Back to Journals » Nature and Science of Sleep » Volume 13
Does Napping Enhance the Consolidation of Clinically Relevant Information? A Comparison of Individuals with Low and Elevated Depressive Symptoms
Authors Lo EBL, Laferriere LJC, Stewart MR, Milanovic M, Kinney M, Bowie CR, Dringenberg HC
Received 17 December 2020
Accepted for publication 19 January 2021
Published 10 February 2021 Volume 2021:13 Pages 141—152
DOI https://doi.org/10.2147/NSS.S297872
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sarah L Appleton
Edwyn BL Lo,1 Lilian JC Laferriere,1 Matthew R Stewart,1 Melissa Milanovic,1 Melinda Kinney,1 Christopher R Bowie,1,2 Hans C Dringenberg1,2
1Department of Psychology, Queen’s University, Kingston, Ontario, Canada; 2Centre for Neuroscience Studies, Queen’s University, Kingston, Ontario, Canada
Correspondence: Hans C Dringenberg
Department of Psychology, Queen’s University, Kingston, Ontario, K7L 3N6, Canada
Tel +1613-533-6215
Fax +1613-433-2499
Email [email protected]
Purpose: Sleep, both overnight and daytime naps, can facilitate the consolidation of declarative memories in healthy humans. However, it is unclear whether such beneficial effects of sleep occur in special populations, such as individuals with elevated neuropsychiatric symptoms, and if they apply to clinically relevant material that may have personal significance to those populations.
Methods: We examined memory retention over a 60-minute interval of wakefulness or nap opportunity in participants with low or elevated scores (≤ 13 and ≥ 21, respectively) on the Beck Depression Inventory-II (BDI-II). Memory for depression-related information was assessed by (a) free-recall of a video depicting a personal experience narrative of the impact of depression on cognition and workplace performance; and (b) a paired-associates task linking depression-related cognitive symptoms to appropriate coping strategies.
Results: The results showed no overall difference in recall between the nap and waking condition. However, across the full sample of participants, there were significant positive correlations between total sleep time and paired associates recall, and slow wave sleep (SWS) percentage and story free recall performance. Unexpectedly, participants with elevated BDI-II scores exhibited better free-recall performance compared to those with low scores.
Conclusion: These results suggest that sleep, specifically SWS, may stabilize memories for clinically relevant information in populations with low and elevated depressive symptoms. The superior recall in participants with elevated-BDI scores may be related to the personal significance and stronger encoding of depression-related information. These observations raise the possibility that mnemonic deficits in depressed patients may be, at least in part, related to the type of information used to assess memory performance.
Keywords: memory, consolidation, depression, napping, sleep, slow-wave sleep
Introduction
A substantial body of experimental work suggests that sleep, whether overnight sleep or daytime naps, facilitates the consolidation of memories in numerous species, including humans.1–11 In humans, beneficial effects of sleep have been detected in tasks assessing non-declarative12–14 or declarative memory,14,15 including memories high in emotional valence.16,17
Several physiological mechanisms have been proposed to mediate the facilitatory impact of sleep on memory stabilization. Studies employing electroencephalogram (EEG) recordings have found correlations between recall performance and specific patterns of sleep-related oscillatory activity, particularly slow wave activity (SWA; delta and slow oscillations) and sleep spindles.13,18–22 In addition, changes in the release of neuromodulators and hormones also appear to contribute to the effects of sleep on memory strength, including lowered levels of acetylcholine23 and cortisol24,25 during the slow-wave sleep (SWS)-rich first half of nocturnal sleep. Together, the available evidence suggests that the oscillatory activity and neurochemical milieu present during sleep act in concert to support the synaptic consolidation of information encoded during preceding waking periods.
The positive impact of sleep on memory formation is well established for healthy individuals. However, much less is known about whether these effects are also present in special populations, such as those afflicted by neuropsychiatric illnesses. Whether sleep is able to support cognitive functions in these populations is a particularly important issue, given that significant sleep disturbances are a common symptom across neuropsychiatric and neurological conditions.26–32 However, it is largely unknown whether sleep in neuropsychiatry patients is able to provide the same benefits to cognition as those seen in healthy individuals.
Major depression constitutes a particularly prevalent neuropsychiatric condition.33 The limited available evidence suggests that, in clinically depressed (medicated) individuals, sleep (napping) can enhance the consolidation of both declarative and non-declarative memories, similar to the effects seen in non-depressed populations.34 Further, in participants exhibiting depressive symptoms, specific sleep stages appear to interact with the emotional valence of the material, such that the consolidation of neutral and negatively charged emotional information appears to benefit from SWS and rapid eye movement sleep (REM-S), respectively.35 However, more information is needed with regard to the role of sleep in supporting memory in depressive illnesses and other neuropsychiatric conditions.
With the present study, we examined whether a short daytime nap affects memory recall in participants who self-reported experiencing either clinically non-significant or elevated levels of depressive symptoms. Given the importance of emotional valence on consolidation processes during sleep,16,17,35 we were particularly interested in assessing memory for information that might carry personal and emotional significance to the participant population reporting depressive symptoms. Thus, a memory test containing clinically relevant, depression-related information was used to examine the effect of napping and wakefulness on subsequent recall performance.
We hypothesized that, regardless of the severity of depressive symptoms (BDI-II scores), participants would show better recall of information after a nap relative to the wake condition. In addition, given the high prevalence of attentional, encoding, and memory deficits in depression,36–42 we hypothesized that recall performance would be lower in individuals with higher BDI-II scores, regardless of the time point of testing (ie, pre- and post-condition).
