Back to Journals » Cancer Management and Research » Volume 13
Diverse Distribution and Gene Expression on the 21-Gene Recurrence Assay in Breast Cancer Patients with Locoregional Recurrence Versus Distant Metastasis
Authors Lu Y, Tong Y, Huang J, Lin L, Wu J, Fei X, Chen X , Shen K
Received 12 April 2021
Accepted for publication 19 July 2021
Published 10 August 2021 Volume 2021:13 Pages 6279—6289
DOI https://doi.org/10.2147/CMAR.S314461
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Yong Teng
Yujie Lu,1,* Yiwei Tong,1,* Jiahui Huang,1 Lin Lin,2 Jiayi Wu,1 Xiaochun Fei,3 Xiaosong Chen,1 Kunwei Shen1
1Department of General Surgery, Comprehensive Breast Health Center, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, People’s Republic of China; 2Department of Clinical Laboratory, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, 200025, People’s Republic of China; 3Department of Pathology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaosong Chen; Kunwei Shen
Department of General Surgery, Comprehensive Breast Health Center, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, People’s Republic of China
Email [email protected]; [email protected]
Background: It remains uncertain whether the 21-gene recurrence score (RS) of a primary tumor has selective prognostic value for locoregional recurrence (LRR) or distant metastasis (DM). The current study aimed to compare the distribution and single-gene expression on the RS panel in breast cancer patients with LRR versus DM.
Methods: Consecutive early breast cancer patients who had been operated on at the Comprehensive Breast Health Center, Ruijin Hospital from January 2009 to December 2016 were retrospectively reviewed. Patients were divided into LRR, DM, and no-recurrence groups according to the first reported recurrent event. Comparison and subgroup analysis of 21-gene RS, RS category, and single-gene expression on the RS panel were conducted among patients with different recurrence status.
Results: A total of 1,287 patients were included, with median follow-up of 61.5 months, and 27, 47, and 1,213 patients were classified as LRR, DM, and no recurrence groups, respectively. RS was significantly diversely distributed among the three groups (P< 0.001). No-recurrence patients (median 22) presented much lower RS than LRR (median 39, P< 0.001) and DM (median 30, P< 0.001) patients. LRR patients had lower PR (P< 0.001), BCL2 (P=0.010), and CEGP1 (P< 0.001) expression, and DM patients had higher STMY3 (P=0.019) expression than no-recurrence patients. Moreover, CEGP1 expression was significantly lower in the LRR group than the DM one (P=0.028).
Conclusion: RS was differently distributed between recurrent and nonrecurrent patients. PR, BCL2, CEGP1, and STMY 3 expression was associated with LRR and DM, while CEGP1 was lower in the LRR group than DM patients, warranting further clinical evaluation.
Keywords: breast cancer, 21-gene recurrence assay, gene expression, locoregional recurrence, distant metastasis
Introduction
Breast cancer is the most common global malignancy in women nowadays.1,2 Approximately 20%–30% of early breast cancer patients will develop disease recurrence, including locoregional recurrence (LRR) or distant metastasis (DM), despite early detection and standard comprehensive treatment.3,4 Disease recurrence, especially in distant sites, is recognized as a major reason for breast cancer–related death.5 Therefore, the prevention and treatment of LRR or DM is an important challenge in breast cancer.
LRR- and DM-risk assessment is primarily based on traditional clinical and histological items.6 Vila et al declared that clinical stage, pathological stage, histological grade, and estrogen receptor (ER) status can stratify risk of LRR,7 and based on a large population from the Netherlands, Maaren et al found that breast cancer subtype was an important factor in DM.8 Nowadays, multigene assays are widely used in breast cancer management.9,10 Several recent studies have demonstrated that clinicopathological profiles and genomic assays have similar prognostic values for DM and LRR.9, 11–15
Isolated LRR at any site has been confirmed as a prognostic factor of DM and inferior disease outcome.16–18 Clinical management is quite different between patients with LRR and DM.19 Treatment options for LRR include complete resection of recurrent lesions, radiotherapy, and systemic treatment based on histological examination and restaging. DM is generally treated with palliative intent and therapy.20 Therefore, identifying patients prone to LRR or DM and strengthening local or systemic treatment accordingly may contribute to improvement in disease outcomes.
