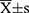Back to Journals » Infection and Drug Resistance » Volume 15
Distribution and Drug Resistance of Pathogenic Bacteria and Prognosis in Patients with Septicemia Bloodstream Infection with Renal Insufficiency
Authors Pan D, Peng P, Fang Y, Lu J, Fang M
Received 6 May 2022
Accepted for publication 20 July 2022
Published 28 July 2022 Volume 2022:15 Pages 4109—4116
DOI https://doi.org/10.2147/IDR.S373665
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Deng Pan,1,* Pin Peng,2,* Yu Fang,3 Jun Lu,3 Minghao Fang3
1Department of Infectious Disease, Xianning Central Hospital, The First Affiliated Hospital of Hubei University of Science and Technology, Xianning, Hubei, People’s Republic of China; 2Intensive Care Unit, Wuhan Asia General Hospital, Wuhan, Hubei, People’s Republic of China; 3Intensive Care Unit, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Minghao Fang, Intensive Care Unit, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, No. 1095 Jiefang Avenue, Wuhan, Hubei, 430030, People’s Republic of China, Tel +86-15071157405, Email [email protected]
Objective: The aim of this study was to investigate the distribution and drug resistance of pathogenic bacteria and the prognosis of patients with sepsis bloodstream infection with renal insufficiency.
Methods: One hundred and twelve patients with septicemic bloodstream infection with renal insufficiency and 112 patients with septic bloodstream infection without renal insufficiency were selected as study group and control group, respectively. We compare the distribution of pathogenic bacteria, analyze the drug resistance of major bacteria, and compare the efficacy, the incidence of septic shock, duration of mechanical ventilation, hospitalization time, and duration of antimicrobial drug administration between the two groups.
Results: A total of 140 pathogenic strains were isolated from blood cultures in the study group, and 136 strains were isolated from blood cultures in the control group. The sepsis bloodstream infection was mainly caused by Gram-negative bacteria, accounting for 59.42% (164/276). Among the gram-negative bacteria, Klebsiella pneumoniae, Escherichia coli, Pseudomonas aeruginosa, and Acinetobacter baumannii had higher resistance rates to levofloxacin, ceftazidime, piperacillin sodium tazobactam, and amikacin. Among the gram-positive bacteria, Streptococcus pneumoniae, Enterococcus, and Staphylococcus aureus had high resistance rates to clindamycin, cefazolin, penicillin G, gentamicin, azithromycin, and levofloxacin. The rate of extended spectrum β-lactamase (ESBLs)-producing enterobacteria and multi-drug resistant Pseudomonas aeruginosa (MDR-PA) infection was significantly higher in the study group than in the control group; there was no difference in multi-drug resistant Acinetobacter baumannii (MDR-AB), vancomycin-resistant enterococci (VRE), and methicillin-resistant Staphylococcus aureus (MRSA) between the two groups. The duration of hospitalization and the duration of antimicrobial drug administration were longer in the study group than in the control group.
Conclusion: The pathogenic bacteria in patients with sepsis bloodstream infection with renal insufficiency are mainly Gram-negative bacteria, are more difficult to be cured, have a longer course of treatment, and need to use antibacterial drugs for a long time.
Keywords: sepsis bloodstream infection with renal insufficiency, pathogenic bacteria, drug resistance, prognosis
A Letter to the Editor has been published for this article.
Introduction
Sepsis is a serious disease with a mortality rate of up to 25% due to multi-organ dysfunction caused by dysregulation of the body’s response to infection and has become a serious health problem worldwide.1 The number of deaths due to sepsis in the United States accounts for 1/3 of the total number of deaths, and the number of deaths due to sepsis in China likewise amounts to more than 1/3 of the total number of deaths in hospitals. Sepsis in the intensive care unit (ICU) accounts for an even higher mortality rate.2 Bloodstream infection sepsis is a systemic inflammatory reactive disease of the organism caused by pathogenic bacteria undergoing blood circulation and multiplying, releasing toxins and various metabolites.3 Older men with multiple comorbidities are more likely to develop bloodstream infection sepsis.4 It is a serious and highly hazardous infectious disease that predisposes patients to organ failure and death. Renal insufficiency is a chronic progressive damage to the kidneys, resulting in renal atrophy, leading to metabolite retention, disruption of acid-base balance and hydromediator balance, and is one of the causative factors of infectious diseases.5 Patients with renal dysfunction are at increased risk for developing and dying of sepsis.6 Early anti-infection should be given to patients with sepsis bloodstream infection with renal insufficiency, which can effectively improve the prognosis level of patients and can reduce the morbidity and mortality rate. The diagnostic criterion for bloodstream infection is a positive blood culture, but due to the long period of blood culture testing and the extremely low positive rate, early anti-infection treatment is still based on empirical medication. Knowledge of the distribution characteristics of pathogenic bacteria and bacterial drug resistance in patients with septicemia bloodstream infection with renal insufficiency can provide a basis for clinical decision-making according to their characteristics and their drug resistance. Therefore, this study will investigate the distribution of pathogenic bacteria in patients with sepsis bloodstream infection with renal insufficiency and the impact of prognosis.