Materials, Instruments, and Methods
Participants and Ethical Approval
This study was carried out in accordance with the Declaration of Helsinki (World Medical Association, 2013) and was reviewed and approved by the General Research Ethics Board (GREB) at Queen’s University in Kingston, Ontario, Canada. Informed, written consent was obtained from all participants prior to the commencement of the experimental procedures.
Participants (58 individuals; 49 females; age range 18 to 27 years; mean age = 24.4 years, SD = 2.2 years) were recruited through advertisements at Queen’s University and in the Kingston, Ontario community. The sample size is similar to or greater than that in previous studies detecting sleep-related enhancements of memory recall in human participants.12–14,16,21 Exclusion criteria were: a current or previous diagnosis of any sleep, psychological, or psychiatric disorders with the exception of depression; use of any prescription medication that could affect sleep, including antidepressant medication; and intermediate (14–21) BDI-II scores (see below). To be included in the study, participants were required to engage in regular napping during the daytime, defined as, on average, napping at least once a week for the past 2 months.
Instruments
Interviews with potential participants were conducted prior to the experiment to obtain informed consent, gather basic demographic information, information regarding napping habits (average duration and frequency) and typical overnight sleep quality, and to assess eligibility to participate (see above). Once eligibility was established, the following tests and questionnaires were administered:
The Wide Range Achievement Test 3 (WRAT-3): Word Reading Subtest43 and the Hopkins Verbal Learning Test-Revised44 (HVLT-R; Form #1) were used to assess estimated intellectual functioning and verbal learning and memory abilities. For the WRAT-3, participants were required to read a list of words out loud and were scored on their pronunciation of each word, based on the guidelines provided by the WRAT-3. For the HVLT-R, participants were read a list of 12 nouns, with four words drawn from three different categories, and asked to repeat as many nouns as possible over three trials.
The Beck Depression Inventory-II45 (BDI-II) was used to assess the severity of depressive symptoms and assign participants to the low- and elevated depressive symptom groups. The total BDI-II score relates to the severity of depressive symptoms as follows: a score of 0–13 indicates minimal depression; 14–19 indicates mild depression; 20–28 indicates moderate depression; and 29–63 indicates severe depression. For the purpose of the current study, the “low BDI-II group” was comprised of participants without a history of clinical depression and a BDI-II score of ≤13. The “elevated BDI-II group” consisted of participants who obtained a BDI-II score of ≥21 or had a diagnosis of clinical depression.
Video
A psychoeducational video (total length of 3:49 min) was created to assess memory for clinically relevant information related to cognitive deficits in depression. The video consisted of two components: (a) a didactic component (2:55 min) providing an introduction to the effects of depression on cognitive functioning, delivered by a registered clinical psychologist (one of the authors, C.R.B.) speaking directly to the viewer; (b) a personal experience part, delivered by an actress who describes her (fictional) experiences with depression and cognitive impairments in the context of her workplace (0:54 min). The personal experience portion of the video was designed to mimic the Story Learning task from the Neuropsychological Assessment Battery46 (NAB). A scoring rubric was developed to assess (the verbally reported) free recall of the personal experience component of the video. Consistent with the Story Learning Task from the NAB, points were given for both accurate recall of the details and the gist of the personal experience story.
Paired Associates Test
Paired associates recall was assessed by presenting participants with a list of ten cognitive processes or abilities known to be affected by depression, each paired with an associated and appropriate behavioural/cognitive coping strategy (“Working Memory – Chunking”; “Isolation – Group Activities”; “Long-Term Memory – Rehearsal”; Divided Attention - Multi-Tasking”; “Planning – Making Lists”; “Emotional Regulation – Breathing”; “Verbal Fluency – Mock Interviews”; “Self-Restraint - Mindfulness”; “Processing Speed – Computer Games”; “Lethargy – Sleep Hygiene”). Pairs were presented as PowerPoint slides (10 slides; 5 sec/slide) that contained both text and a corresponding voice-over recording. Recall was assessed by presenting (on PowerPoint slides) only the first component of each paired associates item (eg, “Planning”) and asking participants to verbally report the corresponding coping strategy (10 sec was allowed to generate each of the ten items). Verbal responses for both the free recall and paired associates task were voice-recorded for subsequent scoring (see below).
Polysomnography
For all participants, polysomnographic activity was recorded during the experimental intervention (60 min of wakefulness or napping opportunity). For the EEG, electrodes (Genuine Grass Gold Disc Electrodes; Natus Neurology, Ireland) were placed on the scalp in accordance with the International 10–20 system and using the following deviations: Fp1 against O1, C4 against O2; ground connections were placed on the left and right mastoid processes. Additional electrodes were placed near the eyes and the masseter to record the electrooculogram (EOG) and electromyogram (EMG), respectively. All signals were amplified (Grass P511 amplifiers, half-amplitude filters set at 0.3 Hz and 10 kHz), digitized (200 Hz; PowerLab 8/30 system running LabChart software, v. 8.1.11, ADInstruments, Toronto, Ont.), and stored for subsequent offline analysis (using LabChart software).
Procedure
All procedures were carried out over two separate appointments (typically 7 days between appointments; the maximal time between appointments was 14 days).
Appointment 1
Participants were given an introduction to the study and experimental procedures, and were asked for their written, informed consent. Subsequently, demographic and sleep-related information was collected, followed by the completion of the WRAT-3, the HVLT-R (Form #1), and the BDI-II. Finally, participants received a tour of the sleep laboratory to familiarize them with the surroundings in an effort to increase comfort if asked to nap during their second appointment. After the participant had left the laboratory, they were randomly assigned to either the napping or waking condition using a random number generator (in Microsoft Excel, v. 16.16.24).
