Back to Journals » International Journal of Women's Health » Volume 6
Does the presence of coexisting diseases modulate the effectiveness of a low-dose estrogen/progestin, ethinylestradiol/drospirenone combination tablet in dysmenorrhea? Reanalysis of two randomized studies in Japanese women
Authors Momoeda M, Hayakawa M, Shimazaki Y, Mizunuma H, Taketani Y
Received 10 July 2014
Accepted for publication 5 September 2014
Published 2 December 2014 Volume 2014:6 Pages 989—998
DOI https://doi.org/10.2147/IJWH.S70935
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Elie Al-Chaer
Mikio Momoeda,1 Masakane Hayakawa,2 Yukio Shimazaki,3 Hideki Mizunuma,4 Yuji Taketani5
1Department of Integrated Women's Health, St Luke's International Hospital, Tokyo, 2Medical Affairs, 3Product Development, Bayer Yakuhin, Ltd, Osaka, 4Department of Obstetrics and Gynecology, Faculty of Medicine, Hirosaki University, Hirosaki, 5Japan Labour Health and Welfare Organization, Kawasaki, Japan
Background: The purpose of this study was to investigate the effectiveness of a combination of ethinylestradiol (EE) and 0.02 mg/drospirenone (DRSP) 3 mg in Japanese women with dysmenorrhea and in particular to determine whether or not the presence of specific coexisting organic diseases (eg, endometriosis, uterine fibroids, uterine adenomyosis) has an impact on treatment.
Methods and results: Four hundred and ten patients with dysmenorrhea aged 20 years or older (315 without coexisting organic disease, 28 with endometriosis, 37 with uterine fibroids, and 46 with uterine adenomyosis [some patients had multiple coexisting organic diseases]) were enrolled and treated with EE/DRSP in either a 16-week comparator study or a 52-week long-term safety study. Evaluations included changes in total dysmenorrhea score, visual analog scale for dysmenorrhea, severity of symptoms, hormone levels, endometrial thickness, and safety outcomes. In both studies, the total dysmenorrhea score was significantly (P<0.001) decreased from baseline during treatment with EE/DRSP. Time-dependent changes in visual analog score for dysmenorrhea and alleviation of symptoms, such as lower abdominal pain, low back pain (lumbago), headache, and nausea/vomiting, were similar in all patient groups with and without any specific coexisting organic diseases. These improvements with EE/DRSP were observed for both short-term (16 weeks) and long-term (52 weeks) use. These effects were associated with suppressed increases in serum estradiol and progesterone levels and decreased endometrial thickness. The safety profile of EE/DRSP was similar in all patients, irrespective of the presence of coexisting organic diseases.
Conclusion: EE/DRSP may be prescribed for patients with dysmenorrhea irrespective of the presence of any specific coexisting organic diseases.
Keywords: dysmenorrhea, organic disease, ethinylestradiol, drospirenone, oral contraceptive
Introduction
Dysmenorrhea is characterized by pain that predominantly occurs in the lower abdomen, but can radiate to the thighs and back, and is experienced during the menstrual period. In some cases, other systemic symptoms such as nausea, vomiting, and breast pain may be present.1–4
When no organic disease is found as the underlying cause of dysmenorrhea, it is commonly classified as primary dysmenorrhea. On the other hand, when organic disease is identified, it is commonly classified as secondary dysmenorrhea. Primary dysmenorrhea is considered to be caused by contraction of the uterine smooth muscle that is induced by excess secretion of prostaglandin associated with changes in hormone levels, and consequent ischemia and distal nerve stimulation.3,4 In those with dysmenorrhea and coexisting organic diseases, production of inflammatory substances in the abdominal cavity due to endometriosis, uterine abnormal contractions due to uterine fibroids, and bleeding from the myometrium due to uterine adenomyosis are all considered to be contributory causes for dysmenorrhea. In some cases, however, the causal relationship between the symptoms of dysmenorrhea and underlying organic disease is unclear.