Recent studies have demonstrated that traditional clinical and pathological features show better prognostic value in DM than LRR.7,1121–23 With multigene assays assessing the risk of disease recurrence,24,25 most showed more prognostic value in DM than LRR.26 Nowadays, the 21-gene recurrence score (RS) assay is most widely used to indicate systemic treatment,27 and shows considerable prognostic value in hormone receptor (HR)–positive, HER2-negative breast cancer patients.2,28,29 Recently, a few investigations have evaluated the prognostic value of RSs in LRR.26,30,31 Mamounas et al demonstrated that RS had prognostic value for LRR risk in ER-positive patients in both node-negative30 and node-positive cohorts31 based on large data from the National Surgical Adjuvant Breast and Bowel Project trials B14 and B20. Inconsistent results were revealed in subsequent smaller studies.32,33 For instance, in a retrospective analysis of a subset of patients of the E2197 trial by the Eastern Cooperative Oncology Group (ECOG), Solin et al found no significant association between RS and LRR after median follow-up of 10 years.34 There is still a lack of consensus as to whether RS has selective prognostic value for LRR in early breast cancer patients. RS has also shown considerable association with disease outcome in recurrent breast cancer patients, as demonstrated in our previous work,29 and showed different prognostic value between LRR and DM subcohorts. However, there were limited data on RS categories or gene-expression distribution among patients with LRR or DM.
In this study, we aimed to evaluate the distribution of RS among patients with LRR or DM, and then to compare single-gene expression with the RS panel between LRR and DM groups.
Methods
Study Population
We retrospectively screened consecutive breast cancer patients treated at the Comprehensive Breast Health Center, Ruijin Hospital Shanghai Jiao Tong University School of Medicine. Eligibility criteria were surgery between January 2009 and December 2016, 21-gene RS results available, as well as single-gene expression on RS panel for the primary tumor, complete clinicopathological and immunohistochemical information on primary tumors, and complete follow-up. Exclusion criteria were history of breast malignancy, malesex, T3–T4 tumor, and more than three positive lymph nodes at time of surgery. Detailed clinical data were retrieved from the Shanghai Jiao Tong University Breast Cancer Database. All patients had signed a consent form for recording their treatment information in our database that may be used in scientific analysis, and our study was approved by the independent ethical committees of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine. All procedures were in accordance with the 1964 Declaration of Helsinki and its later amendments.
Histopathological Analysis and 21-Gene RS Testing
Tumor histopathological examination was conducted by at least two experienced pathologists from the Department of Pathology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine. Positive criteria for immunohistochemical assessment of ER, progesterone receptor (PR), HER2, and Ki67 were as presented in our previous article.35
The 21-gene RS testing was performed in the Department of Clinical Laboratory, as described in our previous work.36 After careful quality control to assure adequate tumor tissue, RNA was extracted from unstained formalin-fixed, paraffin-embedded (FFPE) sections of breast tumors using an RNeasy FFPE RNA kit (Qiagen 73504). An OmniScript RT kit (Qiagen 205111) was used for reverse transcription. Single-gene expression was evaluated by quantitative real-time PCR using an Applied Biosystems 7500 real-time PCR system with Premix Ex Taq (Takara Bio RR390A). Expression of each gene, measured by threshold cycle (CT), was confirmed in triplicate and normalized according to five endogenous reference genes — TFRC, RPLPO, GUS, GAPDH, and ACTB — and recorded as –ΔCT = CTreference – CTgene. ER, HER2, proliferation, and invasion group scores were calculated accordingly.29 Patients were divided into low- (RS <18), intermediate- (18–30), and high-risk (≥31) groups as per our previous work.37,38
Follow-Up
Patient follow-up was carried out annually by specialized breast cancer nurses or staff at our center. Patients were categorized into three groups according to their first presentation of disease relapse: LRR (including recurrence in the chest wall, ipsilateral breast, or regional lymph nodes), DM at any site, and no recurrence.39 Patients with concurrent LRR and DM were put into the DM group. The last follow-up was accomplished in October 2020.
Statistical Analysis
Categorical data were analyzed using univariate ?2 and multivariate logistic regression. Continuous variables were compared among groups by one-way ANOVA. Kruskal–Wallis tests were conducted for abnormally distributed data. Post hoc analyses were conducted between every two groups, using Bonferroni when data showed homogeneity of variance and Games–Howell for heterogeneity of variance. Survival difference by RS category was compared using Kaplan–Meier curves. All statistical analysis was conducted using SPSS 23.0 and GraphPad Prism 8.0. Receiver-operating characteristic (ROC) curves was used to compare the prognostic performance of RS for different recurrence status. Two-sided P<0.05 was considered statistically significant.
Results
Baseline Characteristics
Overall, 1,370 HR-positive/HER2-negative breast cancer patients with 21-gene RS records were retrospectively reviewed, and 1,287 were included for final analysis (Figure 1). At a median follow-up of 61.5 (Q1 46.9, Q3 80.9) months, 74 patients had disease recurrence (Table 1). The first reported recurrence event was LRR in 27 patients and DM in 47.