Data and Methods
Clinical Data
One hundred and twelve patients with sepsis bloodstream infection with renal insufficiency admitted to our hospital from January 2020 to February 2021 were used as the study group, and 112 patients with sepsis bloodstream infection without renal insufficiency admitted to our hospital during the same period were selected as the control group. This study program is in accordance with the relevant requirements of the Declaration of Helsinki of the World Medical Association.7
Inclusion Criteria
(1) meeting the diagnostic criteria of the third international consensus definition of sepsis (Sepsis-3):8 life-threatening organ dysfunction with a dysregulated host response to infection and a SOFA score ≥2; (2) meeting the diagnostic criteria of the US CDC 1996 for bloodstream infection; (3) renal insufficiency using the diagnostic criteria of Nephrology: blood creatinine 178–707 μmol/L and glomerular filtration rate 10–50 mL/min; all patients and family members gave informed consent and those who signed the informed consent form.
Exclusion Criteria
Exclusion criteria include patients with acute and chronic infectious diseases; those with combined malignant neoplasm, immune system or severe psychiatric diseases; and those with combined infections at other sites.
Methods
Pathogenic Culture and Multi-Drug Resistant Bacteria Detection
In strict accordance with the aseptic practice, 5–10 mL of venous blood was drawn from patients in the early morning on an empty stomach and placed in EDTA anticoagulation tubes, and the serum was separated by centrifugation at 3000 r/min for 5 min under room temperature for 30 min. The strains were identified by VITEK-II microbial automatic identification instrument. A drop of the bacteria was incubated at 37℃ with shaking and then placed on standard bacteriology media. Then, all plates were incubated at 37℃ for 24–48 h. After incubation, each plate was examined for bacterial identification and antimicrobial susceptibility testing.9 K-B agar diffusion method was used for the drug sensitivity test of the antibacterial drugs, and the drug sensitivity results were judged according to the American Clinical Laboratory Standardization Institute (CLSI) 2015 standard.10 Multi-drug resistant bacteria refer to bacteria that present resistance to 3 or more types of antimicrobial drugs at the same time, mainly including multi-drug resistant Acinetobacter baumannii (MDR-AB), multi-drug resistant Pseudomonas aeruginosa (MDR-PA), extended spectrum β-lactamase (ESBLs) producing Enterobacteriaceae, vancomycin-resistant enterococci (VRE), methicillin-resistant Staphylococcus aureus (MRSA). Pathogenic bacteria exclude fixed values with contaminating strains, and the same strains were calculated according to the same strains in the same patient who sent specimens multiple times for detection.
Treatment Method
Patients in both groups were treated with appropriate antibacterial drugs based on pathogenic bacteria and drug sensitivity test results, and patients with severe renal insufficiency were treated with bedside hemodialysis.
Observation Index
We compare the distribution of drug-resistant bacteria in the two groups.
Analyze the drug resistance of major gram-negative bacteria.
we compare the efficacy, incidence of septic shock, duration of mechanical ventilation, hospital stay, and duration of antimicrobial drug administration between the two groups.
The efficacy was determined: Significant effect: after 7 days of treatment, clinical symptoms and signs such as rapid heartbeat and respiratory rate disappeared or improved significantly, body temperature returned to below 38℃, and white blood cell count and blood culture returned to normal; Effective: clinical symptoms and signs such as rapid heartbeat and respiratory rate improved, and white blood cell count and blood culture were basically close to normal; Ineffective: none of the above clinical symptoms and signs improved or worsened. Total effective rate = apparent rate + effective rate.
Statistics
SPSS 23.0 software was applied for statistical analysis of the data. All data were subjected to normality test and chi-square test, and the measurement data were expressed by Mean ± SD. t-test was used to measure data between groups, and the difference was considered statistically significant at P<0.05.
