Back to Journals » Breast Cancer: Targets and Therapy » Volume 13
Does the Presence of Cytokeratin Positive Individual Tumor Cells (N0(I+)) in Sentinel Lymph Nodes Affect Clinical Outcomes in Breast Cancer Patients Treated with Accelerated Partial Breast Irradiation
Authors Smith J , Leonard C , Carter DL, Tole S
Received 4 May 2021
Accepted for publication 9 August 2021
Published 29 August 2021 Volume 2021:13 Pages 513—517
DOI https://doi.org/10.2147/BCTT.S318197
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pranela Rameshwar
Jamie Smith,1 Charles Leonard,1 Dennis L Carter,2 Shannon Tole1
1Rocky Mountain Cancer Centers Littleton, Denver, CO, USA; 2Rocky Mountain Cancer Centers Aurora, Denver, Co, USA
Correspondence: Jamie Smith
Rocky Mountain Cancer Centers Littleton, 790 Fillmore St., Denver, Co, 80206, USA
Tel +1 303-304-1187
Email [email protected]
Purpose: To report a primary objective clinical outcome of ipsilateral breast cancer recurrence following accelerated partial breast irradiation (APBI) with N0(i+) (single tumor cells or clusters < 2mm) in sentinel lymph nodes. The secondary objective was to observe any incidence of ipsilateral breast failure.
Patients and Methods: Between March 2004 and April 2016, a total of 747 patients were enrolled in one of two APBI (Accelerated Partial Breast Irradiation) breast protocols (Phase II NCT01185145 and Phase III NCT01185132). Nineteen patients with N0(i+) disease were treated between February 2005 and December 2015. Patient eligibility included a primary invasive or DCIS tumor size < 3 cm, N0(i+) disease, and margin width of > 2 mm. All enrolled patients presented in this report had sentinel lymph node examinations. Clinical outcomes of ipsilateral breast, axillary and combined regional (breast or axillary) recurrences were analyzed.
Results: Median follow-up for all patients was 5 years (1– 8 years). No patient experienced either ipsilateral breast or axillary recurrence.
Conclusion: There has been scarce information/reporting of the treatment of patients with cytokeratin positive individual tumor cells N0(i+) with APBI. The authors have presented data which suggest that the successful outcomes of these patients might warrant further study.
Keywords: breast sentinel nodes, breast conservation therapy
Introduction
Accelerated partial breast radiotherapy (APBI) has the benefit of a shortened treatment time and reduced radiation exposure to surrounding tissues when compared to whole breast irradiation (WBI). Currently, it is felt to be an acceptable alternative to breast radiotherapy for the post-lumpectomy adjuvant management of breast cancer.1–6
The National Comprehensive Cancer Network (NCCN) panel accepts the updated 2016 version of the American Society of Radiation Oncology (ASTRO) APBI guidelines, which now defines patients “suitable” for APBI to be the following: 50 years or older with invasive ductal carcinoma (IDCA) measuring ≤2 cm (T1 disease) with negative margin widths of ≥2 mm and node negative, no lymphovascular invasion, estrogen receptor (ER) positive, and BRCA 1/2 negative. In the ASTRO guidelines patients are categorized into “suitable”, “cautionary”, and “unsuitable” groups.1 Recently, these guidelines were revised and expanded to include characteristics previously felt to be “cautionary” into the “suitable” category.3 Additionally, the GEC-ESTRO Brachytherapy Committee has also published recommended APBI clinical guidelines. These guidelines state that APBI could be offered as standard therapy to node negative eligible patients > 50 years of age who have T1 invasive ductal carcinoma with a minimum of 2 mm margins.4 To date there is scarce information regarding the treatment of patients with cytokeratin positive individual tumor cells N0(i+) (single tumor cells or clusters < 2mm) following APBI treatment protocols. Previous analyses of N0(i+) cells range between 10–13% for positive sentinel lymph and 4.9–14.6% in non-sentinel lymph nodes.7
This is a retrospective analysis to observe any incidence of ipsilateral breast failure of a total of 747 patients who received APBI. Nineteen of the 747 patients with isolated tumor cells N0(i+) in sentinel lymph node sampling were estrogen and progesterone receptor positive, with HER 2/neu negative T1 infiltrating ductal tumors. Therefore, other than the isolated tumor cells found in a sentinel node, these patients were generally considered to be in the “suitable” category as defined by ASTRO guidelines. There were 5 exceptions which included 2 women who were younger than 50, 2 with infiltrating lobular carcinoma, and with a 1 mm anterior margin at skin.