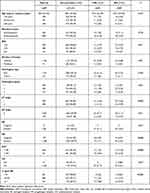 |
Table 1 Baseline characteristics of study participants and impact factors for recurrence status |
 |
Figure 1 Flowchart of included patients. Abbreviations: CBHC, Comprehensive Breast Health Center; RS, recurrence score; IHC, immunohistochemical. |
Clinicopathological features are presented in Table 1. Median age was 58 (24–92) years. At the time of initial surgery, 825 patients were postmenopausal. Regarding histopathological features, pT1 tumors were found in 903 patients, and 84.85% of patients (n=1,092) were node-negative. ER- and PR-positive tumors were observed in 1,281 and 1,108 patients, respectively. Ki67 in 49.03% patients was >14% (n=631). Lymphovascular invasion was found in 41 patients. On 21-gene RS, 298 (23.15%), 605 (47.01%), and 384 (29.84%) patients were categorized into the low-, intermediate-, and high-risk groups, respectively.
Clinicopathologic features were compared based on patients’ recurrence status. Univariate analysis (Table 1) showed that AJCC pT stage (P=0.003), PR (P=0.046), and Ki67 (P=0.004) were distributed differently among the groups. Multivariate logistic regression analysis found AJCC pT stage was significantly associated with recurrence status (P=0.049, Table 2). DM patients were less likely to have pT1 tumors (OR 0.50, 95% CI 0.28–0.91; P=0.023) than no-recurrence patients.
 |
Table 2 Multivariate analysis of impact factors for recurrence status |
Association of Surgery, Adjuvant Treatment, and Recurrence Status
Mastectomy was conducted in 59.13% of enrolled patients, and showed no impact on recurrence status (P=0.917, Table 3). Axillary lymph node–dissection was performed on 38.77% of patients and significantly more in LRR and DM patients than the no-recurrence population (P=0.020). In terms of adjuvant treatment, 565 (43.9%) patients received radiation therapy after the first operation. A smaller proportion of LRR patients experienced radiation therapy (29.63%, eight of 27) than DM (46.81%, 22 of 47) and no-recurrence patients (44.11%, 535 of 1 213); however, no statistically significant difference was observed (P=0.299). Regarding systemic treatment, half the patients (50.27%) received chemotherapy in an adjuvant setting, and almost all (96.50%) received endocrine therapy. Adjuvant chemotherapy was applied in 51.85%, 70.21%, and 49.46% of patients with LRR, DM, and no recurrence, respectively (P=0.020). There was no significant difference in usage of adjuvant endocrine therapy (P=0.508).
 |
Table 3 Local and systemic treatment based on recurrence status |
Association Between 21-Gene RS and Recurrence Status
The distribution of 21-gene RS and RS categories among patients with different recurrence status is shown in Figure 2. In general, no-recurrence patients (median, 22) had much lower RS than LRR (median 39, P<0.001; Figure 2A) and DM (median 30, P<0.001) patients, while the latter two groups had similar RS (P=0.266). RS risk was differently distributed among the three groups on univariate analysis (P<0.001, Figure 2B). The proportion of high-risk patients was lowest in the no-recurrence group (16.4%: P<0.001 compared to LRR, P<0.001 compared to DM) and highest in the LRR group (70.4%, P=0.035 compared to DM).
Multivariate analysis showed that the 21-gene RS category was independently associated with patient recurrence (P<0.001, Table 2). Compared to no-recurrence patients, LRR patients were more likely to be high-risk (low-risk vs high-risk, OR 0.04, 95% CI 0.01–0.27, P=0.001; intermediate-risk vs high-risk, OR 0.13, 95% CI 0.05–0.32, P<0.001). Similarly, DM patients were also more likely to be high-risk compared to no-recurrence ones (low-risk vs high-risk, OR 0.09, 95% CI 0.03–0.29, P<0.001; intermediate-risk vs high-risk, OR 0.31, 95% CI 0.17–0.58, P<0.001). ROC curves of the prognostic value of RS for LRR and DM are shownd in Supplementary Figure S1. Compared to prognostic performance for DM (3-year AUC 0.778, 5-year AUC 0.736), RS showed higher AUC values on prognosis for LRR (3-year AUC 0.852, 5-year AUC 0.822).