Appointment 2
The second appointment was scheduled around each participant’s typical nap time. During this appointment, participants watched the psychoeducational video, immediately followed by a (pre-condition) free recall test for the content of the personal experience portion of the video. Participants were instructed to repeat the verbal content as completely and accurately as possible, using the same words as those in the video. Subsequently, participants watched the paired associates slides, again followed by an immediate recall test. All responses were recorded with an audio recorder for subsequent scoring purposes.
Next, participants were prepared for the EEG, EOG, and EMG recordings, informed of their experimental condition (nap or wake), and taken into the experimental room for the following 60 minutes. Participants assigned to the nap condition were asked to lie down and fall asleep, while those in the wake condition watched an episode of Planet Earth Season I (British Broadcasting Corporation, 2006) to prevent participants from falling asleep or actively rehearsing information from the memory tests.
Following the 60-minute period, participants returned to the main lab area, where all electrodes were removed, followed by a second round of recall tests (post-condition; typically starting around 10 min after the end of the experimental intervention; free recall of the personal story, followed by the paired associates test). Finally, participants were debriefed, given the opportunity to ask questions regarding the study, and received compensation (monetary or course credit, as per Departmental policies) for their time.
Data Scoring and Analysis
The voice recordings of all recall tests were independently scored by two researchers, both of whom were blind to the group assignment and experimental condition of the participants (inter-rater reliability scores of r = 0.97 and 0.96 for pre-condition and post-condition tests, respectively). For the free recall test, a scoring rubric (out of 42 points) was used, which followed the scoring for the Story Learning Task from the NAB,46 awarding points for accurately repeating the details of the personal experience portion of the video, as well as recalling the gist of the story. For the paired associates task (out of 10 points), a point was given for each correct coping strategy reported by the participant that matched the associated depression-related cognitive deficit.
Polysomnographic recordings were scored (in successive 30 s epochs) by an experienced rater and in accordance with the American Academy of Sleep Medicine Manual.47 Parameters included sleep onset latency, number of awakenings, total sleep time (TST), minutes spent in each sleep stage, and percent of total sleep duration spent in each sleep stage. Polysomnographic recordings for participants assigned to the wake condition were checked to ensure that participants did not fall asleep, in which case their data were excluded from the analyses.
For statistical analyses, independent samples t-tests were used to compare participants in the different experimental conditions with regard to demographic information, general cognitive performance, and depressive symptom severity. Mixed-model analyses of variance (ANOVA) were used to compare recall performance between the two participant groups (low- and elevated BDI-II scores), the two time-points of testing (pre- and post-condition), and the two experimental conditions (nap and wake). In addition, for participants assigned to the nap condition, Pearson’s correlations were computed to assess the relationship between recall performance and a range of sleep parameters (see above). All statistical analyses were performed using SPSS software (v. 25; IBM, Armonk, New York).
Results
Demographic Information and General Cognitive Performance
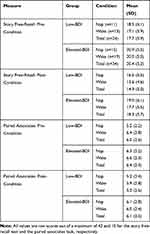
Demographic information, as well as self-reported napping habits and overnight sleep quality for participants with low and elevated depressive symptoms (BDI-II scores of ≤13 and ≥21, respectively), is shown in Table 1. Participants with elevated BDI-II scores reported more frequent napping, longer nap length, and worse sleep quality relative to those with low BDI-II scores. In addition, the elevated BDI-II group had a significantly lower proportion of male participants (6%) when compared to the low BDI-II group (29%).
 |
Table 1 Mean (Standard Deviation) Values for Participants’ Demographic and Sleep-Related Information |
Performance on the WRAT-3 and HVLT-R were similar for participants with low and elevated BDI-II scores and assigned to either the wake or nap condition (Table 2). Thus, estimates of intellectual functioning, as assessed by these tests, did not appear to be notably different between the two experimental groups and conditions. Participants in the low- and elevated BDI groups had mean BDI-II scores of 7.9 and 23.4, respectively, corresponding to a minimal and moderate level of depressive symptoms.45
 |
Table 2 Mean (Standard Deviation) Values for General Cognitive Assessments and BDI-II Scores |
Sleep Characteristics of Nappers
Participants assigned to the nap condition (n = 26) were given a 60-minute sleep opportunity in a quiet, private sleep room. During this time, EEG, EOG, and EMG activity was monitored online and stored for subsequent analysis and sleep stage scoring using AASM guidelines.47 Sleep characteristics are summarized in Table 3; as shown, participants spent the majority of time (about 21 minutes, or 48% of the TST) in stage 2 sleep. None of the participants entered REM-S during the 60-minute sleep opportunity. Interestingly, participants with elevated BDI-II scores took significantly longer to fall asleep compared to those in the low BDI-II group (14.7 vs 10.1 minutes; t(24) = −2.29, p = 0.03). None of the other sleep parameters differed significantly between the two BDI-II groups (all p’s > 0.7).
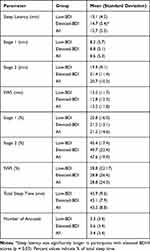 |
Table 3 Sleep Characteristics (Mean and Standard Deviation) for Participants with Low (n = 11) and Elevated (n = 15) BDI-II Scores Assigned to the Nap Condition |
Recall Performance
Recall performance was assessed at two time points: pre-condition, ie, immediately after the exposure to the psychoeducational video (personal story free recall) and the paired associates list; and post-condition, ie, after the 60-min experimental condition (nap vs wake). The raw recall scores for both tests are summarized in Table 4.
