Back to Journals » OncoTargets and Therapy » Volume 12
DNA-PKcs Mediates An Epithelial-Mesenchymal Transition Process Promoting Cutaneous Squamous Cell Carcinoma Invasion And Metastasis By Targeting The TGF-β1/Smad Signaling Pathway
Authors Zhang J, Jiang H, Xu D, Wu WJ , Chen HD , He L
Received 12 February 2019
Accepted for publication 26 August 2019
Published 7 November 2019 Volume 2019:12 Pages 9395—9405
DOI https://doi.org/10.2147/OTT.S205017
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Takuya Aoki
Juan Zhang,1,* Hui Jiang,1,* Dan Xu,1,* Wen-Juan Wu,1 Hong-Duo Chen,2 Li He1
1Department of Dermatology, First Affiliated Hospital of Kunming Medical University, Institute of Dermatology & Venereology of Yunnan Province, Kunming, People’s Republic of China; 2Department of Dermatology, The First Hospital of China Medical University, Shenyang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Li He
Department of Dermatology, First Affiliated Hospital of Kunming Medical University, Institute of Dermatology & Venereology of Yunnan Province, 295 Xi Chang Road, Kunming 650032, People’s Republic of China
Email [email protected]
Hong-Duo Chen
Department of Dermatology, The First Hospital of China Medical University, 155 North Nanjing Street, Shenyang 110001, People’s Republic of China
Tel +86-13840597383
Email [email protected]
Purpose: DNA-dependent protein kinase catalytic subunit (DNA-PKcs) has attracted extensive attention in various types of malignant tumors. However, the role of DNA-PKcs in cutaneous squamous cell carcinoma (cSCC) development has not been elucidated. In this study, we investigated the role of DNA-PKcs in cSCC and the molecular mechanisms of TGF-β1-induced cSCC progression mediated by DNA-PKcs.
Methods: We performed bioinformatic analysis and RT-PCR to examine the DNA-PKcs expression level in cSCC. Then, we downregulated DNA-PKcs using a DNA-PK-specific inhibitor or small interfering RNA (siRNA) to explore the effects of DNA-PKcs on SCL-1 cell migration and invasion. To further investigate the mechanism by which DNA-PKcs promotes cSCC progression, TGF-β1 and the TGF-β receptor (TGF-βR) I/II dual inhibitor LY2109761 were used to examine whether DNA-PKcs participates in TGF-β1/Smad signaling.
Results: DNA-PKcs expression was upregulated in cSCC. DNA-PK inhibition or expression knockdown resulted in inhibited migration and invasion and altered epithelial-mesenchymal transition (EMT) marker expression patterns in SCL-1 cells. Importantly, TGF-β1 mediated EMT induction in cSCC cells, and DNA-PKcs was identified as a TGF-β1-responsive gene. TGF-β1 promoted DNA-PKcs transcription, and DNA-PKcs enhanced the TGF-β1-induced EMT program involved in cSCC invasion and metastasis by phosphorylating Smad3.
Conclusion: This study is the first to show that DNA-PKcs mediates EMT to promote cSCC aggressiveness by targeting the TGF-β1/Smad signaling pathway, which provides insight into how DNA-PKcs impacts cSCC progression and identifies a new therapeutic target.
Keywords: cutaneous squamous cell carcinoma, DNA-dependent protein kinase catalytic subunit, epithelial-mesenchymal transition, transforming growth factor-β1, E-cadherin
Introduction
Cutaneous squamous cell carcinoma (cSCC) is the second most common nonmelanoma skin cancer after basal cell carcinoma. Its high incidence and treatment cost impose a great burden on individuals and society,1–3 and the metastatic rate of cSCC is approximately 5%.4 Exposure to chronic ultraviolet (UV) radiation from the sun is the most important environmental factor involved in the occurrence of cSCC.5
DNA-dependent protein kinase catalytic subunit (DNA-PKcs) is a catalytic subunit of the DNA-dependent protein kinase (DNA-PK) holoenzyme that is involved in DNA double-strand break (DSB) repair after UV radiation.6,7 Our previous study also found that the phosphorylation levels of DNA-PKcs (T2647 and T2609) were significantly increased in normal human epidermal keratinocytes after exposure to different doses of UVB radiation.8 Recently, DNA-PKcs has attracted extensive attention because its aberrant expression has been detected in various types of malignant tumors.9–11 However, the role of DNA-PKcs in cSCC development has not been elucidated.