For patients with dysmenorrhea and coexisting organic disease, physicians may select surgery, depending on the underlying condition and the diagnosis. When alleviation of symptoms is considered clinically important, treatment options are based on those used for patients with primary dysmenorrhea (ie, without organic disease). The most commonly used treatments include nonsteroidal anti-inflammatory drugs and combined (estrogen/progestin) hormonal contraceptives.1–4 Until now, however, the efficacy of combined hormonal contraceptives has usually been examined in patients with primary and secondary dysmenorrhea separately.
Japan is the first country to approve ethinylestradiol (EE)/drospirenone (DRSP) as a low-dose combined hormonal contraceptive with a specific indication for the treatment of dysmenorrhea. The use of EE/DRSP is not limited by the presence of coexisting organic disease when relief of symptoms is prioritized. DRSP is a novel α-spirolactone derivative that has a receptor-binding profile similar to that of natural progesterone, but is different from other synthetic progestogens. DRSP not only has progestogenic effects but also antimineralocorticoid and antiandrogenic effects.5 EE/DRSP is administered in a 24/4-day regimen (24 days of active treatment followed by a 4-day hormone-free interval) rather than the more commonly used 21/7-day regimen (21 days of active treatment followed by a 7-day hormone-free interval). Because the hormone-free interval is shorter and the fluctuation of endogenous hormone levels is smaller, the 24/4-day regimen is expected to inhibit follicular maturation more strongly during the hormone-free interval, and to inhibit ovulation, often a cause of dysmenorrhea, more reliably than 21/7-day regimens.6,7
To investigate the efficacy and safety of EE 0.02 mg/DRSP 3 mg for treating both primary and secondary dysmenorrhea in Japanese women, two randomized, controlled clinical trials were conducted. The first was a 16-week double-blind, randomized, placebo-controlled, comparative study (Comparative study) that investigated the optimal dose of DRSP required and aimed to confirm the efficacy of EE/DRSP in treating dysmenorrhea. The second was a 52-week single-blind, randomized, active-controlled study (Long-term study) that investigated the intracyclic bleeding profile of EE and its long-term safety in patients with dysmenorrhea treated by EE/DRSP for 52 weeks. Results from these studies have been published in Japanese domestic journals, in the Japanese language, and demonstrated that EE/DRSP was effective in alleviating symptoms and generally well tolerated over 13 cycles in patients with primary or secondary dysmenorrhea.8,9
The present study aimed to clarify, by reanalyzing these two clinical studies, whether or not the presence of specific coexisting diseases (ie, endometriosis, uterine fibroids, and uterine adenomyosis) can modulate the effect of EE/DRSP on symptoms seen in patients with dysmenorrhea.
Materials and methods
Study design
The rationale, design, and conduct of the two studies have been reported previously.8,9 In brief, both the Comparative (undertaken in 12 study centers from July 2007 to January 2009; ClinicalTrials.gov identifier NCT00511797) and Long-term (undertaken in 26 study centers from February 2007 to August 2009; NCT00461305) studies were conducted with approval of the study protocols by the institutional review board at each study center and in accordance with the International Conference on Harmonization Good Clinical Practice guidelines. Written informed consent was obtained from all patients prior to study entry.
Study participants
Both studies included women aged 20 years or older with a total dysmenorrhea score (described in detail below) of at least 3 points. The patients also had to have normal menstrual cycles (cycle length of 28±3 days for the Comparative study and 25–38 days for the Long-term study) in the last two menses prior to enrollment and desire contraceptive protection. To identify specific coexisting organic diseases (ie, secondary dysmenorrhea), all patients underwent gynecological examination, including bimanual examination and diagnostic imagining (transvaginal ultrasound).
Study treatments
In the Comparative study, patients were randomly assigned to one of four treatment groups for 4 cycles: EE 0.02 mg/DRSP 1 mg, EE 0.02 mg/DRSP 2 mg, EE 0.02 mg/DRSP 3 mg (YAZ®; Bayer HealthCare Pharmaceuticals, Berlin, Germany), or placebo. In the Long-term study, patients were randomly assigned to either EE 0.03 mg/DRSP 3 mg for six cycles or EE 0.02 mg/DRSP 3 mg for 13 cycles. In both studies, patients were administered active tablets (or all placebo in the placebo group) once daily for 24 days, followed by inert tablets once daily for 4 days in a 28-day cycle. Irrespective of whether or not withdrawal bleeding had finished, the next cycle was then started from the 29th day and the same tablet intake repeated for all subsequent cycles. Although concomitant drug use was restricted in both studies to those usually prohibited in clinical trials of hormonal drugs, patients were permitted to use their regular analgesics, if necessary. Only data from the EE 0.02 mg/DRSP 3 mg groups in both studies, as the approved optimal dose for the treatment of patients with dysmenorrhea, and placebo group are presented in this report.