As shown in Figure 1A, for the story free recall test, recall scores for both groups and experimental conditions were higher for the pre-condition test compared to the post-condition test, indicative of a general decline in memory recall over the 60 min-period allowed for the experimental intervention (main effect of time, F(1, 57) = 35.4, p < 0.01). However, contrary to the widely reported beneficial effects of napping on memory consolidation, there was no significant main effect of experimental condition (nap vs wake; F(1, 54) = 1.3, p = 0.26), and no significant two- or three-way interactions between any of the variables (time, group, and condition; all p’s > 0.25). Interestingly, and in contrast to our hypothesis, participants in the elevated BDI group showed better recall performance across time and conditions than participants in the low-BDI group (Figure 1A; main effect of group, F(1, 54) = 4.4, p = 0.04).
 |
Figure 1 Recall performance (mean scores out of 42 and 10 in (A and B), respectively) in participants with low (≤13) and elevated (≥21) scores on the Beck Depression Inventory-II (BDI) before and after the experimental condition (Nap vs Wake). (A) For the story free recall test, recall scores declined from pre- to post-condition testing for all participants. Further, the elevated BDI-group had higher recall scores than the low-BDI group. (B) For the paired associates test, recall performance also declined over time (pre- to post-condition). Error bars are omitted for clarity; standard deviations and group sizes are reported in Table 4. |
For the paired associates task (see Table 4 for raw scores), recall performance also declined over time (Figure 1B; main effect of time, F(1, 54) = 9.14, p < 0.01), but there were no main effects of group or condition, and none of the two- or three-way interaction effects reached statistical significance (all p’s > 0.15).
Correlations Between Sleep Parameters, Recall Performance, and BDI-II Scores
In order to perform correlational analyses between memory recall and sleep variables, all recall scores were converted to difference scores (pre-condition scores minus post-condition scores, with higher values indicating greater memory decay and negative values indicative of enhanced recall over the 60-min experimental intervention period).
For the story free recall test, there was a small negative correlation between memory decay over time and TST, indicative of a trend of reduced forgetting with longer TST (Figure 2A). However, this correlation did not reach statistical significance (r = −0.21, p = 0.31). It is noteworthy that the trend toward a (non-significant) negative association between TST and forgetting was also present in both groups of participants (ie, low- and elevated BDI scores; data not shown).
For the paired associates task, the analysis revealed a significant negative correlation between TST and memory decay across the entire sample (r = −0.45, p = 0.02), indicating that longer TST was associated with reduced memory decay (Figure 2B). Again, the negative association between TST and memory decay was also notable in both participant groups, even though these moderately sized correlations did not reach statistical significance in these smaller samples sizes (low BDI: r = −0.43, p = 0.19; elevated BDI: r = −0.48, p = 0.07).
For participants who entered SWS (n = 19) during the 60-min sleep opportunity, there was a significant negative correlation between memory decay on the story free recall test and the percentage of TST spent in SWS (% SWS) (Figure 3; r = −0.52, p = 0.02); the correlation between % SWS and memory decay on the paired associates test, while also negative, did not reach statistical significance (r = −0.35, p = 0.14). Further exploratory analyses showed that, in nappers who entered SWS, there were significant negative correlations between TST and memory decay on the story free-recall test (r = −0.50, p = 0.03) and the paired associates task (r = −0.51, p = 0.03). In contrast, there were no significant associations between TST and memory decay in nappers who did not enter SWS (free-recall test: r = −0.02, p = 0.98; paired associates task: r = −0.38, p = 0.4).
A final set of analyses examined possible associations of memory decay with BDI-II scores. There was no significant correlation between BDI-II scores and memory decay for the story free-recall test (r = −0.05, p = 0.7), but the (negative) correlation between these variables approached significance for the paired associates task (r = −0.26, p = 0.052).
Discussion
The present investigation examined the effects of napping on memory stability in groups of participants with low (≤13) and elevated (≥21) scores on the BDI-II, a well-established measure of depressive symptom severity.45 In contrast to our hypothesis, there was no significant overall difference in memory recall between participants allowed to nap compared to those who stayed awake during the 60-min intervention period. However, correlational analyses revealed that, among nappers, there were significant negative correlations between memory decay and TST, as well as the percent time spent in SWS. These observations indicate that longer sleep, particularly SWS, during the 60-min sleep opportunity was associated with better recall of information, consistent with our predictions and the reported beneficial effects of napping on measures of declarative memory consolidation.48–50
Participants with elevated BDI-II scores exhibited better recall performance than those with low scores across both time points of testing (ie, pre- and post-condition). We were surprised by this set of results, given the consistent finding of cognitive and memory deficits in individuals with depressive symptoms,36–42,51 with symptom severity relating to the degree of cognitive dysfunction.52 In this regard, it is important to note that the material used to assess memory was related to the topic of depressive illness and some of the associated cognitive impairments. As such, it is possible that this content of our memory tests was particularly relevant, personally meaningful, or emotionally engaging to individuals with a higher depressive symptom severity. Memories for emotionally charged material tend to be more accurate and stable over time, due to the activation of the sympathetic nervous system, amygdala, and central cholinergic system, among others.53–58 This concerted hormonal and neural activation during periods of emotional arousal reinforces the induction and maintenance of synaptic plasticity in relevant memory networks of the brain,59,60 thus enhancing the recall of information at later time points.57,61,62
It is also noteworthy that recall performance in the elevated BDI-II group was superior for both the pre- (ie, immediately after the presentation of the material) and post-condition tests. Thus, it appears that the initial encoding and/or storage in working memory was already more effective in those with higher depressive symptoms. The observation that both BDI-II groups exhibited similar performance on the estimates of intellectual functioning and verbal learning and memory (HVLT-R and WRAT-3) suggests that the superior recall in the elevated BDI-II group may not be due to gross differences in general cognitive abilities between the two participant groups. Together, these results raise the possibility that the nature of experimental stimuli (eg, neutral vs emotionally engaging) can influence cognitive performance. Consequently, the severity of cognitive deficits seen in depression may be, at least in part, related to the specific tests and materials used to assess cognitive functioning, a hypothesis that should be explored in more detail in future studies.