Epithelial-mesenchymal transition (EMT) is a biological event during which epithelial cells lose their polarity and cell-cell adhesions and acquire a mesenchymal phenotype, and EMT is thought to play an important role in pathological processes such as wound healing and cancer progression.12,13 Transforming growth factor (TGF)-β1 is a member of the TGF-β superfamily and is the most widely distributed cytokine in skin tissue.14 TGF-β1 acts as an inhibitor in normal tissues,15,16 but studies utilizing transgenic mice have found that TGF-β1 signaling accelerates cSCC,17,18 although the mechanism remains elusive. Previous studies have indicated that TGF-β1 acts as a potent inducer of EMT as well as a factor for the maintenance of EMT in various epithelial cells.19,20
In this study, we investigated the role of DNA-PKcs in cSCC and the molecular mechanisms of TGF-β1-induced cSCC progression involving DNA-PKcs. We found that DNA-PKcs expression was upregulated in cSCC and that downregulation of DNA-PKcs expression inhibited migration and invasion and altered the expression patterns of EMT markers in SCL-1 cells. More interestingly, DNA-PKcs was identified as a TGF-β1-responsive gene. We further revealed that TGF-β1 promoted the activation of DNA-PKcs and that DNA-PKcs enhanced the TGF-β1-induced EMT program involved in cSCC invasion and metastasis by phosphorylating Smad3.
Materials And Methods
Skin Tissue Sample Collection
cSCC (n=3) and matched normal control (n=3) skin tissue samples were collected from patients who underwent routine skin tumor resection at the First Affiliated Hospital of Kunming Medical University (Kunming, China). None of these patients received any form of treatment. All patients provided written informed consent, and the study protocol was approved by the Ethics Committee of our institution (First Affiliated Hospital of Kunming Medical University) in compliance with the Declaration of Helsinki.
Real-Time Quantitative Reverse Transcription PCR (RT-PCR)
Total RNA was extracted from tissue samples and cells using TRIzol reagent (Invitrogen, MA, USA) and reverse-transcribed into cDNA using a FastKing-RT Reagent kit (Tiangen, Beijing, China) according to the manufacturer’s protocols. RT-PCR analysis was conducted using SYBR Green Master Mix (Tiangen, Beijing, China) with a Rotor-Gene PCR system (Qiagen, Germany). The primers are listed in Table 1. The conditions for amplification were as follows: 95°C for 15 mins, followed by 40 cycles of 95°C for 10 seconds, 60°C for 20 seconds, and 72°C for 30 seconds. Relative mRNA levels were calculated based on the threshold cycle (Ct) following normalization to the level of GAPDH and averaged among three replicates. The relative expression of the RT-PCR results was determined using the comparative CT (2 −ΔΔCt) method.
 |
Table 1 Primer Sequences |
Cell Culture And Treatment
Immortalized human epidermal keratinocytes (HaCaT cells) and the cSCC cell line SCL-1 were supplied by the Department of Dermatology at the First Hospital of China Medical University, and was approved by our institutional ethics committee. The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin and 100 mg/L streptomycin in a 5% CO2 incubator at 37°C. For the inhibitor and small interfering RNA (siRNA) studies, the cells were seeded in different plates and incubated until they attached firmly to the flask, and then the cells were treated with the DNA-PK inhibitor NU7026 (Selleckchem, Shanghai, China) or transfected with a specific siRNA targeting DNA-PKcs (RiboBio, Guangdong, China). The sequences of the three DNA-PKcs-specific siRNA (si-h-PRKDC) oligonucleotides were as follows: si-h-PRKDC_001, 5ʹ-GCCAGTTTATCAATCTGAT-3ʹ; si-h-PRKDC_002, 5ʹ-GGCCTTATGTACAGCATCA-3ʹ; and si-h-PRKDC_003, 5ʹ-GATCGCACCTTACTCTGTT-3ʹ. The si-h-PRKDC transfection efficiency was determined by RT-PCR. For the TGF-β1 or TGF-β receptor I/II (TGF-βR I/II) dual inhibitor LY2109761 study, the cells were treated with 5 ng/mL TGF-β1 (Sigma-Aldrich) or 300 nmol/L LY2109761 (Selleckchem, Shanghai, China).
