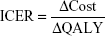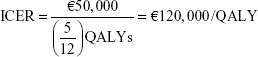Back to Journals » Patient Preference and Adherence » Volume 10
Do new cancer drugs offer good value for money? The perspectives of oncologists, health care policy makers, patients, and the general population
Authors Dilla T, Lizan L, Paz S, Garrido P , Avendaño C, Cruz-Hernandez JJ, Espinosa J, Sacristan JA
Received 4 August 2015
Accepted for publication 15 October 2015
Published 18 December 2015 Volume 2016:10 Pages 1—7
DOI https://doi.org/10.2147/PPA.S93760
Checked for plagiarism Yes
Editor who approved publication: Dr Johnny Chen
Tatiana Dilla,1 Luís Lizan,2 Silvia Paz,2 Pilar Garrido,3 Cristina Avendaño,4 Juan J Cruz-Hernández,5 Javier Espinosa,6 José A Sacristán1
1Medical Department, Lilly, Madrid, 2Outcomes’10, Jaime I University, Castellón, 3Medical Oncology Department, University Hospital Ramon y Cajal, Madrid, 4Clinical Pharmacology Department, Puerta de Hierro-Majadahonda Hospital, Madrid, 5Salamanca Institute for Biomedical Research, University Hospital of Salamanca, Salamanca, 6Medical Oncology Department, General Hospital Ciudad Real, Ciudad Real, Spain
Background: In oncology, establishing the value of new cancer treatments is challenging. A clear definition of the different perspectives regarding the drivers of innovation in oncology is required to enable new cancer treatments to be properly rewarded for the value they create. The aim of this study was to analyze the views of oncologists, health care policy makers, patients, and the general population regarding the value of new cancer treatments.
Methods: An exploratory and qualitative study was conducted through structured interviews to assess participants’ attitudes toward cost and outcomes of cancer drugs. First, the participants were asked to indicate the minimum survival benefit that a new treatment should have to be funded by the Spanish National Health System (NHS). Second, the participants were requested to state the highest cost that the NHS could afford for a medication that increases a patient’s quality of life (QoL) by twofold with no changes in survival. The responses were used to calculate incremental cost-effectiveness ratios (ICERs).
Results: The minimum improvement in patient survival means that justified inclusions into the NHS were 5.7, 8.2, 9.1, and 10.4 months, which implied different ICERs for oncologists (€106,000/quality-adjusted life year [QALY]), patients (€73,520/QALY), the general population (€66,074/QALY), and health care policy makers (€57,471/QALY), respectively. The costs stated in the QoL-enhancing scenario were €33,167, €30,200, €26,000, and €17,040, which resulted in ICERs of €82,917/QALY for patients, €75,500/QALY for the general population, €65,000/QALY for oncologists, and €42,600/QALY for health care policy makers, respectively.
Conclusion: All estimated ICER values were higher than the thresholds previously described in the literature. Oncologists most valued gains in survival, whereas patients assigned a higher monetary value to treatments that enhanced QoL. Health care policy makers were less likely to pay more for therapeutic improvements compared to the remaining participants.