Efficacy evaluations
Dysmenorrhea score
The total dysmenorrhea score10 is a rating scale based on the verbal rating scales of Andersch and Milson11 and Biberoglu and Behrman,12 and consists of both the severity of dysmenorrhea evaluated from the aspects of activities of daily living and the use of analgesics. In both studies, the severity of dysmenorrhea was scored using the following criteria: 0, none (none); 1, mild (slightly interferes with work [including schoolwork or housework]); 2, moderate (interferes sufficiently with work [including schoolwork or housework] such that the patient wants to lie down and rest); and 3, severe (necessary to stay in bed all day, cannot do any work [including schoolwork or housework]). The use of analgesics was scored using the following criteria: 0, none (none); 1, mild (used analgesics for 1 day during the previous [or current] menstrual period); 2, moderate (used analgesics for 2 days during the previous [or current] menstrual period); and 3, severe (used analgesics for 3 days or more during the previous [or current] menstrual period). The total dysmenorrhea score (range 0 to 6) is the sum of the two subscores for the severity of dysmenorrhea and use of analgesics.
Visual analog scale for dysmenorrhea
In both studies, the severity of pain during menstruation was evaluated using the visual analog scale (VAS) for dysmenorrhea. The VAS uses an unmarked scale on a line of 100 mm in length, where 0 mm represents “no pain” and 100 mm represents “worst pain a patient has ever experienced”. The VAS score was, therefore, determined by measuring in millimeters (scale 0–100 mm) from the left-hand end of the line to the point that the patient marked.
Severity of lower abdominal pain, low back pain, headache, and nausea or vomiting
In both studies, the severity of lower abdominal pain, low back pain (lumbago), headache, and nausea or vomiting during menstruation was rated using the following criteria: none (none), mild (can be easily endured), moderate (noticeable, but does not interfere with daily activities), and severe (interferes with daily activities).
Serum hormone levels and endometrial thickness
In the Comparative study only, the serum estradiol levels in the follicular phase, serum progesterone levels in the luteal phase, and endometrial thickness were measured at baseline and cycle 4.
Safety evaluations
For both studies, all reported adverse events (including any abnormal laboratory values) were recorded and assessed. Adverse drug reactions were defined as adverse events for which a causal relationship to the study drug could not be ruled out (ie, other than those considered “none”) by the investigators or subinvestigators.
Statistical analysis
For the Comparative study, the population sample size was determined to be 232 patients (58 per treatment arm) based on the following assumptions: a dropout rate of 15%; a placebo group effect of -1.5; an EE/DRSP group treatment effect of -2.5; an equal number of patients in all treatment groups; a common standard deviation of 1.5; a significance level of α=2.5% (one-sided); and a power of 90% for the one-sided t-test of the hypothesis that EE/DRSP is better than placebo versus the alternative hypothesis that EE/DRSP is worse than placebo at a significance level of 2.5%. For the Long-term study, in order to assess the long-term safety of EE 0.02 mg/DRSP 3 mg, at least 300 patients for 6 months and 100 patients for one year were required. The population sample size was determined to be 330 patients in this group assuming a dropout rate of 10%.
The comparison between the values prior to administration of the study drug and those after administration of the study drug were analyzed by a one-sample t-test. A two-sample t-test was used for the comparison between EE 0.02 mg/DRSP 3 mg and placebo groups in the Comparative study. All significance levels were 5% (two-sided).
Results
The Comparative study enrolled 249 patients of whom 61 received EE 0.02 mg/DRSP 3 mg and 58 received placebo, while the Long-term study enrolled 420 patients of whom 349 received EE 0.02 mg/DRSP 3 mg. All patients who received EE 0.02 mg/DRSP 3 mg and placebo were included in the full analysis set, which was used for efficacy and safety evaluations. In total, 51 (83.6%) patients who received EE/DRSP and 47 (81.0%) patients who received placebo in the Comparative study and 254 (72.8%) patients who received EE/DRSP in the Long-term study completed the studies.