Scratch Wound Healing Assay
For the scratch assay, SCL-1 cells were grown to confluence in 6-well plates, and a wound was scratched into the cell monolayer with a sterilized 200 µl pipette tip. The culture medium was then removed, and the cells were washed with phosphate-buffered saline (PBS) and cultured with the indicated treatment. The width of the wound was measured under a microscope at 0 and 48 h after the scratch to assess the migratory ability of the cells.
Transwell Migration And Invasion Assays
SCL-1 cells (2 × 105 and 5 × 104 for the migration and invasion assays, respectively) were seeded in the top chamber of a noncoated (for migration) or Matrigel-coated (for invasion) Transwell chamber with an 8 µm pore membrane (BD Biosciences). Medium supplemented with the indicated treatment was added to the lower chamber as a chemoattractant for 48 h. Nonmigrated/noninvaded cells on the upper surface of the membrane were removed using cotton swabs. The migrated/invaded cells attached to the lower surface were fixed, stained with the Haematoxylin and Eosin Stain Kit (Keygen, Nanjing, China), mounted on slides and counted under a microscope.
Immunofluorescence
To assess immunofluorescence in cultured cells, SCL-1 cells were fixed with 4% formaldehyde in PBS, permealized with 0.5% Triton X-100 and blocked with 1% BSA in PBS. The cells were incubated with an anti-E-cadherin primary antibody overnight at 4°C and then incubated with a Cy3-labeled goat anti-mouse secondary antibody (Abcam, Cambridge, UK). For staining of the cytoskeleton, the cells were incubated with Alexa Fluor 594-conjugated phalloidin for 30 min at room temperature. Finally, the cells were stained with DAPI for nuclear staining, and images of the cells were captured with a fluorescence microscope equipped with a CCD camera (Nikon ECLIPSE Ti, Japan).
Western Blotting
Cells were harvested and washed twice with PBS, and then total protein was isolated using RIPA cell lysis buffer (Beyotime, Jiangsu, China) and quantified by using the BCA Protein Assay Kit (Beyotime, Jiangsu, China). Protein from each group was loaded onto 4-15% SDS-polyacrylamide gel electrophoresis (PAGE) gels and then transferred onto polyvinylidene fluoride membranes (Millipore, MA, USA). The membranes were blocked with 5% nonfat milk for 2 h and incubated with antibodies. Horseradish peroxidase-conjugated secondary antibodies were added to the incubation. Specific antigen-antibody interactions were detected using an enhanced chemiluminescence (ECL) detection reagent (Keygen, Nanjing, China). Western blotting bands were quantitated using ImageJ software (National Institutes of Health, Bethesda, MD). β-actin was used as the internal control.
Statistical Analysis
Data are presented as the mean ± standard deviation (SD). SPSS for Windows (version, 20.0; SPSS, Inc. Chicago, IL, USA) and GraphPad Prism (version 7.0, USA) were used to analyze all values. Student’s t-test was used to test all differences, and significance was set at p< 0.05.
Results
DNA-PKcs Expression Was Significantly Upregulated In cSCC Human Skin Tissue And SCL-1 Cells
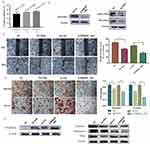
First, we used the Oncomine database (www.oncomine.com) to analyze the data collected from the gene expression microarray dataset, identifying the differential expression of DNA-PKcs in cSCC (Figure 1A and B).21,22
Second, we measured the DNA-PKcs expression levels in cSCC tissue samples kept in RNAlater® RNA Stabilization Solution (Invitrogen, MA, USA) by RT-PCR. We found that DNA-PKcs expression was increased in cSCC tissue compared to normal skin tissue. (Figure 1C)
Finally, at the cellular level, to examine whether the expression of DNA-PKcs was upregulated in cSCC cells, we determined the PRKDC mRNA expression in normal keratinocyte HaCaT and cSCC cell line SCL-1 cells. The results in Figure 1D show that the PRKDC mRNA levels were increased in SCL-1 cells compared to the HaCaT cells. These data suggest that DNA-PKcs plays an important role in the progression of cSCC.