For both memory measures (story free recall and paired associates) used in the present study, recall performance showed a clear decline over the two time-points of testing (pre- and post-condition). Thus, the encoded information appeared to be prone to decay, possibly due, at least in part, to interference by incoming sensory information and ongoing memory encoding.63 Such interference effects should be reduced by napping, leading to less forgetting when compared to periods of active wakefulness.63 Thus, the absence of a significant difference in recall accuracy between the nap and wake condition was unexpected. However, for several of the memory measures, recall performance was significantly correlated with TST (paired associates recall) and % SWS (story free recall). Thus, sleep, and specifically SWS, appears to reduce the decay of declarative memory, including the type of clinically relevant information probed in the current investigation. The absence of an overall benefit of napping on recall may be due to the fact that a substantial portion of nappers did not obtain sufficient SWS to allow for the effective memory stabilization over the 60-minute period of sleep opportunity.
The observations that napping and SWS appeared to protect against declarative memory decay are consistent with previous work. In line with the current investigation, Tucker et al allowed participants about 1 hour of napping opportunity, which contained a mix of stages 1, 2, and SWS.50 Declarative memory recall, as assessed by a paired associates task, was enhanced in nappers compared to the wake condition, an effect that correlated positively with the amount of SWS obtained during the nap. Similarly, Lau et al noted a significant correlation between SWS obtained during a daytime nap and measures of associative and relational memory, two specific aspects of declarative memory.49 Lahl et al also noted enhanced recall of words over a 60-min nap opportunity compared to waking, even though this effect did not correlate with the amount of SWS.48 These studies, together with the results presented here, offer support for the notion that the stability of declarative memories can benefit from relatively short daytime naps, particularly when these naps contain a sufficient amount of SWS (even though other sleep stages, including stage 2 sleep, have also been implicated in nap-induced declarative memory consolidation21).
It is important to acknowledge that, despite the large body of work linking sleep and memory, the precise contributions of sleep in the consolidation process remain somewhat unclear. For example, recent work indicates that quiet wakefulness (eg, immobility, resting, meditation) can also facilitate memory stabilization,64–67 leading to the suggestion that sleep, rather than playing a unique or critical role, is one of the several states of relative behavioral and/or cognitive inactivity that all support memory consolidation.63,68 In the present study, participants in the wake condition watched a nature documentary (Planet Earth) during the 60-min intervention period. A nature documentary was chosen with the aim of presenting material that was not personally engaging or emotionally arousing, while still being able to prevent sleep and the conscious rehearsal of information. However, given the verbal content and rich visual imagery, movies of this type will certainly engage numerous perceptual and cognitive brain networks. As such, it remains to be determined whether, for the memory tests used here, states of quiet wakefulness can provide beneficial effects on memory recall, similar to those seen with sleep.
The large majority of experimental work on sleep and memory has been conducted in healthy populations. In contrast, relatively little is known with regard to the role of sleep in populations suffering from psychiatric conditions, particularly depressive symptoms. Some reports have detected impairments in sleep-related consolidation of implicit memories (motor skill learning) in medicated patients diagnosed with major depression.69,70 Thus, it is possible that sleep loses its effectiveness to support the consolidation of some types of learning in clinically depressed individuals; of course, the possible role of various confounding factors, such as the medication status, also needs to be considered.
Recently, Harrington et al compared overnight memory consolidation for neutral and emotionally valent images in individuals with low (0–6) and higher (14–27) BDI-II scores.35 Given that their sample did not include medicated patients, these researchers were able to focus more directly on depressive symptom severity in the absence of effects related to pharmacological treatments. Interestingly, sleep produced a greater enhancement of recognition performance in participants with higher BDI scores compared to the low-BDI group.35 Further, recognition of neutral and negative images benefitted mostly from early (mostly SWS) and later (mostly REM-S) nocturnal sleep, respectively, consistent with the proposed role of REM-S in consolidating emotionally charged information.16,17 We observed that the correlations between memory strength and TST and % SWS generally held across the entire sample, even if the separate analyses for low- and elevated BDI groups often failed to reach statistical significance, likely due to the small sample sizes. In other words, participants with elevated depressive symptoms appeared to benefit from sleep, similar to individuals with low symptom levels. Thus, the findings of Harrington et al,35 together with the present results, suggest that sleep continues to support some consolidation processes in individuals with depressive symptoms; whether this holds true for those with more severe forms of (clinical) depression is currently unknown.