Keywords: oncology, cost, cost-effectiveness, cost-effectiveness threshold, ICER, clinically meaningful outcomes, Spain
Background
Cancer is one of the leading causes of mortality and morbidity worldwide, contributing to 8.2 million deaths in 20121 and an estimated 169.3 million years of healthy life lost.2 Despite the considerable burden of this disease, important advances in cancer prevention, early diagnosis, screening, and treatment have reduced the overall mortality associated with this disease. Remarkable progress toward prolonging survival and reducing adverse events has been achieved through pharmaceutical treatments that can be translated into quality of life (QoL) improvements and potential cost savings.3,4
Improving health and controlling rising health care costs are top priorities for most health care systems. In the current health care environment, which is driven by cost-containment measures, new cancer treatments that provide lifespan extension and/or QoL improvement have raised concerns regarding whether National Health Systems (NHS) can afford these therapeutic advances. The cost-effectiveness ratios of new cancer treatments have been scrutinized, given the trend of increasing costs of recently approved cancer drugs.5
Knowledge regarding cost-effectiveness helps to establish the monetary value of new treatments and influence the decisions of oncologists and health policy makers. In a cost-effectiveness analysis, the additional consumption of medical resources is divided by the health benefits (eg, quality-adjusted life years [QALYs]) gained from health care interventions to calculate the incremental cost-effectiveness ratio (ICER). In general, an intervention is considered cost-effective if the ICER (cost per QALY) is below a predetermined threshold. For instance, common thresholds used in reimbursement and coverage decisions are £20,000–£30,000/QALY for the UK,6 $50,000/QALY for the USA,7 and €30,000/QALY for Spain.8
Decisions regarding rationing and allocating scarce health care resources should reflect the society’s opinion regarding willingness to pay for the value that the interventions produce.9 In oncology, establishing the value of new cancer treatments is challenging. Although some recent advances in defining clinically meaningful outcomes for some types of cancer have been made,10 a clear definition of the different perspectives regarding the drivers of innovation in oncology is required to enable new cancer treatments to be properly rewarded for the value they create.
Thus far, few studies have analyzed the views of doctors and/or health care policy makers regarding the willingness to pay for new cancer treatments based on the analysis of their potential health benefits.11–14 Some studies have determined the implicit ICERs set by oncologists to determine whether a new treatment is efficient.15–18 To our knowledge, this study is the first to incorporate the perceptions of costs and outcomes for other agents that may influence the decision-making process and that also represent the interests of society as a whole.
Spain’s NHS represents a publicly funded system that offers universal public health care coverage to Spanish citizens. Most oncology drugs are administered at hospitals and provided free of charge to patients. We conducted an exploratory and qualitative study to describe the attitudes of oncologists, health care policy makers, patients, and the general population toward costs and outcomes of cancer drugs in Spain to understand their perceptions regarding the costs and value of new cancer agents.
Methods
Study participants
All study participants were selected using a nonprobabilistic sampling method. Oncologists with a work experience >10 years and working for the Spanish NHS were invited to participate in the study. Health care policy makers with at least a single experience of political and legislative and local, regional, or national activity were contacted. Patient contact was made through local cancer associations and through the Spanish Cancer Federation. All participants who were invited to participate in the study expressed a clear interest in the subject of the study. In order to obtain the number of participants required within the established period and to assure that all participants were able to understand the questionnaire and willing to participate in the study, a convenience sample of the general population was used. This sample included employees of technological companies, research institutes, universities, and governmental institutions.
All participants were assured of their anonymity and confidentiality, and no incentives were offered to any of the participants for completing the questionnaire. This study followed the principles of the Declaration of Helsinki. Given the nature of the study it did not require an ethics committee approval.
Structured interview
Two hypothetical decision-making scenarios frequently presented in oncology clinical practice were included in the structured interview: a life-prolonging scenario (scenario 1) and a QoL-enhancing scenario (scenario 2). Illustrative vignettes were used for both scenarios in order to facilitate the understanding of each question. During the interview, general information about the respondents was also collected.
The structured interview was carried out between December 2013 and February 2014.
Description of the scenarios
To allow comparison with findings from other studies, the life-prolonging scenario followed an approach similar to that described by Nadler et al.15 Participants had to address a hypothetical situation in which a new drug for metastatic lung cancer had an additional cost of €50,000 per year compared to the standard treatment. Specifically, the standard treatment had a cost of €25,000, and the new treatment had a cost of €75,000; both treatments had the same safety profile. The standard treatment would provide a 1-year survival rate without changing the health-related QoL. Then, the respondents were asked to identify the minimum survival benefit that the new treatment should provide to be funded by the NHS. Survival benefits were presented in a list that included the following options: 1 day, 1 month, 2–4 months, 4–6 months, 9–12 months, or >12 months.