DNA-PKcs Increased The Migratory And Invasive Abilities Of cSCC Cells And Promoted EMT
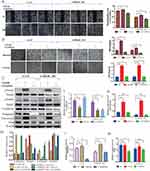
To confirm the effect of siRNA transfection on DNA-PKcs expression, we assessed the PRKDC mRNA level. The results showed that the mRNA levels were efficiently reduced by 46.4% with si-PRKDC_001, 67.1% with si-PRKDC_002 and 72.1% with si-PRKDC_003 (Figure 2A). si-PRKDC_003 had the highest transfection efficiency, so it was used in a follow-up experiment. The DNA-PK inhibitor NU7026 was also used to treat SCL-1 cells to assess the downregulation of DNA-PKcs. When the effects of NU7026 and si-PRKDC_003 on DNA-PKcs were examined, the protein levels of DNA-PKcs were found to be significantly downregulated after NU7026 or si-PRKDC_003 treatment (Figure 2B).
To determine the role of DNA-PKcs in cSCC progression, DNA-PKcs knockdown cells were made through NU7026 treatment or si-PRKDC_003 transfection. Scratch wound healing assays and Transwell invasion and migration assays were conducted, and the results are shown in Figure 2C and D. The knockdown of DNA-PKcs decreased the invasion and migration of SCL-1 cells.
Since EMT is a key event associated with tumor invasion and metastasis and the invasion and metastasis of cSCC cells could be altered by DNA-PKcs, we hypothesized that DNA-PKcs participated in the EMT process in cSCC. We found that DNA-PKcs expression downregulation in SCL-1 cells increased the expression of the epithelial marker E-cadherin and decreased the expression of the mesenchymal markers, Vimentin, Fibronectin and Slug at the protein level (Figure 2E). These results suggest that DNA-PKcs promotes EMT in cSCC.
TGF-β1 Induced SCL-1 Cell EMT And Promoted The Activation Of DNA-PKcs
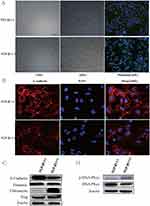
As TGF-β1 has been identified as an important inducer of EMT, we thus intended to investigate whether TGF-β1 promoted EMT and invasion in cSCC. We treated SCL-1 cells with or without 5 ng/mL TGF-β1 for 48 h. Following TGF-β1 stimulation, compared with the control cells, the SCL-1 cells exhibited a spindle-shaped, fibroblast-like morphology with mesenchymal phenotypic changes (Figure 3A). Furthermore, we examined the expression of the EMT markers. We found that in the presence of TGF-β1, the E-cadherin level was decreased in SCL-1 cells, while mesenchymal markers Fibronectin and Slug were increased. (Figure 3B and C).
SCL-1 cells were also used to confirm the expression of DNA-PKcs. Compared with that in the corresponding control cells, the phosphorylation level of DNA-PKcs (T2609) in the SCL-1 cells stimulated with TGF-β1 was significantly increased after 48 h of treatment (Figure 3D).
DNA-PKcs Promoted EMT And Invasion Partly Via TGF-β1/Smad Signaling
The role of DNA-PKcs in cSCC has not been elucidated. Here, we explored the possible mechanism by which DNA-PKcs affects EMT in cSCC cells. Several reports have shown that the TGF-β1/Smad signaling pathway plays a vital role in EMT;19,20 thus, we proposed that DNA-PKcs accelerated EMT and cell invasion via TGF-β1/Smad signaling. We treated cells with and without TGF-β1 as described above and measured the protein levels of Smad2 and Smad3. As shown in Figure 4C and D, TGF-β1 activated p-Smad2/3 signaling. In addition, the knockdown of DNA-PKcs decreased the p-Smad3 levels in SCL-1 cells. These data suggest that DNA-PKcs can affect the TGF-β1/Smad3 signaling pathway.
To validate the effect of DNA-PKcs on TGF-β1/Smad3 signaling with a loss-of-function approach, we used LY2109761, a TGF-β receptor inhibitor, to repress p-Smad3 expression. As shown in Figure 4C–H, the E-cadherin level was restored by using LY2109761, while mesenchymal marker markers Vimentin and Slug were decreased.
In addition, the numbers of cells invading through the Transwell membrane and migrating to close the scratch wound were significantly increased after TGF-β1 stimulation and reduced following LY2109761 inhibition (Figure 4A and B). These results indicate that LY2109761 participates in EMT reversion by blocking the Smad3 pathway in cSCC.
