of DA, to produce amide linkages between DA and MPA or MUA on the QDs. The DA-functionalized InP/ZnS QDs were conjugated with polystyrene microbeads with amine functional groups through an EDC/sulfo-NHS coupling reaction using a previously described method.22
Fabrication of PDMS microfluidic chip with beads-QDs-DA
A PDMS microfluidic chip with a line of pillars was fabricated using a protocol described in a previous paper.23 SU-8 masters were prepared via a photoresist process, and the microfluidic chip was prepared by thermal curing of PDMS. A PDMS slide with channels and holes was attached to a glass slide by O2 plasma treatment. The surfaces of the PDMS microfluidic chip and glass slides were both activated by O2 plasma, using plasma cleaner, at 10.5 W for 1 minute. After the plasma treatment, they were bonded in an oven at 80°C for 40 minutes. The punched holes on the channel were used as the inlet and outlet, and the silicon tubes (OD 1.6 mm and OD 0.8 mm) were inserted to introduce beads-QDs-DA into the channel. The beads with QDs were packed into a microchannel blocked by pillars using syringe pump. Since the size of the beads is larger than the space between pillars, the beads could not penetrate the pillars in the microchannel of the PDMS chip. The flow rate of the solution was kept at 50 μL/min using a syringe pump. The fluorescence intensity was measured by image scanning over specific areas of the channel. After injection of Zn2+ solution, the fluorescence images on the microchannel were quantified by using image analysis program (IM-1 2005) Ratio Fluorescence Imaging System (PTI), equipped with a xenon lamp and a fluorescent microscope (Olympus IX71).
Results and discussion
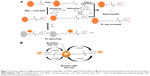
In this study, MPA- and MUA-capped InP/ZnS QDs were prepared, and DA was linked to the QD surfaces and PL quenching as shown in Figure 1. In Figure 1A, paths i and ii show schematic representation of PL quenching and recovery by the addition of Zn2+ cations and adenosine, respectively, to the system. The PL intensity of the DA-QDs is quenched by Zn2+ because of the strong coordination interactions between DA and Zn2+. Zn2+ ions induce electron transfer from the QDs, and PL quenching occurs. In the presence of adenosine, the Zn2+ cations preferentially bind to adenosine, and electron transfer from the QDs decreases; therefore, the PL intensity of the QDs-DA is recovered. The PL quenching and recovery also depend on the chain length of the organic ligand on the QD surfaces. MPA and MUA both were thiol-substituted carboxylic acids with three and eleven carbon chain lengths, respectively. Organic QDs surface was modified with this ligand to produce water soluble QDs for biological assays.
Figure 1B shows the representation of PL quenching mechanism. Fluorescence quenching was broadly explained by two possible mechanisms.24 One is dynamic quenching, which results from the collision between the fluorophore and a quencher, and the second one is static quenching, which results from the ground-state complex formation between the fluorophore and a quencher. According to studies on the mechanisms of fluorescence quenching of QDs and metal ions,25–27 the above process can be attributed to effective electron transfer from the QDs to the Zn2+ ions, and fluorescence recovery can be attributed to effective electron transfer from Zn2+ ions to QDs after addition of adenosine, based on the strong affinity of zinc for nitrogen atoms, which correspondingly yields the nonradiative recombination of excited electrons (e−) in the conduction bands and holes (h+) in the valence band on the surface of QDs.
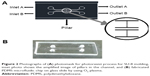
Figure 2A and B shows photographs of the photomask for the photoresist process for SU-8 molding and the fabricated PDMS microfluidic chip on the glass slide by using O2 plasma, respectively. The chip (25 mm ×12 mm) was fabricated using a conventional photo process. The channel width and height were 400 μm and 50 μm, respectively, and the distance between pillars was 25 μm. The elliptical pillar was constructed with a long diameter of 100 μm and a short diameter of 50 μm. The chip had two inlets and two outlets, with two channels. The channels were packed with the beads-QDs-DA. Figure 2A inset photo shows the amplified image of pillars in the channel. The gaps between the pillars were small enough to block the beads but large enough for buffer solution to flow through the channels.