Further subgroup analysis was performed to compare RS distribution among LRR, DM, and no-recurrence patients, stratified by tumor size, nodal status, and application of adjuvant chemotherapy and radiation therapy (Figure 3). LRR and DM patients showed consistently similar RS to each other, but both were significantly higher than no-recurrence patients, regardless of tumor size (Figure 3A), adjuvant chemotherapy use (Figure 3C), or radiation-therapy application (Figure 3D). However, for node-negative patients, RS was significantly higher in LRR than DM (P=0.033, Figure 3B) and no-recurrence (P<0.001) patients. For the pN1 group, a significantly higher RS in the DM group (P=0.011) and numerically higher RS in the LRR group (P=0.060) were observed compared to no-recurrence ones.
Single-Gene Expression in 21-Gene RS Panel by Recurrence Status
Single-gene expression of the 21-gene RS panel was compared among LRR, DM, and no-recurrence groups. We found that PR (P<0.001, Figure 4), BCL2 (P=0.016), CEGP1 (P=0.004), and STMY3 (P=0.034) were differently distributed among LRR, DM, and no-recurrence patients. In detail, PR (P<0.001), BCL2 (P=0.010), and CEGP1 (P<0.001) were significantly lower in LRR patients than the no-recurrence group. Lower CEGP1 (P=0.028) was observed in the LRR group than the DM group, contributing to the RS distinction between the LRR and DM groups. STMY3 was higher in DM patients than no-recurrence ones (P=0.019).
Group scores were calculated from normalized single-gene expression levels. LRR patients had significantly higher proliferation (median 7.328 vs 6.947, P=0.006; Supplementary Figure S2A), higher invasion (8.420 vs 7.837, P=0.020; Supplementary Figure S2B), aand ER scores (8.156 vs 8.779, P=0.020; Supplementary Figure S2D) than no-recurrence patients. Compared to no-recurrence patients, invasion scores were significantly higher in DM patients (8.240 vs 7.837, P=0.034). Proliferation, invasion, and ER scores were similar between LRR and DM patients (all P>0.05). HER2 scores were generally similar among the groups (P=0.533, Supplementary Figure S2C).
Discussion
In this study, we included 1,287 patients from 5,854 consecutive early HR-positive/HER2-negative breast cancer patients with single-gene expression data on the 21-gene RS test. We found that RS was differently distributed among patients with different recurrence status. LRR and DM patients showed consistently similar RS to each other, both significantly higher than no-recurrence ones. Regarding single-gene expression, CEGP1 was the only gene differently expressed between the LRR and DM groups. Other differently expressed genes included PR and BCL2 between the LRR and no-recurrence groups and STMY3 between the DM and no-recurrence groups. To our knowledge, this is the first study to compare the distribution and single-gene expression of the 21-gene RS panel among patients with different recurrence types, especially between LRR and DM.
Breast cancer is a biologically heterogeneous disease, resulting in varying outcomes.40 Disease recurrence happened in patients is a major factor in poor prognosis, while risk factors and clinical management are different between patients with recurrence in locoregional and distant organs.41 Traditional clinicopathological parameters, such as tumor size, axillary lymph–node metastasis, lymphovascular invasion, histological grade, and biomarker status, are regularly taken into consideration when estimating LRR risk.42 Prognostic models based on these have been constructed and validated in a small retrospective database, which showed limited application14 because of unavoidable selection bias in training and validating cohorts. To date, no consensus has been reached on the optimum prognostic model to distinguish the risk of LRR and DM. The 21-gene RS is well known to be related to DM,43 and association with LRR has been reported in several previous studies.26,30,31,44 From a single institutional database, Turashvili et al observed 4-yearf LRR of 0.84%, 2.72%, and 2.80% in low-, intermediate-, and high-RS groups, respectively.45 Moreover, in a recent analysis of Southwest Oncology Group (SWOG) Intergroup trial S8814, higher RS was associated with increased LRR rate in HR-positive breast cancer patients with regional lymph–node metastasis.44 Although these studies confirmed the prognostic value of RS for LRR, none evaluated whether RS were capable of separating LRR risk from DM. In this study, pT1–pT2 breast cancer patients with no more than three positive axillary lymph nodes were retrospectively involved. We found significantly higher risk in patients who developed LRR during follow-up than no-recurrence patients with other clinical outcomes and a trend of higher risk thano DM patients. In addition, we also confirmed the prognostic value of RS for both LRR and DM risk in our cohort regardless of node status, which was in line with the aforementioned studies. Additionally, considering RS as a continuous variable, we also noticed a significantly higher RS in LRR and DM patients than no-recurrence patients. Our finding is consistent with previous results:30,31 RS may have prognostic value for LRR in early breast cancer patients, but we did not find evidence that RS can distinguish the risk of LRR from DM.