Likewise, EMT was reversed, and cell invasion was decreased when DNA-PKcs was silenced in SCL-1 cells (Figure 2E). These results reveal that DNA-PKcs plays a key role in the regulation of cSCC EMT and cell mobility. Interestingly, when the Smad3 pathway was blocked, downregulating DNA-PKcs expression caused a significant decrease in cell migration and invasion in SCL-1 cells, while E-cadherin expression was obviously decreased. This indicated that in addition to its role in TGF-β1/Smad signaling, DNA-PKcs may also participate in other cellular pathways, such as the TGF-β2/Smad and PI3K/Akt signaling pathways, to regulate EMT. Taken together, our results prove that DNA-PKcs promotes cSCC EMT and invasion partly by modulating TGF-β1/Smad3 signaling at the cellular level.
Discussion
As previously reported, DNA-PKcs has been detected as a key component of the nonhomologous end-joining (NHEJ) repair process.23 Recently, DNA-PKcs has attracted extensive attention because its aberrant expression has been detected in various types of malignant tumors, especially hepatocellular carcinoma, colorectal cancer, pancreatic cancer and glioblastoma.9–11,23 DNA-PKcs has also been linked to the occurrence, development and prognosis of these malignant tumors.24,25 However, the role of DNA-PKcs in cSCC development has not been elucidated. In the present study, we examined the role of DNA-PKcs in cSCC development. Our findings suggested that DNA-PKcs played an important role in cSCC progression because DNA-PKcs promoted cSCC cell migration and invasion. Moreover, similar to the findings of L. Anandi,26 DNA-PKcs expression in the human cSCC SCL-1 cell line was correlated with EMT, indicating that DNA-PKcs facilitates EMT and participates in the processes of cSCC progression and metastasis. This finding is supported by the observation that DNA-PKcs deficiency decreased SCL-1 cell migration and invasion in vitro. These observations indicate that DNA-PKcs may serve as a valuable biomarker for monitoring cSCC development in humans.
Tumor progression and metastasis are facilitated by many different mechanisms. For instance, EMT is associated with cancer cell invasion and metastasis. E-cadherin expression was decreased and Vimentin expression was upregulated in cSCC both in vitro and in vivo (Figure 1E–H). DNA-PKcs appears to regulate cSCC tumor metastasis by mediating EMT in SCL-1 cells, which may be one of the mechanisms underlying cSCC progression and metastasis. DNA-PKcs expression downregulation significantly increased E-cadherin expression and decreased mesenchymal markers expression in SCL-1 cells. These observations indicate that DNA-PKcs facilitates EMT in cSCC as a means of promoting metastasis.
TGF-β comprises three isoforms, TGF-β1, TGF-β2 and TGF-β3, which can regulate the growth, differentiation, and migration of nearly all cell types.14,15 TGF-β1 has been implicated as both a potent inducer and a maintenance factor of EMT.19,20 Several reports have indicated that multiple genes and proteins are associated with TGF-β1/Smad signaling-induced EMT,27–29 but the involvement of DNA-PKcs in the migration and invasion behaviors of cSCC cells as well as the impact of DNA-PKcs had remained uninvestigated. To investigate the roles of DNA-PKcs, we used TGF-β1 (5 ng/mL, 48 h) to induce EMT in the cSCC cell line SCL-1. We observed that the SCL-1 cells cultured without TGF-β1 retained their cobblestone-like morphology with tight cell-cell contacts, whereas the cells cultured with TGF-β1 displayed an elongated fibroblast-like morphology with scattered distribution in the culture. We then examined EMT markers by using immunofluorescence and immunoblotting. The SCL-1 cells cultured with TGF-β1 exhibited a significant downregulation of the expression of the epithelial marker E-cadherin, while mesenchymal markers Fibronectin and Slug were increased. In this TGF-β1-induced EMT model, we detected the upregulation of the p-DNA-PKcs (T2609) protein level (Figure 3D). We also observed a simultaneous downregulation of the phosphorylated Smad3 level, as revealed by experiments with the TGF-βR I/II inhibitor LY2109761 (Figure 4C and D).