Figure 3A–C shows the FTIR spectra of MPA, L-DA, and beads-QDs-DA, respectively. The peak at 2,580 cm−1 in (A) arises from the S–H stretching vibration of MPA. This peak is not observed in (C), which confirms covalent bond formation between the thiol group of MPA and the metal atom in the QD. The peaks at 1,654 cm−1 and 2,978 cm−1 in (A) are attributed to the C=O stretching of carboxylic groups and C–H stretching of methylene groups, respectively. The peaks at 3,206 and 2,977 cm−1 in (B) are attributed to N–H stretching of amine functional groups and C–H stretching of aromatic benzene rings, respectively. The peaks at 1,684 cm−1 and 1,610 cm−1 in (C) are attributed to the –CONH amide band I group and the N–H amide band II group, respectively, and the peak at 1,230 cm−1 is attributed to the –C–N stretching of amide bond, these arise from conjugation of DA with the MPA-capped QDs.
  | Figure 3 FTIR spectra of (A) MPA, (B) L-DA, and (C) beads-QDs-DA. |
Figure 4A and B, respectively, shows photographs of the QDs before and after surface modification with MPA under ultraviolet (UV) illumination. The InP/ZnS QDs are dispersed in a two-layered mixture of n-hexane (upper) and deionized water (lower). The figure shows that before the surface treatment, the QDs were dispersed in the organic phase, but after treatment with MPA, they were dispersed in the water phase since the MPA ligand was water soluble. These surface modified QDs were used for the detection of adenosine in presence of Zn2+.
  | Figure 4 Photographs of QDs (A) before and (B) after surface modification with MPA under UV illumination. |
Figure 5 shows the fluorescence spectra of the QDs-DA, QDs-DA-Zn2+, and QDs-DA-Zn2+-adenosine after surface modification with (A) MPA and (B) MUA. Inset photographs depict the (i) QDs-DA, (ii) QDs-DA-Zn2+, and (iii) QDs-DA-Zn2+ with adenosine system. After the addition of 10 μmol L−1 Zn2+, the PL intensity of the QDs-DA decreased. When 10 μmol L−1 adenosine was added to the QDs-DA-Zn2+ system, the PL intensity increased significantly. The PL intensity was quenched by ~62% by electron transfer from the QDs to the Zn2+ cation; 90% of the PL intensity was recovered in the case of beads-QDs-MPA-DA and 78% in the case of beads-QDs-MUA-DA after addition of adenosine, as a result of preferential binding of Zn2+ to adenosine.
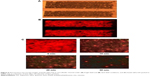
Figure 6 shows the fluorescence microscope images of beads-QDs-DA in the microfluidic channel under (A) bright field and (B) dark field conditions, and (C) as a function of time after introduction of Zn2+ solution. The figure shows that the QDs are well bound to the polystyrene bead surfaces, and the microfluidic chip channels are filled with beads-QDs-DA. All reaction solutions were injected into the microchannel by using syringe pump. Initially, the microchannel was washed with DI water, and Zn2+ and adenosine were injected into the microchannel for 30 minutes. After injection of Zn2+ using a syringe pump, the fluorescence microscope images were recorded as a function of time. The fluorescence intensity was measured by image scanning over specific areas of the channel. After injection of Zn2+ solution, the fluorescence images on the microchannel were quantified by using image analysis program (IM-1 2005) and redrawn as intensity line profile and also the fluorescence intensity per minute at 360 pixel of line profiles were averaged and converted to intensity profile as a function of time as shown in Figure 6. The fluorescence emission intensity from QDs attached to microbeads decreased gradually due to strong affinity between Zn2+ and adenosine. After 30 minutes, it was very difficult to observe further change of the fluorescence intensity, which may be due to the saturation of preferential binding of Zn2+ to adenosine.