Materials and Methods
Between March 2004 and April 2016, a total of 747 patients were enrolled in one of two prospective APBI breast protocols (Phase II NCT01185145 and Phase III NCT01185132). Informed consent according to the Declaration of Helsinki was obtained from every patient for treatment. Patients were treated with 38.5 Gy IMRT or 3D-CRT APBI in 3.85 Gy fraction/BID fractionation for 10 fractions.
Planning volumes were constructed using the following methods. Gross target volumes (GTV) encompassed the surgical bed as defined by the CT/ultrasound, clinical target volumes (CTV) included the GTV with an additional 1 cm, planning target volume (PTV) included the CTV with an additional 1 cm. The CTV was drawn 5mm from the lung-chest wall interface, and both the CTV and PTV were drawn a minimum of 5mm from the surface of the skin. Both contralateral and ipsilateral breasts were contoured to include all breast tissue from the inframammary fold to the clavicle in the medial-lateral direction (approximately mid-axillary line to midsternal line) and the cranial-caudal direction. With the patients lying supine on a spine board, the first CT slice contoured the heart from the pulmonary artery inferiorly to the apex and both lungs were entirely contoured using a Varian Eclipse or ADAC inverse planning nodule. Standardized restrictions for plan optimization were followed according to previously published protocols*. Therapy administration used 6-MV or 15-MV from Varian linear accelerators. Patients’ dose-volume histograms were individually calculated and quantified. Approval of all paired orthogonal and treatment fields occurred before treatment initiation. All patients received treatment in 10 equal fractions administered twice daily over 5 consecutive days.
Nineteen patients with N0(i+) disease were treated between February 2005 and December 2015. Patient eligibility included a primary invasive or DCIS tumor size < 3 cm, N0 or N0(i+) disease, margin width of > 2 mm and planning volume < 25% of ipsilateral breast volume. All enrolled patients presented in this report had sentinel lymph node examinations performed either by Tc99lymphoscintigraphy or injection of 5cc lymphazurin blue dye injection. Two 4mm sections of positive sentinel lymph nodes were embedded in paraffin for analysis, then sectioned at 4 µm thickness. At least three H and E levels as well as adequately controlled pan-cytokeratin staining were performed on each block for evaluation by the pathologist. Clinical outcomes of ipsilateral breast, axillary and combined regional (breast or axillary) recurrences were analyzed.
Results
Patient characteristics are noted in Table 1 and treatment characteristics are listed in Table 2. Median follow-up for all patients was 5 years (1–8 years). Seven patients were enrolled onto a Phase II protocol examining the use of intensity modulation for accelerated partial breast irradiation (NCT01185145). Twelve patients were subsequently enrolled in a phase III protocol which randomized patients to one of two accelerated partial breast treatment arms, intensity modulation or 3-dimensional planning. The majority of patients (14/19) were >60 years of age (median, 65). The majority of patients were postmenopausal (17/19). Most of this patient population did not receive chemotherapy (14/19) and all were administered hormone therapy. Tumor size ranged from 0.2 cm to 2.5 cm (median, 1.3 cm). Seventeen of the 19 patients had infiltrating ductal histologies and two patients had infiltrating lobular tumors. All but one patient were estrogen and progesterone positive; this patient was estrogen receptor positive but progesterone receptor negative. Two patients were HER2/neu positive and seventeen patients were HER2/neu negative. The median margin size was 6 mm (range 1–20 mm). There were three patients with T2 tumors and the remaining patients were T1. All patients had pathologically proven involvement of at least one node with isolated tumor cells and were staged by sentinel node procedure and examination. The number of dissected nodes ranged from 1–8 with a median number of 2 lymph nodes removed. Only one patient had more than one positive lymph node (that patient had a total of 2 positive lymph nodes).