RS is regularly applied in HR-positive/HER2-negative early breast cancer with no lymph-node metastasis nowadays, and in a recent study we proved that RS still had prognostic value in node-positive patients.28 Here, we revealed that patients who develop LRR as the first recurrence event have a trend of higher RS and a higher ratio of high-risk RS than patients who develop DM as the first recurrence event. Traditional clinicopathological parameters related to RS, such as age, menopausal status, tumor size, histological grade, and PR status, were well balanced between LRR and DM patients in our cohort. The phenomenon might contribute to the difference in molecular and biological mechanisms between LRR and DM. Metastasis is frequently considered the final and fatal step in the progression of carcinoma, including tumor-cell escape from primary lesion, intravasation, survival in blood or lymphatic circulation, extravasation into a secondary site, angiogenesis, and uninhibited growth.46 Previous studies have indicated that some circulating tumor cells can seed primary tumors based on attraction signals, leading to residual tumor lesions or local recurrence.47 Preclinical studies have revealed that self-seeding is preferentially mediated by aggressive BCL2-expressing circulating tumor cells and attracted by tumor-derived inflammatory cytokines in vivo.48 In the present analysis, we found a significant difference in expression of PR, BCL2, CEGP1, and STMY3 among the three groups. LRR patients had the lowest ER-group scores and DM patients higher invasion-group score. Dissimilarly to previous in vitro examination, we did not find any connection between STMY3 expression and LRR occurrence. Instead, we first noticed that LRR patients had significantly lower expression of CEGP1, a critical factor in adult angiogenesis, than DM and no-recurrence patients. Cheng et al demonstrated that CEGP1 is a prognostic marker for favorable clinical outcomes,49 and here we found it to be a protective marker for LRR in breast cancer, which may be attributable to its anti-BMP activity50 and ability in suppressing cancer-cell mobility and invasiveness.51 On the other side, STMY3, known as one of the stroma-derived factors in remodeling the tumor microenvironment, enhancing migration and invasion of cancer cells,52,53 showed a more aggressive impact on DM than LRR, suggesting a distant organ–directed signal in circulating breast cancer cells. These results may provide us more evidence in understanding different mechanisms of breast cancer cells’ local recurrence and metastasis.
In the current study, we found various distribution of RS among patients with different recurrence status. This was the largest cohort — 5857 consecutive patients — for evaluation of the association between RS distribution and recurrence status in early breast cancer patients. We found that LRR patients had higher RS than patients with other disease outcomes, and we noticed that CEGP1 was differently expressed in LRR and DM patients. The rate of DM was relatively low, for which there are several explanations. First, here we reported on the percentage of patients based on their first invasive disease-free survival event. Only 47 (3.65%) patients had DM as their first relapse event, another 17 patients further developed DM beyond LRR and were put into the LRR group, and the overall rate of DM in our cohort was 4.97% after a median follow-up of 5 years. Second, the population included in the current study was RS-indicated, ie, luminal-like HER2-negative T1 or T2 tumors with no more than three positive lymph nodes. According to results from the TAILORx trial,2 the percentage of patients whose first invasive disease-free survival event was distant recurrence was only 3.16% (307 of 9,717) at median follow-up of 90 months. Five-year invasive disease-free survival in the 195 pN1 patients in our study was 91.3%, which was also consistent with the RxPONDER trial (91.1%).
This study contains several limitations. Selection bias in the study population was unavoidable, which is inherent in retrospective analysis. RS of a part of patients are retrospectively examined that did not influence adjuvant treatment but in some more recent patients, RS was routinely tested to indicate adjuvant chemotherapy. Cutoffs for risk groups were not aligned with the recently published TAILORx study or the RxPonder trial. Last but not least, the smaller number of recurrence events prevented us undertaking further subgroup analysis to address the prognostic value of RS in different adjuvant treatments or to distinguish in more detail different recurrence locations, such as LRR and regional recurrence, or DM in visceral, bone, and brain tissue.
In conclusion, we found that RS was variously distributed in early breast cancer patients with different recurrence status. RS category was independently associated with LRR and DM events, which was mainly attributed to higher proliferation-group scores, higher invasion-group scores, and lower ER-group scores. LRR patients had lower PR, BCL2, and CEGP1 expression than no-recurrence patients. Moreover, CEGP1 expression was significantly lower in the LRR group than the DM one. Our findings provide us more data about risk estimation for LRR for future analysis of potential mechanisms in breast cancer–cell recurrence and metastasis.
Abbreviations
RS, recurrence score; LRR, locoregional recurrence; DM, distant metastasis.