Our data indicated that TGF-β1 not only simultaneously induced the activation of DNA-PKcs and phosphorylation of Smad2/3 in cSCC cells but also activated EMT. Furthermore, the Smad3 phosphorylation inhibitor LY2109761 repressed Smad3 phosphorylation and cell motility and inhibited EMT in SCL-1 cells. Therefore, our results reveal that TGF-β1 promotes DNA-PKcs activation and that DNA-PKcs enhances the TGF-β1-induced EMT program involved in cSCC invasion and metastasis by phosphorylating Smad3.
In summary, we demonstrated that DNA-PKcs expression was upregulated in cSCC and that DNA-PKcs could induce cell migration, invasion and EMT in SCL-1 cells by promoting the activation of TGF-β1/Smad signaling. DNA-PKcs is considered an oncogene and may become a potential target for cSCC prevention and treatment. To our knowledge, this is the first study to reveal the role of DNA-PKcs in TGF-β1-mediated EMT in cSCC cells. There are also some limitations in our research. We studied the role of DNA-PKcs in cSCC, and our results are based on SCL-1 cells in vitro and the Smad signaling pathway. Therefore, it is necessary to investigate any underlying mechanisms, such as non-Smad signaling, and verify these results in vitro.
Ethics Approval And Informed Consent
Informed consent was obtained from all patients, and the study protocol was approved by the Ethics Committee of our institution (First Affiliated Hospital of Kunming Medical University).
Author Contributions
Juan Zhang and Hui Jiang carried out the studies, participated in collecting data, and drafted the manuscript. Hong-Duo Chen and Li He participated in its design. Dan Xu and Wen-Juan Wu performed the statistical analysis and helped to draft the manuscript. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
All authors declare that they have no conflicts of interest in this work.
References
1. Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146(3):283–287. doi:10.1001/archdermatol.2010.19
2. Donaldson MR, Coldiron BM. No end in sight: the skin cancer epidemic continues. Semin Cutan Med Surg. 2011;30(1):3–5. doi:10.1016/j.sder.2011.01.002
3. Chen JG, Fleischer AB
4. Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol. 2013;68(6):957–966. doi:10.1016/j.jaad.2012.11.037
5. Burns EM, Tober KL, Riggenbach JA, Kusewitt DF, Young GS, Oberyszyn TM. Extended UVB exposures alter tumorigenesis and treatment efficacy in a murine model of cutaneous squamous cell carcinoma. J Skin Cancer. 2013;2013:246848. doi:10.1155/2013/246848
6. Ceccaldi R, Rondinelli B, D’Andrea AD. Repair pathway choices and consequences at the double-strand break. Trends Cell Biol. 2016;26(1):52–64.
7. Rastogi RP, Richa KA, Tyagi MB, Sinha RP. Molecular mechanisms of ultraviolet radiation-induced DNA damage and repair. J Nucleic Acids. 2010;2010:592980. doi:10.4061/2010/592980
8. Tu Y, Ji C, Yang B, et al. DNA-dependent protein kinase catalytic subunit (DNA-PKcs)-SIN1 association mediates ultraviolet B (UVB)-induced Akt Ser-473 phosphorylation and skin cell survival. Mol Cancer. 2013;12(1):172. doi:10.1186/1476-4598-12-172
9. Wu L, Zhang J, Wu H, Han E. DNA-PKcs interference sensitizes colorectal cancer cells to a mTOR kinase inhibitor WAY-600. Biochem Biophys Res Commun. 2015;466(3):547–553. doi:10.1016/j.bbrc.2015.09.068
10. Hu H, Gu Y, Qian Y, et al. DNA-PKcs is important for Akt activation and gemcitabine resistance in PANC-1 pancreatic cancer cells. Biochem Biophys Res Commun. 2014;452(1):106–111. doi:10.1016/j.bbrc.2014.08.059
11. Timme CR, Rath BH, O’Neill JW, Camphausen K, Tofilon PJ. The DNA-PK inhibitor VX-984 enhances the radiosensitivity of glioblastoma cells grown in vitro and as orthotopic xenografts. Mol Cancer Ther. 2018;17(6):1207–1216. doi:10.1158/1535-7163.MCT-17-1267
12. Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–196. doi:10.1038/nrm3758
13. Nieto MA. Epithelial plasticity: a common theme in embryonic and cancer cells. Science. 2013;342(6159):1234850. doi:10.1126/science.1234850
14. Jung MC, Shin MK, Hong KK, Jeong KH, Kim NI. Differential expression of TGF-beta isoforms in human kerationocytes by narrow band UVB. Ann Dermatol. 2008;20(3):113–119. doi:10.5021/ad.2008.20.3.113
15. Roberts AB, Sporn MB. Physiological actions and clinical applications of transforming growth factor-beta (TGF-beta). Growth Factors. 1993;8(1):1–9.