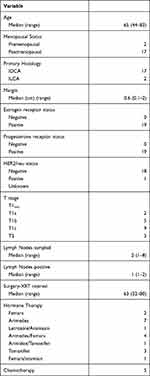 |
Table 1 Patient Characteristics |
 |
Table 2 Treatment Characteristics |
No patient experienced either ipsilateral breast or axillary recurrence nor is any patient deceased.
Discussion
The majority of previous reports have also shown that the presence of N0(i+) disease has not shown any impact on overall survival.11–17,19 There has also been evidence that there is no impact on distant relapse, axillary recurrence or local recurrence.8–13,15–19 However, treatment has not been homogenous. Some patients have had axillary dissections, sentinel node evaluations only or both.8,11–13,15–17 Axillary regional irradiation as well as chemotherapy have also been utilized to treat N0(i+) patients.8,11–13,16,17,19 Patient cohorts have also included a varied mix of patients undergoing breast conservation or mastectomy.11,15–17,19,20 To date, none of these reports concern the use of accelerated partial breast radiotherapy. However, because of this exploratory analysis, further research of this N0(i+) patient group might be warranted, and pertinent for their inclusion into conservative breast cancer treatment with accelerated partial breast irradiation.
Conclusion
The small scale of this study prevents definitive conclusions to be taken. However, the significance of these results in combination with the reasonable follow-up time suggests that accelerated partial breast irradiation may be appropriate in patients with N0(i+) disease, supporting continued research in these patients. These data suggest that APBI in combination with hormonal treatments after sentinel lymph nodes biopsy could effectively treat breast cancer patients with N0(i+) sentinel lymph nodes who have estrogen and progesterone receptor positive, HER 2/neu negative T1 infiltrating ductal tumors. The patients from this study will continue to be monitored for any recurrence. The preliminary findings from this novel study warrant future studies involving larger sample sizes, particularly focused on the clinical outcomes of the newly “suitable” treatment of N0(i+) disease using APBI protocols.
Data Sharing Statement
All patient data including all history/physical as well as protocol treatment and follow-up are reposed and stored indefinitely in an electronic database as well as paper chart here at Rocky Mountain Cancer Centers. Deidentified records/files are available for sharing.
Ethics Statement
All patients were enrolled/consented for one of two treatment protocols approved by WIRB (initial approval 7/7/09) – 20091193, WIRB (initial approval 1/30/04)-20040075).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Smith BD, Arthur DW, Buchholz TA, et al. Accelerated Partial Breast Irradiation Consensus Statement for the American Society for Radiation Oncology (ASTRO). Int J Radiation Oncology Biol Phys. 2009;74:987–1001. doi:10.1016/j.ijrobp.2009.02.031
2. Correa C, Harris EE, Leonardi MC, et al. Accelerated Partial Breast Irradiation: executive summary for the update of an ASTRO Evidence-Based Consensus Statement. Pract Radiat Oncol. 2017;7:73–79. doi:10.1016/j.prro.2016.09.007
3. Polgár C, Limbergen EV, Pötter R, et al. Patient selection for accelerated partial-breast irradiation (APBI) after breast-conserving surgery: recommendations of the Groupe Européen de Curiethérapie-European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) breast cancer working group based on clinical evidence (2009). Radiotherapy Oncol. 2010;94(3):264
4. Lei RY, Leonard CE, Howell KT, et al. Four-year clinical update from a prospective trial of accelerated partial breast intensity-modulated radiotherapy (APBIMRT). Breast Cancer Res Treat. 2013;140(1):119–133. doi:10.1007/s10549-013-2623-x
5. Leonard C, Carter D, Kercher J, et al. Prospective trial of accelerated partial breast intensity-modulated radiotherapy. International Journal of Radiation Oncology*Biology*Physics. 2007;67(5):1291–1298. doi:10.1016/j.ijrobp.2006.11.016
6. Chagpar A, Middleton LP, Sahin AA, et al. Clinical outcome of patients with lymph node‐negative breast carcinoma who have sentinel lymph node micrometastases detected by immunohistochemistry. Cancer. Int J Am Cancer Soc. 2005;103(8):1581–1586.