In the QoL-enhancing scenario, respondents had to decide regarding a new treatment for metastatic lung cancer that improved the QoL by twofold, from 40 to 80 (on a scale from 0 to 100), compared to the standard treatment. Both treatments would provide the same efficacy (1 year), and the annual cost was €25,000 for the standard treatment. Participants were asked to establish the additional cost that the new treatment should have to be funded by the NHS. Selections were made from a list that contained the following values: €0, up to €2,000/year, up to €4,000/year, up to €6,000/year, up to €10,000/year, up to €20,000/year, up to €50,000/year, and >€50,000/year.
Data analysis
In order to understand the different perceptions regarding the value and benefit of a new cancer drug considered by oncologists, health care policy makers, patients, and the general population, the ICER implied by each participant in every hypothetical scenario was calculated.
The results were analyzed in a descriptive form. Data analysis was performed using SPSS v.19 software. The ICER was calculated for each respondent using the following formula:
|
|
The following is a hypothetical example for the life-prolonging scenario (scenario 1):
ΔCost = €50,000 per year (€75,000 for the new treatment - €25,000 for the standard treatment) | (2) |
ΔQALY = (life expectancy [in years] * QoL) for the new treatment - (life expectancy [in years] * QoL) for the standard treatment. For the life expectancy calculation of the QALY term, the midpoint of the selected survival benefit range was used and converted into years. No calculations were made for the QoL component of the QALY term because the QoL in the scenario presented did not change, and as reported previously by Nadler et al,15 quality adjustment =1 when there was no change in QoL.
For instance, assuming that the respondent selected the 4- to 6-month range, the calculation would be as follows:
|
|
A hypothetical response of €50,000 for the QoL-enhancing scenario (scenario 2) in which the new treatment increased the QoL by twofold (from 0.4 to 0.8), with the same life expectancy for both treatments (1 year), would yield the following ICER:
|
|
Results
Participant characteristics
A total of 53 oncologists, 25 health care policy makers, 60 patients, and 50 individuals from the general population participated in the study. Most of the general population participants (88%) were employed; 38% of the general population indicated that their annual per capita income was €9,500–16,000/year, and 34% answered that their annual per capita income was €16,000–30,000/year. The respondent characteristics are shown in Table 1.
  | Table 1 Description of study participants |
Incremental cost-effectiveness ratios
Scenario 1: life-prolonging scenario
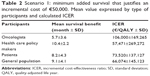
Table 2 describes the responses of participants to the life-prolonging scenario. The results revealed differences among the participants in the minimum mean survival benefit that would justify an incremental cost of €50,000 for a new cancer treatment, with a range from 5.7 months in the case of oncologists to 10.4 months for health care policy makers. Consequently, the ICERs implied by these responses differed among the participants, resulting in values of €57,471/QALY for health care policy makers and €106,000/QALY for oncologists. Implicit ICER values decreased in the following order among the groups of participants: oncologists > patients > general population > health care policy makers.
Scenario 2: quality of life-enhancing scenario
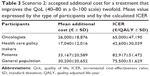
In the QoL-enhancing scenario, the mean willingness to pay for a new drug that improves the QoL by twofold ranged from €17,040 in the case of health care policy makers to €33,167 for patients (Table 3). These mean values yielded ICER values that varied from €42,600/QALY for health care policy makers to €82,917/QALY for patients. The results based on the ICER data decreased among the groups of participants in the following order: patients > general population > oncologists > health care policy makers.
Discussion
To the best of our knowledge, this is the first exploratory and qualitative study that describes the implicit ICERs suggested by oncologists, health care policy makers, patients, and the general population for new cancer treatments. Two different scenarios, a life-prolonging and a QoL-enhancing scenario, were developed to examine whether each of the four groups of participants valued a gain in survival or an improvement in QoL more based on their willingness to pay for specific outcomes. We were also able to detect variations in the responses within a specific scenario.