Data Sharing Statement
All data of this article were retrieved from the Shanghai Jiao Tong University Breast Cancer Database (SJTU-BCDB), and are available on request to the corresponding author, Professor Xiaosong Chen.
Ethics Approval
This study was reviewed and approved by the independent ethical committees of Ruijin Hospital, Shanghai Jiaotong University School of Medicine. All procedures involving human participants were consistent with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Consent for Publication
All authors gave consent for publication.
Acknowledgments
The authors would like to thank the assistance of Ms Yidong Du in inputting SJTU-BCDB and Dr Yitian Xiao from the University of California, Davis in language polishing.
Funding
The authors appreciate the financial support from the National Natural Science Foundation of China (grants 81772797 and 82072897), Shanghai Municipal Education Commission—Gaofeng Clinical Medicine Grant Support (20172007), and the Shanghai Science and Technology Committee Sailing Program (21YF1427400). All these sponsors had no role in study design, collection, analysis, or interpretation of data.
Disclosure
The authors declare no competing interests.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Sparano JA, Gray RJ, Ravdin PM, et al. Clinical and genomic risk to guide the use of adjuvant therapy for breast cancer. N Engl J Med. 2019;380(25):2395–2405. doi:10.1056/NEJMoa1904819
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi:10.3322/caac.21590
4. Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. doi:10.1016/S0140-6736(05)66544-0
5. Tevaarwerk AJ, Gray RJ, Schneider BP, et al. Survival in patients with metastatic recurrent breast cancer after adjuvant chemotherapy: little evidence of improvement over the past 30 years. Cancer. 2013;119(6):1140–1148. doi:10.1002/cncr.27819
6. Voogd A, Nielsen M, Peterse J, et al. Differences in risk factors for local and distant recurrence after breast-conserving therapy or mastectomy for stage I and II breast cancer: pooled results of two large European randomized trials. J Clin Oncol. 2001;19(6):1688–1697. doi:10.1200/JCO.2001.19.6.1688
7. Vila J, Teshome M, Tucker SL, et al. Combining clinical and pathologic staging variables has prognostic value in predicting local-regional recurrence following neoadjuvant chemotherapy for breast cancer. Ann Surg. 2017;265(3):574–580. doi:10.1097/SLA.0000000000001492
8. van Maaren MC, de Munck L, Strobbe LJA, et al. Ten-year recurrence rates for breast cancer subtypes in the Netherlands: a large population-based study. Int J Cancer. 2019;144(2):263–272. doi:10.1002/ijc.31914
9. Poorvu P, Gelber S, Rosenberg S, et al. Prognostic Impact of the 21-gene recurrence score assay among young women with node-negative and node-positive ER-positive/HER2-negative breast cancer. J Clin Oncol. 2020;38(7):725–733. doi:10.1200/JCO.19.01959
10. Sestak I, Buus R, Cuzick J, et al. Comparison of the performance of 6 prognostic signatures for estrogen receptor-positive breast cancer: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2018;4(4):545–553. doi:10.1001/jamaoncol.2017.5524
11. Voduc KD, Cheang MC, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010;28(10):1684–1691. doi:10.1200/JCO.2009.24.9284
12. Warren LE, Ligibel JA, Chen YH, Truong L, Catalano PJ, Bellon JR. Body Mass index and locoregional recurrence in women with early-stage breast cancer. Ann Surg Oncol. 2016;23(12):3870–3879. doi:10.1245/s10434-016-5437-3
13. Nguyen P, Taghian A, Katz M, et al. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol. 2008;26(14):2373–2378. doi:10.1200/JCO.2007.14.4287
14. Corso G, Maisonneuve P, Massari G, et al. Validation of a novel nomogram for prediction of local relapse after surgery for invasive breast carcinoma. Ann Surg Oncol. 2020;27(6):1864–1874. doi:10.1245/s10434-019-08160-7
15. Stuart-Harris R, Dahlstrom JE, Gupta R, Zhang Y, Craft P, Shadbolt B. Recurrence in early breast cancer: analysis of data from 3765 Australian women treated between 1997 and 2015. Breast. 2019;44:153–159. doi:10.1016/j.breast.2019.02.004
16. Taghian A, Jeong JH, Mamounas E, et al. Patterns of locoregional failure in patients with operable breast cancer treated by mastectomy and adjuvant chemotherapy with or without tamoxifen and without radiotherapy: results from five national surgical adjuvant breast and bowel project randomized clinical trials. J Clin Oncol. 2004;22(21):4247–4254.