7. Tallet A, Lambaudie E, Cohen M, et al. Locoregional treatment of early breast cancer with isolated tumor cells or micrometastases on sentinel lymph node biopsy. World J Clin Oncol. 2016;7(2):243. doi:10.5306/wjco.v7.i2.243
8. Chagpar A, Middleton LP, Sahin AA, et al. Clinical outcome of patients with lymph node‐negative breast carcinoma who have sentinel lymph node micrometastases detected by immunohistochemistry. Cancer. 2005;103(8):1581–1586. doi:10.1002/cncr.20934
9. Millis RR, Springall R, Lee AH, Ryder K, Rytina ER, Fentiman IS. Occult axillary lymph node metastases are of no prognostic significance in breast cancer. Br J Cancer. 2002;86(3):396. doi:10.1038/sj.bjc.6600070
10. Karam I, Lesperance MF, Berrang T, Speers C, Tyldesley S, Truong PT. pN0 (i+) breast cancer: treatment patterns, locoregional recurrence, and survival outcomes. Int J Radiation Oncol. 2013;87(4):731–737. doi:10.1016/j.ijrobp.2013.07.028
11. Cox CE, Kiluk JV, Riker AI, et al. Significance of sentinel lymph node micrometastases in human breast cancer. J Am Coll Surg. 2008;206(2):261–268. doi:10.1016/j.jamcollsurg.2007.08.024
12. Herbert GS, Sohn VY, Brown TA. The impact of nodal isolated tumor cells on survival of breast cancer patients. Am j Surg. 2007;193(5):571–574. doi:10.1016/j.amjsurg.2007.01.007
13. McGuckin MA, Cummings MC, Walsh MD, Hohn BG, Bennett IC, Wright RG. Occult axillary node metastases in breast cancer: their detection and prognostic significance. Br J Cancer. 1996;73(1):88–95. doi:10.1038/bjc.1996.16
14. Nasser IA, Lee AK, Bosari S, Saganich R, Heatley G, Silverman ML. Occult axillary lymph node metastases in “node-negative” breast carcinoma. Hum Pathol. 1993;24(9):950–957. doi:10.1016/0046-8177(93)90108-S
15. Pugliese MS, Beatty JD, Tickman RJ, et al. Impact and outcomes of routine microstaging of sentinel lymph nodes in breast cancer: significance of the pN0 (i+) and pN1mi categories. Ann Surg Oncol. 2009;16(1):113–120. doi:10.1245/s10434-008-0121-x
16. Millis RR, Springall R, Lee AH, Ryder K, Rytina ER, Fentiman IS. Occult axillary lymph node metastases are of no prognostic significance in breast cancer. Br J Cancer. 2002;86(3):396–401.
17. Colpaert C, Vermeulen P, Jeuris W, et al. Early distant relapse in ‘node‐negative’breast cancer patients is not predicted by occult axillary lymph node metastases, but by the features of the primary tumour. J Pathol. 2001;193(4):442–449. doi:10.1002/path.829
18. de Mascarel I, MacGrogan G, Debled M, Brouste V, Mauriac L. Distinction between isolated tumor cells and micrometastases in breast cancer: is it reliable and useful? Cancer. 2008;112(8):1672–1678. doi:10.1002/cncr.23368
19. Tan LK, Giri D, Hummer AJ, et al. Occult axillary node metastases in breast cancer are prognostically significant: results in 368 node-negative patients with 20-year follow-up. J Clin Oncol. 2008;26(11):1803–1809. doi:10.1200/JCO.2007.12.6425
20. Querzoli P, Pedriali M, Rinaldi R, et al. Axillary lymph node nanometastases are prognostic factors for disease-free survival and metastatic relapse in breast cancer patients. Clin Cancer Res. 2006;12(22):6696–6701. doi:10.1158/1078-0432.CCR-06-0569
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