In both scenarios, the ICER values were higher than the figures commonly used as thresholds for cost-effectiveness analyses.6–8 The higher ICERs obtained in our study may indicate that actual reimbursement and access decisions may not properly reflect the society’s willingness to pay for health benefits. However, the results obtained in this study must be confirmed by larger studies. Although conventional cost-effectiveness thresholds are often used as a guide to interpret the cost-effectiveness analysis, their scientific basis has been questioned because these thresholds may not reflect the society’s willingness to pay for health benefits and in most of the cases have not been updated since their inception.19 An intense debate regarding whether cost-effectiveness thresholds should be reviewed to reflect societal preferences has been ongoing since the last decade.20,21 A recent study indicated that the threshold should be lowered to £18,317 per QALY;22 however, some organizations, including the World Health Organization, have pushed for a threshold of two to three times the per capita annual income, which would represent a threshold of $110,000–$160,000 per QALY for the USA.23 In Spain, this threshold would represent a value of €44,600–€66,800 per QALY, given the annual per capita income of €22,279 in 2013. In our study, the obtained ICERs were above the cost–utility ratios published in the literature for cancer, which ranged from $27,000 to $48,000 per QALY (values for 2008), depending on the tumor type.24
Analyzing both scenarios together showed that oncologists and health care policy makers presented higher ICER values in the life-prolonging scenario compared with those in the QoL-enhancing scenario. Conversely, patients and the general population yielded higher ICER values in the QoL-enhancing scenario compared with those in the life-prolonging scenario. These findings may indicate the preferences of oncologists and health care policy makers to reward survival benefits versus QoL improvements, whereas patients and the general population value an improvement in the QoL related to cancer treatment more than a survival gain.
In the life-prolonging scenario, oncologists most valued gains in survival, followed by patients, the general population, and health care policy makers. These results endorsed a cost-effectiveness threshold of €106,000 per QALY for oncologists, which was the highest threshold in our study. The implicit ICER assessed by oncologists is consistent with previous studies examining the views of medical oncologists regarding the cost of new cancer medications.15–18 Ubel et al indicated that oncologists required an average of six additional months of life for a cancer drug that costs $75,000, which implied an ICER of $100,000 per QALY, and 7.8 months for a drug that costs $150,000, suggesting an ICER of $192,308 per QALY.17 In a study performed in the USA by Nadler et al, the mean implied cost-effectiveness ratio reported by oncologists for a new cancer drug that provides a survival benefit was $318,773.15 Camps-Herrero et al estimated the cost per QALY that could be adopted in the field of oncology in Spain based on the opinions of 35 experts and determined that 68.8% of the respondents considered a cost per QALY between €30,000 and €100,000 to be acceptable.25
Our study found that the ICERs for patients and the general population for the QoL scenario were among the highest compared to those for oncologists and health care policy makers. Compared to the other groups of participants, patients were willing to pay more for a twofold improvement in QoL. These results are particularly relevant for the current era of patient-centered medicine, in which the preferences of patients for a specific treatment should be considered during the decision-making process.26 For a comprehensive evaluation of treatment efficacy, a patient-reported outcome assessment should be included to fully capture patients’ perceptions of symptoms, functioning, and general well-being.27 In oncology, patient-reported outcome measures still have little effect on the decision-making process, in which the primary endpoints of survival usually drive reimbursement decisions. Similar to the results of the present study, where patients and the general population placed greater importance on the QoL-enhancing scenario than on the life-prolonging treatment scenario, a recent study suggested that end-of-life (EOL) decisions made by cancer patients and their caregivers were significantly affected by their preference for QoL over quantity of life, whereas the decisions made by physicians were not.28
The comparison of both scenarios shows that oncologists placed a significantly higher value per QALY on life-prolonging treatment than on QoL-enhancing interventions in our study. This finding may reflect traditional approaches to medical ethics based on the principle of beneficence, which requires medical professionals to treat patients in a manner that produces the maximum benefit for the patient, resulting in the prioritizing of survival gains versus QoL improvements. In the literature, studies that examined both survival gains and QoL improvements showed that estimated ICERs related to gains in survival were also greater compared with those estimated in QoL-enhancing scenarios.16,18 For the two scenarios analyzed in our study, health care policy makers were less willing to pay for therapeutic improvements compared to the remaining participants. This finding was somewhat anticipated because of their responsibility in financing and delivering health care, particularly in Spain, where cancer treatments are publicly funded and exempted from copayment by the patient. This situation may contrast with other country settings in which out-of-pocket costs may influence treatment recommendations.