16. Mordasky Markell L, Pérez-Lorenzo R, Masiuk KE, Kennett MJ, Glick AB. Use of a TGFβ type I receptor inhibitor in mouse skin carcinogenesis reveals a dual role for TGFβ signaling in tumor promotion and progression. Carcinogenesis. 2010;31(12):2127–2135. doi:10.1093/carcin/bgq191
17. Bian Y, Terse A, Du J, et al. Progressive tumor formation in mice with conditional deletion of TGF-beta signaling in head and neck epithelia is associated with activation of the PI3K/Akt pathway. Cancer Res. 2009;69(14):5918–5926. doi:10.1158/0008-5472.CAN-08-4623
18. Roberts AB, Wakefield LM. The two faces of transforming growth factor beta in carcinogenesis. Proc Natl Acad Sci U S A. 2003;100(15):8621–8623. doi:10.1073/pnas.1633291100
19. Katsuno Y, Lamouille S, Derynck R. TGF-beta signaling and epithelial-mesenchymal transition in cancer progression. Curr Opin Oncol. 2013;25(1):76–84. doi:10.1097/CCO.0b013e32835b6371
20. Chen Q, Yang W, Wang X, et al. TGF-beta1 induces EMT in bovine mammary epithelial cells through the TGFbeta1/Smad signaling pathway. Cell Physiol Biochem. 2017;43(1):82–93. doi:10.1159/000480321
21. Riker AI, Enkemann SA, Fodstad O, et al. The gene expression profiles of primary and metastatic melanoma yields a transition point of tumor progression and metastasis. BMC Med Genomics. 2008;1:13. doi:10.1186/1755-8794-1-13
22. Nindl I, Dang C, Forschner T, et al. Identification of differentially expressed genes in cutaneous squamous cell carcinoma by microarray expression profiling. Mol Cancer. 2006;5:30. doi:10.1186/1476-4598-5-30
23. Pascale RM, Joseph C, Latte G, Evert M, Feo F, Calvisi DF. DNA-PKcs: a promising therapeutic target in human hepatocellular carcinoma? DNA Repair (Amst). 2016;47:12–20. doi:10.1016/j.dnarep.2016.10.004
24. Ren F, Yang ZL, Tan X, et al. DNA-PKcs and Ku70 are predictive markers for poor prognosis of patients with gall bladder malignancies. Appl Immunohistochem Mol Morphol. 2014;22(10):741–747. doi:10.1097/PAI.0000000000000017
25. Pinel B, Duchesne M, Godet J, et al. Mesenchymal subtype of glioblastomas with high DNA-PKcs expression is associated with better response to radiotherapy and temozolomide. J Neurooncol. 2017;132(2):287–294. doi:10.1007/s11060-016-2367-7
26. Anandi L, Chakravarty V, Ashiq KA, Bodakuntla S, Lahiri M. DNA-dependent protein kinase plays a central role in transformation of breast epithelial cells following alkylation damage. J Cell Sci. 2017;130(21):3749–3763. doi:10.1242/jcs.203034
27. Liang Q, Li L, Zhang J, et al. CDK5 is essential for TGF-beta1-induced epithelial-mesenchymal transition and breast cancer progression. Sci Rep. 2013;3:2932. doi:10.1038/srep02932
28. Yang Y, Liu Q, Li Z, et al. GP73 promotes epithelial-mesenchymal transition and invasion partly by activating TGF-beta1/Smad2 signaling in hepatocellular carcinoma. Carcinogenesis. 2018;39(7):900–910. doi:10.1093/carcin/bgy010
29. Wang B, Liu T, Wu J-C, et al. STAT3 aggravates TGF-β1-induced hepatic epithelial-to-mesenchymal transition and migration. Biomed Pharmacother. 2018;98:214–221. doi:10.1016/j.biopha.2017.12.035
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.