Figure 7 shows the fluorescence intensity variations of the QDs as a function of time after 1.5 mL injection of 10 μmol L−1 Zn2+ and adenosine solution. The flow rate was kept at 50 μL/min using a syringe pump. After addition of 10 μmol L−1 Zn2+, the PL intensity of the QDs-DA gradually decreased within 30 minutes and the fluorescence was significantly recovered by the addition of 10 μmol L−1 adenosine. This experiment results also correlated with fluorescence spectra. The PL intensity was quenched by ~60%–65% by electron transfer from the QDs to the Zn2+ cation; 85% of the PL intensity was recovered in the case of beads-QDs-MPA-DA and 75% in the case of beads-QDs-MUA-DA after addition of adenosine, as a result of preferential binding of Zn2+ to adenosine. The figure also shows that for a shorter chain length between Zn2+ and the QDs (MPA), PL quenching was faster. The recovery of PL intensity was higher for a shorter chain length and vice versa. The experiment was repeated three times, with the fluorescence intensity vs time expressed as the mean ± standard deviation, as shown in Figure 7.
Conclusion
In summary, water-soluble InP/ZnS QDs were prepared by surface modification with MPA and MUA ligands. The QDs were immobilized on polystyrene microbeads by an EDC/sulfo-NHS coupling reaction, and DA was conjugated with the QDs. Fluorescence quenching and recovery were observed as a result of electron-transfer interactions between DA and Zn2+. A PDMS microfluidic chip was fabricated with polystyrene beads-QDs-DA packed in the channel. After addition of Zn2+, the PL intensity of the QDs-DA decreased and was recovered by addition of adenosine. A shorter chain length between Zn2+ and the QDs gave faster PL intensity quenching and recovery. The proposed fluorescence bioprobe has potential application in biological systems for the detection of adenosine in solution and serum at low concentration levels.
Acknowledgment
This work was supported by a National Research Foundation (NRF) grant funded by the Korean government (MEST) (NRF-2006-2005382).
Disclosure
The authors report no conflicts of interest in this work.
References
Craig CG, Temple SD, White TD. Is cyclic AMP involved in excitatory amino acid-evoked adenosine release from rat cortical slices? Eur J Pharmacol. 1994;269:79–85. | ||
Fredholm BB, IJzerman AP, Jacobson KA, Linden J, Müller CE. International union of basic and clinical pharmacology. LXXXI. Nomenclature and classification of adenosine receptors – an update. Pharmacol Rev. 2011;63:1–34. | ||
Pijanowska DG, Remiszewska E. pH-based detection of phenylalnine by potentiometric and colorimetric methods. Sensors. 2006;6:428–434. | ||
Hu YF, Zhang ZH, Zhang HB, Luo LJ, Yao SZ. Electrochemical determination of l-phenylalanine at polyaniline modified carbon electrode based on β-cyclodextrin incorporated carbon nanotube composite material and imprinted sol–gel film. Talanta. 2011;84:305–313. | ||
Kand’ár R, Žáková P. Determination of phenylalanine and tyrosine in plasma and dried blood samples using HPLC with fluorescence detection. J Chromatogr B. 2009;877:3926–3929. | ||
Wróbel K, Wróbel K. Determination of aspartame and phenylalanine in diet soft drinks by high-performance liquid chromatography with direct spectrofluorimetric detection. J Chromatogr A. 1997;773:163–168. | ||
Tagliaro F, Moretto S, Valentini R, et al. Capillary zone electrophoresis determination of phenylalanine in serum: a rapid, inexpensive and simple method for the diagnosis of phenylketonuria. Electrophoresis. 1994; 15:94–97. | ||