The findings reported here are preliminary and require further investigations for their confirmation, and to address some of the shortcomings of the current study. Of note, the post-condition memory test was conducted relatively soon (~10 min) after waking and the removal of the EEG electrodes. As such, we were unable to assess whether napping promotes memory retention over longer time intervals. We also cannot rule out the acute impact of varying levels of alertness on post-condition performance, which, in nappers, may range from increased alertness to the existence of sleep inertia.71 It is noteworthy that longer TST and SWS were related to better recall, suggesting only a limited influence of sleep inertia. Nevertheless, it is possible that, by allowing more time between the end of sleep and the recall test, sleep inertia would have been reduced further, potentially leading to a greater boost in recall performance; additional work is required to examine these and related questions. Also, due to the relatively short nap opportunity provided, none of the participants entered REM-S. As such, we were unable to examine the influence of REM-S on the consolidation of depression-related information. Given the suggested role of REM-S in the consolidation of emotional material,16,17,35 future work should employ longer nap times or overnight sleep to assess if REM-S enhances the recall of personally relevant, depression-related information.
In summary, the experiments summarized here suggest that a relatively short daytime nap can enhance the recall for clinically relevant, depression-related information in individuals with low and elevated depressive symptoms. Both TST and the amount of SWS obtained during the nap appear to protect these declarative memories against excessive decay. Future work should assess the impact of sleep on memory consolidation in other clinical samples and different stages of disease progression, such as an acute depressive episode and various stages of remission. In addition, it will be of interest to examine whether similar effects of sleep on memory recall are seen in older patients afflicted by depression, or a combination of mood disorders and age-related cognitive decline. It is conceivable that sleep may play a particularly important, protective role in such populations, but future work is required to critically evaluate this hypothesis.
Cognitive dysfunction is now recognized as a core feature of major mood disorders.36–42,51 Importantly, attentional and memory biases favoring the encoding and storage of negatively charged emotional experiences likely contribute to the vulnerability to, as well as the persistence and relapse of depressive symptoms.72–74 As such, sleep disturbances in depression are positioned to contribute to a number of critical aspects of the disease by: (a) impairing cognitive functioning; (b) impairing the processing and evaluation of emotional experiences;75 and (c) favoring the encoding and consolidation of negative experiences over positive or neutral events (Bovy et al, 2019; Walker and van der Helm, 2009).76,77 The wide-ranging interactions of sleep with core features of depression are consistent with the view that interventions targeting sleep hold significant promise in improving cognitive and emotional capacities in those afflicted by mood disorders.
Disclosure
Dr Christopher R Bowie reports grants from Takeda; personal fees from Lundbeck, Pfizer, and Boehringer Ingelheim, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Buzsáki G. Memory consolidation during sleep: a neurophysiological perspective. J Sleep Res. 1998;7(Suppl. S1):17–23. doi:10.1046/j.1365-2869.7.s1.3.x
2. Chatburn A, Lushington K, Kohler MJ. Complex associative memory processing and sleep: a systematic review and meta-analysis of behavioural evidence and underlying EEG mechanisms. Neurosci Biobehav Rev. 2014;47:646–655. doi:10.1016/j.neubiorev.2014.10.018
3. Diekelmann S. Sleep for cognitive enhancement. Front Syst Neurosci. 2014;8:46. doi:10.3389/fnsys.2014.00046
4. King BR, Hoedlmoser K, Hirschauer F, Dolfen N, Albouy G. Sleeping on the motor engram: the multifaceted nature of sleep-related motor memory consolidation. Neurosci Biobehav Rev. 2017;80:1–22.
5. Klinzing JG, Niethard N, Born J. Mechanisms of systems memory consolidation during sleep. Nat Neurosci. 2019;22:1598–1610.
6. Rasch B, Born J. About sleep’s role in memory. Physiol Rev. 2013;93:681–766.
7. Sousouri G, Huber R. Sleep and plasticity. In: Dringenberg HC, editor. Handbook of Sleep Research. San Diego: Academic Press/Elsevier; 2019:425–442.
8. Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437:1272–1287.
9. Van Den Berg NH, Benoit A, Toor B, Fogel S. Sleep stages and neural oscillations: a window into sleep’s role in memory consolidation and cognitive abilities. In: Dringenberg HC, editor. Handbook of Sleep Research. San Diego: Academic Press/Elsevier; 2019:455–470.
10. Vorster AP, Born J. Sleep and memory in mammals, birds and invertebrates. Neurosci Biobehav Rev. 2015;50:103–119. doi:10.1016/j.neubiorev.2014.09.020
11. Walker MP, Stickgold R. Sleep-dependent learning and memory consolidation. Neuron. 2004;44(1):121–133. doi:10.1016/j.neuron.2004.08.031
12. Fischer S, Hallschmid M, Elsner AL, Born J. Sleep forms memory for finger skills. Proc Natl Acad Sci. 2002;99(18):11987–11991. doi:10.1073/pnas.182178199
13. Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430(6995):78–81. doi:10.1038/nature02663
14. Plihal W, Born J. Effects of early and late nocturnal sleep on declarative and procedural memory. J Cognitive Neurosci. 1997;9(4):534–547. doi:10.1162/jocn.1997.9.4.534