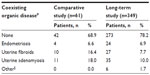
The demographic and baseline characteristics of the patients who received EE 0.02 mg/DRSP 3 mg or placebo in the Comparative study and EE 0.02 mg/DRSP 3 mg in the Long-term study are shown in Table 1. In general, there were no considerable differences in patient characteristics between the Comparative and Long-term studies or between the EE/DRSP and placebo groups in the Comparative study. Of the patients in the EE 0.02 mg/DRSP 3 mg groups, 42 (68.9%) in the Comparative study and 273 (78.2%) in the Long-term study had no coexisting organic diseases (Table 2). Table 2 also shows the number and proportion of patients with specific coexisting organic diseases (endometriosis, uterine fibroids, uterine adenomyosis, and other [which included bicornuate uterus, multiple endometrial polyp, endometrial polyp, and uterine enlargement]). In some patients, two or more coexisting organic diseases were found simultaneously.
  | Table 1 Demographic and baseline characteristics of patients with dysmenorrhea |
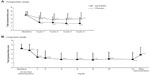
The changes over time in the total dysmenorrhea score are shown in Figure 1. In the Comparative study, the total dysmenorrhea score significantly (P<0.001) decreased from baseline to cycle 1 and this significant decrease was maintained in all cycles until the end of the study in both the EE/DRSP and placebo groups (Figure 1A). For all cycles, the change from baseline in the total dysmenorrhea score was significantly greater in the EE/DRSP group than in the placebo group. In the Long-term study, decreases from baseline in the total dysmenorrhea score were maintained throughout the study from cycle 1 to cycle 13 (Figure 1B).
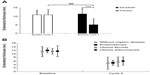
The VAS for dysmenorrheic pain at baseline (before administration of EE/DRSP or placebo) and up to cycle 4 (after administration of treatment) are shown in Figure 2A for the Comparative study and at baseline and up to cycle 13 in Figure 2B for the Long-term study. The treatment-dependent changes in the VAS for dysmenorrheic pain were similar to those observed for the total dysmenorrhea score. In the Comparative study, treatment with EE/DRSP significantly (P<0.001) improved dysmenorrhea as measured by VAS compared with the placebo group. A similar improvement was observed in the Long-term study. In the Long-term study, when stratifying according to the absence or presence of specific coexisting organic diseases, the decrease in the VAS for dysmenorrhea score from baseline was significant (P<0.001) for all patient groups irrespective of coexisting organic diseases (eg, endometriosis, uterine fibroids, and adenomyosis). The effect was maintained throughout 13 cycles of treatment in all patient groups (Figure 3).
  | Figure 2 Time-dependent changes in VAS in patients with dysmenorrhea (A and B). |
Changes in the severity of lower abdominal pain, low back pain, headache, and nausea or vomiting during menstruation in the Comparative study are shown in Figure 4A. Although the incidences of these symptoms were different at baseline, moderate or severe symptoms were much more markedly improved after treatment with EE/DRSP compared with placebo.
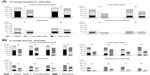  | Figure 4 Changes in the severity of dysmenorrheic symptoms during menstruation in patients with dysmenorrhea. |
Figure 4B shows the changes in severity of lower abdominal pain, low back pain, headache, and nausea or vomiting during menstruation in patients with dysmenorrhea from baseline to cycle 13 in the Long-term study, which is stratified according to those with and without coexisting organic diseases. As in the Comparative study, although the incidences of the symptoms were different at baseline, moderate or severe symptoms were markedly improved after treatment with EE/DRSP and the effect was sustained over 13 cycles. No substantial difference due to coexisting organic diseases (eg, endometriosis, uterine fibroids, and adenomyosis) was observed in improvement of symptoms by treatment with EE/DRSP.