Abbasi F, Mirzadeh H, Katbab A-A. Modification of polysiloxane polymers for biomedical applications: a review. Polym Int. 2001;50:1279–1287. | ||
Owen MJ, Smith PJ. Plasma treatment of polydimethylsiloxane. J Adhes Sci Technol. 1994;8:1063–1075. | ||
Lucas LJ, Chesler JN, Yoon J-Y. Lab-on-a-chip immunoassay for multiple antibodies using microsphere light scattering and quantum dot emission. Biosens Bioelectron. 2007;23:675–681. | ||
Sato K, Tokeshi M, Odake T, et al. Integration of an immunosorbent assay system: analysis of secretory human immunoglobulin A on polystyrene beads in a microchip. Anal Chem. 2000;72:1144–1147. | ||
Baron R, Zayats M, Willner I. Dopamine-, l-DOPA-, adrenaline-, and noradrenaline-induced growth of Au nanoparticles: assays for the detection of neurotransmitters and of tyrosinase activity. Anal Chem. 2005;77:1566–1571. | ||
Brown AS, Gershon S. Dopamine and depression. J Neural Transm. 1993;91:75–109. | ||
Bharali DJ, Lucey DW, Jayakumar H, Pudavar HE, Prasad PN. Folate-receptor-mediated delivery of InP quantum dots for bioimaging using confocal and two-photon microscopy. J Am Chem Soc. 2005;127: 11364–11371. | ||
Kang I, Wise FW. Electronic structure and optical properties of PbS and PbSe quantum dots. J Opt Soc Am B. 1997;14:1632–1646. | ||
Rogach A, Kershaw S, Burt M, et al. Colloidally prepared HgTe nanocrystals with strong room-temperature infrared luminescence. Adv Mater. 1999;11:552–555. | ||
Du H, Chen CL, Krishnan R, et al. Optical properties of colloidal PbSe nanocrystals. Nano Lett. 2002;2:1321–1324. | ||
Fraser KA, Harding MM. The crystal and molecular structure of bis(histidino)nickel(II) monohydrate. J Chem Soc A. 1967:415–420. | ||
Liu Y, Li Y, Wu ZQ, Yan XP. Fabrication and characterization of hexahistidine-tagged protein functionalized multi-walled carbon nanotubes for selective solid-phase extraction of Cu2+ and Ni2+. Talanta. 2009;79:1464–1471. | ||
Liu JW, Yang T, Ma LY, Chen XW, Wang JH. Nickel nanoparticle decorated graphene for highly selective isolation of polyhistidine-tagged proteins. Nanotechnology. 2013;24:505704–505711. | ||
Liu J-W, Yang T, Chen S, Chen XW, Wang JH. Nickel chelating functionalization of graphene composite for metal affinity membrane isolation of lysozyme. J Mater Chem B. 2013;1:810–818. | ||
Yoo JH, Jongsung K. The specific hybridization of p53 gene on bead-quantum dot complex in microfluidic chip. J Nanosci Nanotechnol. 2011;11:7082–7085. | ||
Yoo JH, Jongsung K. Identification of p53 gene by using CdSe/ZnS conjugation and hybridization. J Nanosci Nanotechnol. 2011;11:4343–4346. | ||
Lakowicz JR, Weber G. Quenching of fluorescence by oxygen. Probe for structural fluctuations in macromolecules. Biochemistry. 1973; 12:4161–4170. | ||
Wang J, Liang J, Sheng Z. A novel strategy for selective detection of Ag+ based on the red-shift of emission wavelength of quantum dots. Microchim Acta. 2009;167:281–287. | ||
Cai ZX, Yang H, Zhang Y. Preparation, characterization and evaluation of water-soluble l-cysteine-capped-CdS nanoparticles as fluorescence probe for detection of Hg(II) in aqueous solution. Anal Chim Acta. 2006;559:234–239. | ||
Chen J, Gao YC. A, novel fluorescent array for mercury(II) ion in aqueous solution with functionalized cadmium selenide nanoclusters. Anal Chim Acta. 2006;577:77–84. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.