The results of this study have several implications and they highlight that the implicit cost-effectiveness thresholds varied widely across oncologists, health care policy makers, patients, and the general population, reflecting a lack of consensus about this issue.
Nowadays, understanding the value that these individuals place on innovation of new cancer drugs and their willingness to pay for them is becoming more important since it may enrich the discussion related to cost-effectiveness threshold and its implications.
Given that the market price depends in part on the willingness of third-party payers to reimburse treatment, the lower ICER awarded by health care policy makers may have an influence on the pricing of new cancer drugs.
Results also reflect that although oncologists are aware of the cost of new cancer drugs, it does not necessarily affect their decision-making process related to prescription.
When drugs offer the potential to increase the survival or improve the QoL, refusing to fund these drugs is politically difficult.29 Oncology is one of the exclusive therapeutic areas in which exceptions are being made to bring new therapeutic options to patients. For instance, in Canada, oncology drugs are adopted at the highest thresholds of acceptability.30 Some exceptions have also been made in the UK, escaping the rigid application of cost-effectiveness thresholds because of the introduction of new criteria for appraising EOL treatments.31 In this regard, a study that estimated the plausible cost-effectiveness threshold of the National Institute for Health and Care Excellence following the application of the EOL criteria suggested that the cost-effectiveness threshold could be ~£50,000/QALY.32 Moreover, the approval of the Cancer Drug Fund is another example of an exception made in the UK for paying for expensive oncology medications that have not been recommended for coverage by the National Institute for Health and Care Excellence. The fund’s approval, as well as its recent renewal, has generated an intense debate regarding its lack of support for evidence-based decision making.33 In conclusion, decisions regarding which drugs to fund to treat feared diseases such as cancer sometimes depend on political and social acceptability.29
Regarding cancer drugs, in addition to the cost-effectiveness thresholds explored in the present study, other factors should be considered in the decision-making process about their funding by NHS. Priority settings are critical in aligning drug funding with national health needs. Once the important priority goals are determined, future actions to achieve those goals will be identified easily, thereby facilitating the decision-making process. Cost opportunity should also be considered since, relative to needs, resources are scarce. Involving patients and the general population in the decision-making process is also desirable. However, only a few current health care systems have established mechanisms for seeking this information from patients or the general population.
This study has several limitations. Most of the associations included in the study were breast cancer associations, which resulted in an overrepresentation of women in our patient sample. A convenience sample of the general population was taken to guarantee an acceptable response rate. Because most of the respondents were employed and had a university degree, plausible problems related to a misunderstanding of the questionnaire were minimized.
Even though the sample size of the study is questionable, it should be considered that most methodologists openly recognize the lack of standards for sample size in qualitative studies. There is a vast range of sample sizes for all research designs, with the most common sample size being between 20 and 30 interviews.34
Finally, to make better informed decisions, additional information such as burden of illness, budget impact based on the number of patients expected to treat, and whether the new treatment addressed an unmet need should be added to each of the presented scenarios.