After treatment with EE/DRSP, all except two patients showed estradiol levels of 100 pg/mL or lower in the Comparative study. The majority of patients were within the threshold value of 30 pg/mL13 or below, irrespective of the absence or presence of coexisting organic diseases, indicating that follicular maturation was suppressed during the follicular phase after EE/DRSP treatment (Table 3). All except for one patient were within the threshold progesterone level value of 1.5 ng/mL13 or lower in cycle 4, indicating that ovulation was inhibited (Table 3). One patient who had higher estradiol and progesterone levels at cycle 4 had lower serum DRSP levels than the limit of quantification measured in cycles 2, 3, and 4, suggesting that this patient did not take the investigational product appropriately. Overall, the results suggest that the presence of specific coexisting organic diseases does not have an impact on the effect of EE/DRSP in suppressing follicular maturation or inhibiting ovulation.
In the Comparative study, the average of endometrial thickness decreased from approximately 12 mm at baseline to less than 6 mm by the end of treatment with EE/DRSP (Figure 5). The decreases were almost equal in all patient groups irrespective of the absence or presence of specific coexisting organic diseases.
As previously described,8,9 the most common adverse drug reactions were headache and nausea, which are commonly reported with the use of estrogen/progestin hormonal combinations including oral contraceptives. Although dysmenorrhea, metrorrhagia, and genital hemorrhage were reported as adverse events in all subgroups, these events were reported at similar rates across the subgroups, in the ranges of 18.4%–25.0%, 21.4%–32.4%, and 14.3%–30.4%, respectively. Overall, the tolerability of EE/DRSP in treating dysmenorrhea was comparable across all the subgroups.
Discussion
In this additional analysis of data from two randomized trials conducted in Japan, treatment with EE 0.02 mg/DRSP 3 mg was shown to be effective and well tolerated in patients with dysmenorrhea irrespective of the absence or presence of specific coexisting organic diseases. EE/DRSP significantly improved activities of daily living, including schoolwork and housework, and shortened the number of days of analgesic use as measured by the total dysmenorrhea score. EE/DRSP also alleviated pain experienced during menstruation as measured by the VAS for dysmenorrhea score. In addition, marked improvements were observed in symptom severity of lower abdominal pain, low back pain, headache, and nausea or vomiting. These improvements were observed from cycle 1 and were maintained through cycle 4 in the Comparative study and through cycle 13 in the Long-term study. No clinically significant difference was observed in the effectiveness of EE/DRSP for dysmenorrhea when treating those with and without specific coexisting organic diseases.
The finding that EE/DRSP is effective for treating patients with dysmenorrhea irrespective of the absence or presence of specific coexisting organic diseases may indicate that there is a common cause of dysmenorrhea that is independent of the existence of organic disease. It has been reported that oral contraceptives can prevent or improve dysmenorrhea directly by limiting endometrial growth and reducing the amount of endometrial tissue available for prostaglandin and leukotriene production, and indirectly by inhibiting ovulation and subsequent progesterone secretion.4 In addition, it has been demonstrated that low-dose combined hormonal contraceptives can suppress uterine peristalsis and contraction during withdrawal bleeding in healthy women, including those with primary dysmenorrhea.14 In the present studies, EE/DRSP suppressed increases in serum estradiol and progesterone levels, and decreased endometrial thickness in patients with dysmenorrhea irrespective of the presence of endometriosis, uterine fibroids, and/or uterine adenomyosis. These results suggest that estrogen/progestin combinations inhibit follicular maturation and ovulation as well as endometrial proliferation, irrespective of specific coexisting organic diseases. Although a number of mechanisms have been proposed for the cause of dysmenorrhea-associated pain, it seems reasonable to hypothesize that uterine contraction induced by prostaglandins would be an important mechanism for the associated pain. EE/DRSP improves the symptoms of dysmenorrhea irrespective of the presence of specific coexisting organic diseases by suppressing prostaglandin production through inhibition of follicular maturation, ovulation, and endometrial proliferation.
The incidence and type of adverse drug reactions were similar to those that have been reported for other combined hormonal contraceptives. In the Long-term study, over 13 cycles the rate of discontinuation due to adverse events was 7.4%, which is also comparable with other combined hormonal contraceptives.15 There were no meaningful differences observed in the incidence of adverse drug reactions between patients with dysmenorrhea without organic disease, and those with endometriosis, uterine fibroids, or uterine adenomyosis.