Results
Comparison of General Information Between the Two Groups
General data such as age, gender, BMI, disease causation, liver function, and antimicrobial drug use were compared between the two groups and no statistical differences were found, as shown in Table 1.
 |
Table 1 Comparison of General Information of Each Group |
Comparison of the Distribution of Pathogenic Drug Bacteria
A total of 140 pathogenic strains were isolated from blood cultures in the study group, and 136 strains were isolated from blood cultures in the control group. The sepsis bloodstream infection was mainly caused by Gram-negative bacteria, and Gram-negative bacteria accounted for 59.42% (164/276). Gram-negative bacteria in the study group were lower than those in the control group, and Gram-positive bacteria were significantly higher than those in the control group, as shown in Table 2.
 |
Table 2 Comparison of the Distribution of Drug-Resistant Bacteria Between the Two Groups n (%) |
Drug Resistance in Common Gram-Negative Bacteria
Among the Gram-negative bacteria isolated from both groups, Klebsiella pneumoniae had higher resistance rates to ceftriaxone, levofloxacin, ceftazidime, piperacillin sodium tazobactam, amikacin, minocycline, amoxicillin, and the lowest resistance rate to tigecycline; Escherichia coli had higher resistance rates to levofloxacin, ceftazidime, ceftriaxone, piperacillin sodium tazobactam, amikacin, imipenem, Cefoperazone sodium sulbactam sodium, minocycline, and the lowest resistance rate to amoxicillin and tigecycline; Pseudomonas aeruginosa had a higher resistance rate to levofloxacin, cefoperazone sodium sulbactam sodium, ceftazidime, piperacillin sodium tazobactam, amikacin, imipenem; Acinetobacter baumannii had a higher resistance rate to ceftazidime, levofloxacin, piperacillin sodium tazobactam, imipenem, cefoperazone sodium sulbactam sodium, amikacin, amoxicillin and tigecycline, as shown in Table 3.
 |
Table 3 Drug Resistance in Common Gram-Negative Bacteria n (%) |
Drug Resistance of Common Gram-Positive Bacteria
Among the Gram-negative bacteria isolated from the two groups of patients, Streptococcus pneumoniae had higher resistance rates to clindamycin, cefazolin, penicillin G, gentamicin, azithromycin, levofloxacin, cotrimoxazole, and rifampin; Enterococci had higher resistance rates to clindamycin, azithromycin, cefazolin, levofloxacin, gentamicin, penicillin G, and vancomycin; Staphylococcus aureus had higher resistance rates to penicillin G, clindamycin, azithromycin, cefazolin, gentamicin, levofloxacin, rifampicin and compound sulfamethoxazole had higher resistance rates, as shown in Table 4.
 |
Table 4 Drug Resistance of Common Gram-Positive Bacteria n (%) |
Comparison of Multi-Drug Resistant Bacterial Infections Between the Two Groups
The two groups of multi-drug resistant bacteria infections were compared, and it was found that the rate of ESBLs-producing Enterobacteriaceae and MDR-PA infections was significantly higher in the study group than in the control group, while the difference was not statistically significant when comparing MDR-AB, VRE and MRSA in the two groups, see Table 5.
 |
Table 5 Comparison of Multi-Drug Resistant Bacterial Infections Between the Two Groups |
Comparison of the Efficacy of the Two Groups
In addition, we compared the total effective rate of treatment between the two groups and found no difference in the total effective rate between the two groups, as shown in Table 6.
 |
Table 6 Comparison of the Efficacy of the Two Groups n (%) |
Comparison of the Incidence of Septic Shock, Duration of Mechanical Ventilation, Length of Hospitalization, and Duration of Antimicrobial Drug Administration Between the Two Groups
The length of hospital stay and duration of antimicrobial drug administration in the two groups were counted, and it was found that the length of hospital stay and duration of antimicrobial drug administration in the study group were significantly longer than those in the control group; there was no difference in the incidence of septic shock and duration of mechanical ventilation between the two groups, as shown in Table 7.
 |
Table 7 Comparison of the Incidence of Septic Shock, Duration of Mechanical Ventilation, Length of Hospital Stay, and Duration of Antimicrobial Drug Administration Between the Two Groups ( |
Discussion
Sepsis is a common complication of severe trauma, burns, shock, infection and major surgical procedures, with typical clinical symptoms, such as drowsiness, impaired consciousness, oliguria and cough, which progresses more rapidly and can develop into a multi-organ dysfunction syndrome and even lead to death without timely and effective treatment.11 Bloodstream infection is a clinical emergency, and the disease progresses rapidly.12 Renal insufficiency is a group of diseases caused by the destruction of renal tissues, the decrease in glomerular filtration function, and the decrease in the ability of the kidneys to detoxify from various causes, resulting in impairment of the body’s ability to regulate electrolyte balance and excrete metabolites.13 The sepsis bloodstream infection with renal insufficiency will not only increase the condition, but also increase the difficulty of treatment, which is extremely detrimental to the prognosis of patients.14 Currently, anti-infective therapy is often used to treat patients with sepsis bloodstream infection with renal insufficiency, and anti-infective therapy requires analysis of the distribution of pathogenic bacteria in patients in order to improve clinical efficacy. Recent studies pointed out that renal insufficiency is one of the main high-risk factors for associated multi-drug resistant bacterial infections, and the distribution of pathogenic bacteria may not be identical between patients with hemorrhagic infection with renal insufficiency and non-renal patients.15,16 At present, there is a lack of clinical guidelines and expert consensus on the treatment of patients with septicemic bloodstream infection with renal insufficiency, and the relevant literature is scarce, which is therefore not conducive to the empirical use of anti-infective therapy and the selection of antimicrobial drugs in clinical practice.