17. Wapnir I, Anderson S, Mamounas E, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in five national surgical adjuvant breast and bowel project node-positive adjuvant breast cancer trials. J Clin Oncol. 2006;24(13):2028–2037. doi:10.1200/JCO.2005.04.3273
18. Tanis E, van de Velde C, Bartelink H, van de Vijver M, Putter H, van der Hage J. Locoregional recurrence after breast-conserving therapy remains an independent prognostic factor even after an event free interval of 10 years in early stage breast cancer. Eur J Cancer. 2012;48(12):1751–1756. doi:10.1016/j.ejca.2012.02.051
19. Belkacemi Y, Hanna NE, Besnard C, Majdoul S, Gligorov J. Local and regional breast cancer recurrences: salvage therapy options in the new era of molecular subtypes. Front Oncol. 2018;8:112. doi:10.3389/fonc.2018.00112
20. Holleczek B, Stegmaier C, Radosa JC, Solomayer EF, Brenner H. Risk of loco-regional recurrence and distant metastases of patients with invasive breast cancer up to ten years after diagnosis - results from a registry-based study from Germany. BMC Cancer. 2019;19(1):520. doi:10.1186/s12885-019-5710-5
21. Hunt KK, Ballman KV, McCall LM, et al. Factors associated with local-regional recurrence after a negative sentinel node dissection: results of the ACOSOG Z0010 trial. Ann Surg. 2012;256(3):428–436. doi:10.1097/SLA.0b013e3182654494
22. Millar EK, Graham PH, O’Toole SA, et al. Prediction of local recurrence, distant metastases, and death after breast-conserving therapy in early-stage invasive breast cancer using a five-biomarker panel. J Clin Oncol. 2009;27(28):4701–4708. doi:10.1200/JCO.2008.21.7075
23. Jwa E, Shin KH, Kim JY, et al. Locoregional recurrence by tumor biology in breast cancer patients after preoperative chemotherapy and breast conservation treatment. Cancer Res Treat. 2016;48(4):1363–1372. doi:10.4143/crt.2015.456
24. Fitzal F, Filipits M, Rudas M, et al. The genomic expression test EndoPredict is a prognostic tool for identifying risk of local recurrence in postmenopausal endocrine receptor-positive, her2neu-negative breast cancer patients randomised within the prospective ABCSG 8 trial. Br J Cancer. 2015;112(8):1405–1410. doi:10.1038/bjc.2015.98
25. Geffen D. Should decisions on adding adjuvant chemotherapy in early-stage ER-positive breast cancer be based on gene expression testing or clinicopathologic factors or both? Ann Oncol. 2018;29(5):1096–1098. doi:10.1093/annonc/mdy115
26. Bustamante Eduardo M, Popovici V, Imboden S, et al. Characterization of molecular scores and gene expression signatures in primary breast cancer, local recurrences and brain metastases. BMC Cancer. 2019;19(1):549. doi:10.1186/s12885-019-5752-8
27. Sparano JA, Gray RJ, Makower DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;379(2):111–121. doi:10.1056/NEJMoa1804710
28. Tong Y, Wu J, Huang O, et al. 21-gene recurrence score and adjuvant chemotherapy decision for breast cancer patients with positive lymph nodes. Sci Rep. 2019;9(1):13123. doi:10.1038/s41598-019-49644-6
29. Lu Y, Tong Y, Huang J, et al. Primary 21-gene recurrence score and disease outcome in loco-regional and distant recurrent breast cancer patients. Front Oncol. 2020;10:1315. doi:10.3389/fonc.2020.01315
30. Mamounas EP, Tang G, Fisher B, et al. Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: results from NSABP B-14 and NSABP B-20. J Clin Oncol. 2010;28(10):1677–1683. doi:10.1200/JCO.2009.23.7610
31. Mamounas EP, Liu Q, Paik S, et al. 21-gene recurrence score and locoregional recurrence in node-positive/ER-positive breast cancer treated with chemo-endocrine therapy. J Natl Cancer Inst. 2017;109(4):djw259. doi:10.1093/jnci/djw259
32. Jegadeesh NK, Kim S, Prabhu RS, et al. The 21-gene recurrence score and locoregional recurrence in breast cancer patients. Ann Surg Oncol. 2015;22(4):1088–1094. doi:10.1245/s10434-014-4252-y
33. Thaker NG, Hoffman KE, Stauder MC, et al. The 21-gene recurrence score complements IBTR! Estimates in early-stage, hormone receptor-positive, HER2-normal, lymph node-negative breast cancer. Springerplus. 2015;4(1):36. doi:10.1186/s40064-015-0840-y