There are no studies that have examined the use of oral contraceptives for treating both primary and secondary dysmenorrhea. In general, combined oral contraceptives are recommended for the management of primary dysmenorrhea in women who wish to use contraception,16 although a Cochrane review concluded that there is limited evidence to suggest that combined oral contraceptives can provide pain improvement.17 The same review also highlighted that for secondary dysmenorrhea the underlying cause of the pain must be resolved. Results from this study and a recent preliminary study,18 however, suggest that EE 0.02 mg/DRSP 3 mg may be a promising treatment for the management of endometriosis. This study also suggests that EE/DRSP may be a potential option in the management of uterine fibroids and uterine adenomyosis.
It should be noted that the numbers of patients in the specific coexisting organic diseases subgroups were small, particularly in the Comparative study, but similar results were observed in the Long-term study, which had more patients in these subgroups. In particular, evaluation of changes in total pelvic pain were difficult because of low baseline values, and changes in headache, nausea, and vomiting were also difficult because a high proportion of patients did not report these symptoms at baseline. In the Comparative study, there was a high response in the placebo group, with significant improvements observed from baseline for total dysmenorrhea score over all four cycles and VAS for dysmenorrhea scores in cycles 2, 3, and 4. Treatment with EE/DRSP did, however, produce significantly greater improvements in total dysmenorrhea score over placebo. Further studies are warranted in larger populations to confirm these findings in patients with specific coexisting organic diseases and to determine whether these observations are generalizable to other populations.
Conclusion
Regardless of the absence or presence of specific coexisting organic diseases, EE 0.02 mg/DRSP 3 mg significantly improved daily activities and dysmenorrheic pain as compared with placebo or baseline levels in patients with dysmenorrhea in both short-term (16 weeks) and long-term (52 weeks) use. EE/DRSP was shown to inhibit follicular maturation and ovulation as well as endometrial proliferation, irrespective of coexisting organic diseases. Therefore, EE/DRSP may be used for the treatment of both primary and secondary dysmenorrhea.
Acknowledgments
The authors would like to thank the following doctors for participation in the studies: Shoko Koga (Koga Clinic), Kiyoko Iesaka (Iesaka Clinic), Kazunori Kinoshita (Seijo Kinoshita Hospital), Seiko Iizuka (Kosugi Clinic), Ruriko Tsushima (Women’s Wellness Ginza Clinic), Yuta Murakami (Ikebukuro Clinic), Yukari Utsugisawa (Women’s Clinic Luna), Kazuhisa Ideta (Chayamachi Ladies Clinic), Masahide Shiotani (Hanabusa Women’s Clinic), Chisato Kiuchi (Kiuchi Ladies Clinic), Hideki Hanashi and Masataka Karube (NS Clinic), Masayoshi Ishihara (Ishihara OB and GYN), Fumiyasu Nagaike (Nagaike Clinic), Chieko Kakuta (Kakuta Chieko Ladies Clinic), Katsumi Yazaki (Yazaki Clinic), Masuo Yoshioka (Suzuran Clinic), Masao Takane (Takane Clinic), Miyako Ito and Kanako Hanaoka (Ito Medical Clinic), Takuya Irie (C:z Ladies Clinic), Ken Yoshikawa (Kichijyouji Lady’s Clinic), Motoyasu Furuya (Machida Higashiguchi Clinic), Hiroko Mouri (Rinkan Clinic), Takeo Kanou (Kano’s Clinic for Women), Tadashi Iida (Iida Ladies Clinic), Sakie Niwa (Sakie Ladies Clinic), Kazume Kawabata (Kawabata Women’s Clinic), and Takeshi Yoshimura (Yoshimura Ladies Clinic). The authors would also like to thank Noboru Fujomoto (formerly Bayer Yakuhin, Ltd) for his critical review of the manuscript. Clare Wheatcroft, of inScience Communications, Springer Healthcare, provided editorial support on behalf of Bayer HealthCare Pharmaceuticals.