Despite these limitations, the strength of this study lies in adapting a similar methodology used in other oncology studies and in including a broader number of stakeholders who may have an influence on the decision-making process. The use of the QALY as a measure of health benefits also allowed comparisons to be made. Moreover, the simple and indirect approach used in this study to obtain cost-effectiveness thresholds in the form of cost per QALY is also a strength of this study and can be translated to other research areas.
Conclusion
In conclusion, decisions regarding the prioritization and selection of new cancer treatments based on their cost-effectiveness require using a predefined threshold to interpret study results. These thresholds should reflect the society’s opinion regarding the willingness to pay for specific outcomes. Discrepancies in perceptions among oncologists, health care policy makers, patients, and the general population should be considered when establishing thresholds for new oncology treatments, which thus merit further investigation.
Acknowledgments
The authors are extremely grateful to all the study participants who contributed their time to participate in this research. The preliminary results with interim findings were presented at the ISPOR 17th Annual European Congress (Amsterdam, November 8–12, 2014). The actual paper, however, has never been published. The study was funded by Eli & Lilly and Co.
Authors contributions
TD and JAS conceived the study and participated with the rest of the authors in its design and coordination, drafted the questionnaires, and helped to draft the manuscript. LL and SP developed and administered the electronic versions of the questionnaires and conducted data analyses. All authors read and approved the final manuscript. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
TD and JAS are employees of Eli Lilly. The authors report no other conflicts of interest in this work.
References
Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No 11. Lyon: International Agency for Research on Cancer; 2013. Available from: http://globocan.iarc.fr | ||
Soerjomataram I, Lortet-Tieulent J, Parkin DM, et al. Global burden of cancer in 2008: a systematic analysis of disability-adjusted life-years in 12 world regions. Lancet. 2012;380:1840–1850. | ||
Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19:1893–1907. | ||
Patel JD, Krilov L, Adams S, et al. Clinical cancer advances 2013: annual report on progress against cancer from the American society of clinical oncology. J Clin Oncol. 2014;32:129–160. | ||
Kantarjian HM, Fojo T, Mathisen M, Zwelling LA. Cancer drugs in the United States: Justum Pretium – the just price. J Clin Oncol. 2013;31:3600–3604. | ||
National Institute for Health and Clinical Excellence. Guide to the Methods of Technology Appraisal. London: National Institute for Health and Clinical Excellence (NICE); 2008. | ||
Neumann PJ, Sandberg EA, Bell CM, Stone PW, Chapman RH. Are pharmaceuticals cost-effective? A review of the evidence? Health Aff (Millwood). 2000;19:92–109. | ||
Sacristán JA, Oliva J, Del Llano J, Prieto L, Pinto JL. [What is an efficient health technology in Spain?]. Gac Sanit. 2002;16:334–343. | ||
King JT Jr, Tsevat J, Lave JR, Roberts MS. Willingness to pay for a quality-adjusted life year: implications for societal health care resource allocation. Med Decis Making. 2005;25:667–677. | ||
Ellis LM, Bernstein DS, Voest EE, et al. American society of clinical oncology perspective: raising the bar for clinical trials by defining clinically meaningful outcomes. J Clin Oncol. 2014;32:1277–1280. | ||
Slevin ML, Stubbs L, Plant HJ, et al. Attitudes to chemotherapy: comparing views of patients with cancer with those of doctors, nurses, and general public. BMJ. 1990;300:1458–1460. | ||
Berry SR, Bell CM, Ubel PA, et al. Continental divide? The attitudes of US and Canadian oncologists on the costs, cost-effectiveness, and health policies associated with new cancer drugs. J Clin Oncol. 2010;28:4149–4153. | ||