34. Solin LJ, Gray R, Goldstein LJ, et al. Prognostic value of biologic subtype and the 21-gene recurrence score relative to local recurrence after breast conservation treatment with radiation for early stage breast carcinoma: results from the Eastern Cooperative Oncology Group E2197 study. Breast Cancer Res Treat. 2012;134(2):683–692. doi:10.1007/s10549-012-2072-y
35. Zhu S, Wu J, Huang O, et al. Clinicopathological features and disease outcome in breast cancer patients with hormonal receptor discordance between core needle biopsy and following surgical sample. Ann Surg Oncol. 2019;26(9):2779–2786. doi:10.1245/s10434-019-07480-y
36. Wu J, Fang Y, Lin L, et al. Distribution patterns of 21-gene recurrence score in 980 Chinese estrogen receptor-positive, HER2-negative early breast cancer patients. Oncotarget. 2017;8(24):38706–38716. doi:10.18632/oncotarget.16313
37. Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24(23):3726–3734. doi:10.1200/JCO.2005.04.7985
38. Geyer CE
39. Hudis CA, Barlow WE, Costantino JP, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol. 2007;25(15):2127–2132. doi:10.1200/JCO.2006.10.3523
40. McGuire A, Lowery AJ, Kell MR, Kerin MJ, Sweeney KJ. Locoregional recurrence following breast cancer surgery in the trastuzumab era: a systematic review by subtype. Ann Surg Oncol. 2017;24(11):3124–3132. doi:10.1245/s10434-017-6021-1
41. Elsayed M, Alhussini M, Basha A, Awad AT. Analysis of loco-regional and distant recurrences in breast cancer after conservative surgery. World J Surg Oncol. 2016;14(1):144. doi:10.1186/s12957-016-0881-x
42. Corso G, Maisonneuve P, Santomauro G, et al. Ipsilateral breast tumor reappearance and contralateral breast cancer after primary breast cancer treatment: a comprehensive retrospective study of 15,168 patients. Oncology. 2018;95(3):147–155. doi:10.1159/000488764
43. Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351(27):2817–2826. doi:10.1056/NEJMoa041588
44. Woodward WA, Barlow WE, Jagsi R, et al. Association between 21-gene assay recurrence score and locoregional recurrence rates in patients with node-positive breast cancer. JAMA Oncol. 2020;6(4):505–511. doi:10.1001/jamaoncol.2019.5559
45. Turashvili G, Chou JF, Brogi E, et al. 21-Gene recurrence score and locoregional recurrence in lymph node-negative, estrogen receptor-positive breast cancer. Breast Cancer Res Treat. 2017;166(1):69–76. doi:10.1007/s10549-017-4381-7
46. Minn A, Gupta G, Siegel P, et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436(7050):518–524. doi:10.1038/nature03799
47. Kim M-Y, Oskarsson T, Acharyya S, et al. Tumor self-seeding by circulating cancer cells. Cell. 2009;139(7):1315–1326. doi:10.1016/j.cell.2009.11.025
48. Comen E, Norton L, Massague J. Clinical implications of cancer self-seeding. Nat Rev Clin Oncol. 2011;8(6):369–377. doi:10.1038/nrclinonc.2011.64
49. Cheng CJ, Lin YC, Tsai MT, et al. SCUBE2 suppresses breast tumor cell proliferation and confers a favorable prognosis in invasive breast cancer. Cancer Res. 2009;69(8):3634–3641. doi:10.1158/0008-5472.CAN-08-3615
50. Lin Y-C, Chen -C-C, Cheng C-J, Yang R-B. Domain and functional analysis of a novel breast tumor suppressor protein, SCUBE2. J Biol Chem. 2011;286(30):27039–27047. doi:10.1074/jbc.M111.244418
51. Lin YC, Lee YC, Li LH, Cheng CJ, Yang RB. Tumor suppressor SCUBE2 inhibits breast-cancer cell migration and invasion through the reversal of epithelial-mesenchymal transition. J Cell Sci. 2013;127(1):85–100.
52. Min KW, Kim DH, Do SI, et al. Diagnostic and prognostic relevance of MMP-11 expression in the stromal fibroblast-like cells adjacent to invasive ductal carcinoma of the breast. Ann Surg Oncol. 2013;20(Suppl 3):S433–S442. doi:10.1245/s10434-012-2734-3
53. Zhang X, Huang S, Guo J, et al. Insights into the distinct roles of MMP-11 in tumor biology and future therapeutics (Review). Int J Oncol. 2016;48(5):1783–1793. doi:10.3892/ijo.2016.3400
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.