Author contributions
YS was involved in the initial conception and design of this project. MM advised on preparation and revision of the protocol and data handling. HM and YT contributed to the design of the studies and provided advice on data handling. MH and MM contributed toward interpretation of reanalyzed data and drafting the manuscript. All authors provided critical review of the manuscript and confirm that they have read and approved the final version to be published.
Disclosure
MH and YS are employees of Bayer Yakuhin, Ltd. MM, HM, and YT received a medical advisory fee from Bayer Yakuhin, Ltd when the two studies were planned and conducted. The two studies were funded by Bayer Yakuhin, Ltd, Osaka, Japan.
References
French L. Dysmenorrhea. Am Fam Physician. 2005;71:285–291. | |
Dawood MY. Primary dysmenorrhea: advances in pathogenesis and management. Obstet Gynecol. 2006;108:428–441. | |
Proctor M, Farquhar C. Diagnosis and management of dysmenorrhea. BMJ. 2006;332:1134–1138. | |
Harel Z. Dysmenorrhea in adolescents and young adults: from pathophysiology to pharmacological treatments and management strategies. Expert Opin Pharmacother. 2008;9:2661–2672. | |
Rapkin AJ, Winer S. Drospirenone: a novel progestin. Expert Opin Pharmacother. 2007;8:989–999. | |
Klipping C, Duijkers I, Trummer D, Marr J. Suppression of ovarian activity with a drospirenone-containing oral contraceptive in a 24/4 regimen. Contraception. 2008;78:16–25. | |
Sulak PJ, Scow RD, Preece C, Riggs MW, Kuehl TJ. Hormone withdrawal symptoms in oral contraceptive users. Obstet Gynecol. 2000;95:261–266. | |
Momoeda M, Mizunuma H, Taketani Y. [Treatment of functional and organic dysmenorrhea: efficacy and safety of drospirenone/ethinylestradiol combination tablet: Sanka to Fujinka]. Obstet Gynecol. 2010;77:977–988. Japanese. | |
Momoeda M, Mizunuma H, Taketani Y. [Long term efficacy and safety of drospirenone / ethinylestradiol combination (YAZ) tablets for patients with dysmenorrhea: Shinryo to Shinyaku]. Med Consult and New Remedies. 2010;47:1003–1015. Japanese. | |
Harada T, Momoeda M, Taketani Y, Hoshiai H, Terakawa N. Low-dose oral contraceptive pill for dysmenorrhea associated with endometriosis: a placebo-controlled, double-blind, randomized trial. Fertil Steril. 2008;90:1583–1588. | |
Andersch B, Milson I. An epidemiologic study of young women with dysmenorrhea. Am J Obstet Gynecol. 1982;144:655–660. | |
Biberoglu KO, Behrman SJ. Dosage aspects of danazol therapy in endometriosis: short-term and long-term effectiveness. Am J Obstet Gynecol. 1981;139:645–654. | |
Hoogland HJ, Skouby SO. Ultrasound evaluation of ovarian activity under oral contraceptives. Contraception. 1993;47:583–590. | |
Kido A, Togashi K, Kataoka K, et al. The effect of oral contraceptives on uterine contractility and menstrual pain: an assessment with cine MR imaging. Hum Reprod. 2007;22:2066–2071. | |
Guang-Sheng F, Mei-Lu B, Li-Nan C, et al. Efficacy and safety of the combined oral contraceptive ethinylestradiol/drospirenone (Yasmin) in healthy Chinese women: a randomized, open-label, controlled, multicenter trial. Clin Drug Investig. 2010;30:387–396. | |
Zahradnik HP, Hanjalic-Beck A, Groth K. Nonsteroidal anti-inflammatory drugs and hormonal contraceptives for pain relief from dysmenorrhea: a review. Contraception. 2010;81:185–196. | |
Wong CI, Faraquhar C, Roberts H, Proctor M. Oral contraceptive pill for primary dysmenorrhea. Cochrane Database Syst Rev. 2009;4:CD002120. | |
Mabrouk M, Solfrini S, Frascà C, et al. A new oral contraceptive regimen for endometriosis management: preliminary experience with 24/4-day drospirenone/ethinylestradiol 3 mg/20 mcg. Gynecol Endocrinol. 2012;28:451–454. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.