15. Gais S. Declarative memory consolidation: mechanisms acting during human sleep. Learn Mem. 2004a;11(6):679–685. doi:10.1101/lm.80504
16. Wagner U. Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Learn Mem. 2001;8(2):112–119. doi:10.1101/lm.36801
17. Wagner U, Degirmenci M, Drosopoulos S, Perras B, Born J. Effects of cortisol suppression on sleep-associated consolidation of neutral and emotional memory. Biol Psychiatry. 2005;58(11):885–893. doi:10.1016/j.biopsych.2005.05.008
18. Cox R, Hofman WF, Talamini LM. Involvement of spindles in memory consolidation is slow wave sleep-specific. Learn Mem. 2012;19(7):264–267. doi:10.1101/lm.026252.112
19. Marshall L, Helgadóttir H, Mölle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444(7119):610–613. doi:10.1038/nature05278
20. Perrault AA, Khani A, Quairiaux C, et al. Whole-night continuous rocking entrains spontaneous neural oscillations with benefits for sleep and memory. Curr Biol. 2019;29(3):402–411. doi:10.1016/j.cub.2018.12.028
21. Piosczyk H, Holz J, Feige B, et al. The effect of sleep-specific brain activity versus reduced stimulus interference on declarative memory consolidation. J Sleep Res. 2013;22(4):406–413. doi:10.1111/jsr.12033
22. Tamaki M, Huang T-R, Yotsumoto Y, et al. Enhanced spontaneous oscillations in the supplementary motor area are associated with sleep-dependent offline learning of finger-tapping motor-sequence task. J Neurosci. 2013;33(34):13894–13902. doi:10.1523/JNEUROSCI.1198-13.2013
23. Gais S, Born J. Low acetylcholine during slow-wave sleep is critical for declarative memory consolidation. Proc Natl Acad Sci. 2004b;101(7):2140–2144. doi:10.1073/pnas.0305404101
24. Plihal W, Born J. Memory consolidation in human sleep depends on inhibition of glucocorticoid release. NeuroReport. 1999;10(13):2741–2747. doi:10.1097/00001756-199909090-00009
25. Wagner U, Born J. Memory consolidation during sleep: interactive effects of sleep stages and HPA regulation. Stress. 2008;11(1):28–41. doi:10.1080/10253890701408822
26. Gagnon JP, Lafrenière A, Rauchs G, Petit D, Carrier J. Sleep in normal aging, Alzheimer’s disease, and mild cognitive impairment. In: Dringenberg HC, editor. Handbook of Sleep Research. San Diego: Academic Press/Elsevier; 2019:677–692.
27. Jagannath A, Peirson SN, Foster RG. Sleep and circadian rhythm disruption in neuropsychiatric illness. Curr Opin Neurobiol. 2013;23(5):888–894. doi:10.1016/j.conb.2013.03.008
28. Moretto U, Palagini L. Sleep in major depression. In: Dringenberg HC, editor. Handbook of Sleep Research. San Diego: Academic Press/Elsevier; 2019:693–706.
29. Murphy MJ, Peterson MJ. Sleep disturbances in depression. Sleep Med Clin. 2015;10(1):17–23. doi:10.1016/j.jsmc.2014.11.009
30. Tesler N, Gerstenberg M, Huber R. Developmental changes in sleep and their relationships to psychiatric illnesses. Curr Opinion Psychiatry. 2013;26(6):572–579. doi:10.1097/YCO.0b013e328365a335
31. Vadnie CA, McClung CA. Circadian rhythm disturbances in mood disorders: insights into the role of the suprachiasmatic nucleus. Neural Plast. 2017;2017:1–28. doi:10.1155/2017/1504507
32. Wulff K, Gatti S, Wettstein JG, Foster RG. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat Rev Neurosci. 2010;11(8):589–599. doi:10.1038/nrn2868
33. Knoll A, MacLennan RN. Prevalence and correlates of depression in Canada: findings from the Canadian Community Health Survey.. Can Psychol. 2017;58(2):116–123. doi:10.1037/cap0000103
34. Seeck-Hirschner M, Baier PC, Sever S, Buschbacher A, Aldenhoff JB, Göder R. Effects of daytime naps on procedural and declarative memory in patients with schizophrenia. J Psychiatr Res. 2010;44(1):42–47. doi:10.1016/j.jpsychires.2009.05.008
35. Harrington MO, Johnson JM, Croom HE, Pennington K, Durrant SJ. The influence of REM sleep and SWS on emotional memory consolidation in participants reporting depressive symptoms. Cortex. 2018;99:281–295. doi:10.1016/j.cortex.2017.12.004
36. Austin M-P, Mitchell P, Goodwin GM. Cognitive deficits in depression: possible implications for functional neuropathology. Br J Psychiatry. 2001;178(3):200–206. doi:10.1192/bjp.178.3.200
37. Bearden CE, Glahn DC, Monkul ES, et al. Patterns of memory impairment in bipolar disorder and unipolar major depression. Psychiatry Res. 2006;142(2–3):139–150. doi:10.1016/j.psychres.2005.08.010
38. Burt DB, Zembar MJ, Niederehe G. Depression and memory impairment: a meta-analysis of the association, its pattern, and specificity.. Psychol Bull. 1995;117(2):285–305. doi:10.1037/0033-2909.117.2.285
39. Golinkoff M, Sweeney JA. Cognitive impairments in depression. J Affect Disord. 1989;17(2):105–112. doi:10.1016/0165-0327(89)90032-3
40. Lam RW, Kennedy SH, McIntyre RS, Khullar A. Cognitive dysfunction in major depressive disorder: effects on psychosocial functioning and implications for treatment. Can J Psychiatry. 2014;59:649–654.
41. Marazziti D, Consoli G, Picchetti M, Carlini M, Faravelli L. Cognitive impairment in major depression. Eur J Pharmacol. 2010;626:83–86.
42. Reppermund S, Ising M, Lucae S, Zihl J. Cognitive impairment in unipolar depression is persistent and non-specific: further evidence for the final common pathway disorder hypothesis. Psychol Med. 2009;39:603–614.
43. Wilkinson GS, Robertson GJ. Wide Range Achievement Test (WRAT3). Lutz: Psychological Assessment Resources; 2006.
44. Shapiro AM, Benedict RH, Schretlen D, Brandt J. Construct and concurrent validity of the Hopkins Verbal Learning Test–revised. Clin Neuropsychol. 1999;13:348–358.
45. Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, Texas: Psychological Corporation; 1996.
46. Stern RA, White T. Neuropsychological Assessment Battery Administration, Scoring, and Interpretation Manual. Lutz: Psychological Assessment Resources; 2003.
47. Berry RB, Brooks R, Gamaldo CE. For the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 2.2. Darien, Illinois: American Academy of Sleep Medicine; 2015.
48. Lahl O, Wispel C, Willigens B, Pietrowsky R. An ultra short episode of sleep is sufficient to promote declarative memory performance. J Sleep Res. 2008;17:3–10.
49. Lau H, Rucker MA, Fishbein W. Daytime napping: effects on human direct associative and relational memory. Neurobiol Lean Mem. 2010;93:554–560.
50. Tucker MA, Hirota Y, Wamsley EJ, Lau H, Chaklader A, Fishbein W. A daytime nap containing solely non-REM sleep enhances declarative but not procedural memory. Neurobiol Learn Mem. 2006;86:241–247.
51. Rock PL, Roiser JP, Riedel WJ, Blackwell AD. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol Med. 2014;44:2029–2040.
52. McDermott LM, Ebmeier KP. A meta-analysis of depression severity and cognitive function. J Affect Disord. 2009;119:1–8.
53. Dolcos F, LaBar KS, Cabeza R. Remembering one year later: role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proc Natl Acad Sci. 2005;102:2626–2631.
54. Hamann SB, Ely TD, Grafton ST, Kilts CD. Amygdala activity related to enhanced memory for pleasant and aversive stimuli. Nat Neurosci. 1999;2:289–293.
55. Isenberg N, Silbersweig D, Engelien A, et al. Linguistic threat activates the human amygdala. Proc Natl Acad Sci. 1999;96:10456–10459.
56. Kensinger EA, Corkin S. Memory enhancement for emotional words: are emotional words more vividly remembered than neutral words? Mem Cognit. 2003;31:1169–1180.
57. McGaugh JL. Memory consolidation and the amygdala: a systems perspective. Trends Neurosci. 2002;25:456–461.
58. Packard MG, Cahill L, McGaugh JL. Amygdala modulation of hippocampal-dependent and caudate nucleus-dependent memory processes. Proc Natl Acad Sci USA. 1995;91:8477–8481.
59. Akirav I, Richter-Levin G. Priming stimulation in the basolateral amygdala modulates synaptic plasticity in the rat dentate gyrus. Neurosci Lett. 1999;270:83–86.
60. Dringenberg HC, Oliveira D, Habib D. Predator (cat hair)-induced enhancement of hippocampal long-term potentiation in rats: involvement of acetylcholine. Learn Mem. 2008;15:112–116.
61. LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nat Rev Neurosci. 2006;7:54–64.
62. McGaugh JL. Memory - a century of consolidation. Science. 2000;287:248–251.
63. Mednick SC, Cai DJ, Shuman T, Anagnostarasm S, Wixted J. An opportunistic theory of cellular and systems consolidation. Trends Neurosci. 2011;34:504–514.
64. Brokaw K, Tishler W, Manceor S, et al. Resting state EEG correlates of memory consolidation. Neurobiol Learn Mem. 2016;130:17–25.
65. Deuker L, Olligs J, Fell J, et al. Memory consolidation by replay of stimulus-specific neural activity. J Neurosci. 2013;33:19373–19383.
66. Gottselig JM, Hofer-Tinguely G, Borbély AA, et al. Sleep and rest facilitate auditory learning. Neuroscience. 2004;127:557–561.
67. Mednick SC, Makovski T, Cai DJ, Jiang YV. Sleep and rest facilitate implicit memory in a visual search task. Vision Res. 2009;49:2557–2565.
68. Dringenberg HC. Sleep and memory consolidation: conceptual and methodological challenges. In: Dringenberg HC, editor. Handbook of Sleep Research. San Diego: Academic Press/Elsevier; 2019:489–501.
69. Dresler M, Kluge M, Genzel L, Schüssler P, Steiger A. Impaired off-line memory consolidation in depression. Eur Neuropharmacol. 2010;20:553–561.
70. Nishida M, Nakashima Y, Nishikawa T. Slow sleep spindle and procedural memory consolidation in patients with major depressive disorder. Nat Sci Sleep. 2016;8:63–72.
71. Wertz AT, Ronda JM, Czeisler CA, Wright KP. Effects of sleep inertia on cognition. JAMA. 2006;295:159–164.
72. Baert S, De Raedt R, Schacht R, Koster EH. Attentional bias training in depression: therapeutic effects depend on depression severity. J Behav Ther Exp Psychiatry. 2010;41:265–274.
73. Harrington MO, Pennington K, Durrant SJ. The ‘affect tagging and consolidation’ (ATaC) model of depression vulnerability. Neurobiol Learn Mem. 2017;140:43–51.
74. Gotlib IH, Joormann J. Cognition and depression: current status and future directions. Ann Rev of Clin Psychol. 2010;6:285–312.
75. Cote KA, Lustig KA, MacDonald KJ. The role of sleep in processing emotional information. In: Dringenberg HC, editor. Handbook of Sleep Research. San Diego: Academic Press/Elsevier; 2019:505–518.
76. Bovy L, Tendolkar I, Fernández G, Dresler M. Sleep emotional memory, and depression. In: Dringenberg HC, editor. Handbook of Sleep Research. San Diego: Academic Press/Elsevier; 2019:519–531.
77. Walker MP. van der Helm E. Overnight therapy? The role of sleep in emotional brain processing. Psychol Bull. 2009;135:731–748.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.