Neumann PJ, Palmer JA, Nadler E, Fang C, Ubel P. Cancer therapy costs influence treatment: a national survey of oncologists. Health Aff (Millwood). 2010;29:196–202. | ||
Greenberg D, Hammerman A, Vinker S, Shani A, Yermiahu Y, Neumann PJ. Oncologists’ and family physicians’ views on value for money of cancer and congestive heart failure care. Isr J Health Policy Res. 2013;2:44. | ||
Nadler E, Eckert B, Neumann P. Do oncologists believe new cancer drugs offer good value? Oncologist. 2006;11:90–95. | ||
Kozminski MA, Neumann PJ, Nadler ES, Jankovic A, Ubel PA. How long and how well: oncologists’ attitudes toward the relative value of life-prolonging v quality of life-enhancing treatments. Med Decis Making. 2011;31:380–385. | ||
Ubel PA, Berry SR, Nadler E, et al. In a survey, marked inconsistency in how oncologists judged value of high-cost cancer drugs in relation to gains in survival. Health Aff (Millwood). 2012;31:709–717. | ||
Greenberg D, Hammerman A, Vinker S, Shani A, Yermiahu Y, Neumann PJ. Which is more valuable, longer survival or better quality of life? Israeli oncologists’ and family physicians’ attitudes toward the relative value of new cancer and congestive heart failure interventions. Value Health. 2013;16:842–847. | ||
Braithwaite RS, Meltzer DO, King JT Jr, Leslie D, Roberts MS. What does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule? Med Care. 2008;46:349–356. | ||
Towse A. Should NICE’s threshold range for cost per QALY be raised? Yes. BMJ. 2009;338:b181. | ||
Raftery J. Should NICE’s threshold range for cost per QALY be raised? No. BMJ. 2009;338:b185. | ||
Claxton K, Martin S, Soares M, et al. Methods for the Estimation of the NICE Cost Effectiveness Threshold. York: Centre for Health Economics; University of York; 2013:81. | ||
Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness – the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371:796–797. | ||
Greenberg D, Earle C, Fang CH, Eldar-Lissai A, Neumann PJ. When is cancer care cost-effective? A systematic overview of cost-utility analyses in oncology. J Natl Cancer Inst. 2012;102:82–88. | ||
Camps-Herrero C, Paz-Ares L, Codes M, et al. Social value of a quality-adjusted life year (QALY) in Spain: the point of view of oncologists. Clin Transl Oncol. 2014;16:914–920. | ||
Sacristán JA. Patient-centered medicine and patient-oriented research: improving health outcomes for individual patients. BMC Med Inform Decis Mak. 2013;13:6. | ||
Basch E, Abernethy AP, Mullins CD, et al. Recommendations for incorporating patient-reported outcomes into clinical comparative effectiveness research in adult oncology. J Clin Oncol. 2012;30:4249–4255. | ||
Rocke DJ, Beumer HW, Taylor DH, Thomas S, Puscas L, Lee WT. Physician and patient and caregiver health attitudes and their effect on medicare resource allocation for patients with advanced cancer. JAMA Otolaryngol Head Neck Surg. 2014;140(6):497–503. | ||
Raftery JP. Paying for costly pharmaceuticals: regulation of new drugs in Australia, England and New Zealand. Med J Aust. 2008;188:26–28. | ||
Rocchi A, Menon D, Verma S, Miller E. The role of economic evidence in Canadian oncology reimbursement decision-making: to lambda and beyond. Value Health. 2008;11:771–783. | ||
National Institute for Health and Clinical Excellence. Appraising Life-Extending, End of Life Treatments. London: NICE; 2009. | ||
Moïse P, Fassler P. Estimating NICE’s cost-effectiveness threshold for end-of-life cancer treatments [abstract]. Value Health. 2011;14:A173. | ||
Lakdawalla DN, Jena AB, Doctor JN. Careful use of science to advance the debate on the UK cancer drugs fund. JAMA. 2014;311:25–26. | ||
Marshall B, Cardon P, Poddar A, et al. Does sample size matter in qualitative research?: a review of qualitative interviews in is research. J Comput Inform Syst. 2013;54(1):11–22. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.