One study pointed out that the infecting pathogens in patients with bloodstream infection sepsis were predominantly Gram-negative bacteria.17 Our study showed that sepsis bloodstream infection was mainly caused by Gram-negative bacteria, with Gram-negative bacteria accounting for 59.42% (164/276), which is consistent with the results of the above study, suggesting that Gram-negative bacteria were the predominant infecting pathogens in patients with sepsis bloodstream infection with renal insufficiency. However, some studies have also found that Gram-positive bacteria are the predominant infecting pathogens in patients with septicemia bloodstream infection.18 This discrepancy may be due to geographical differences, and therefore clinicians should refer to the local distribution of blood culture pathogens during the clinical selection of antimicrobial drugs for empirical use. It is worth noting in the clinic that because Gram-negative bacteria are widely distributed in the body, they can cause infection in the organism under certain conditions, and bloodstream infections caused by them are more serious and have a higher morbidity and mortality rate than those induced by Gram-positive bacteria.
Gram-negative bacilli have increasing rates of resistance to antimicrobial drug-resistant drugs. When grouped and analyzed in ICU, non-ICU inpatient and emergency departments, the highest detection rates were Klebsiella pneumoniae, Escherichia coli, Pseudomonas aeruginosa, and Acinetobacter baumannii, respectively.19 Klebsiella pneumoniae has extensive drug resistance, limited choice of therapeutic drugs and easy dissemination, which poses a great challenge to clinical infection treatment.20 A study by Lee21 et al pointed out that Klebsiella pneumoniae had a high rate of resistance to amikacin and others; Acinetobacter baumannii had a high resistance rate of about 70% to imipenem and a relatively low rate of resistance to tigecycline.22 A study by Huseh23 et al noted that Escherichia coli had high resistance to levofloxacin, ceftriaxone, and ceftazidime. The results of this study suggested that Klebsiella pneumoniae had a high resistance rate to ceftriaxone, levofloxacin, ceftazidime, piperacillin, amikacin, minocycline, and amoxicillin, and the lowest resistance rate to tigecycline; Escherichia coli resistance rate to levofloxacin, ceftazidime, ceftriaxone, piperacillin, amikacillin, imipenem, cefoperazone, and minocycline, and to amoxicillin and tigecycline lowest. The resistance rate of Pseudomonas aeruginosa to levofloxacin, cefoperazone, ceftazidime, piperacillin, amikacin and imipenem was higher; the resistance rate of Acinetobacter baumannii to ceftazidime, levofloxacin, piperacillin, imipenem, cefoperazone, amikacin, amoxicillin and tigecycline was higher. In recent years, hospital-acquired infections induced by Gram-positive bacteria have been increasing year by year. The resistance of different Gram-positive bacteria to antibacterial drugs varies.24 But we can still find some similarities. Biagio Santella et al found that Gram-positive bacteria were highly resistant to penicillin G and oxacillin,9 and the same high level of resistance to penicillin G was found for Gram-positive bacteria in our study. Therefore, in the clinical treatment of patients with sepsis bloodstream infection with renal insufficiency, sensitive antimicrobial drugs should be selected according to the results of pharmacological examination, and antimicrobial drugs that have developed resistance should be avoided as much as possible, and the drug regimen should be adjusted timely according to the actual situation of patients.
Our study has some limitations. The sample size of this study was small and the age of patients was not differentiated, and the age of patients should be differentiated at a later stage on the basis of expanding the sample size. In addition, all patients in the study were from our hospital, which could not reflect the effect of geographic differences in different pathogenic bacteria on the results, and these need to be further studied in the future.
In conclusion, the pathogenic bacteria in patients with sepsis bloodstream infection with renal insufficiency are predominantly Gram-negative bacteria, and patients with sepsis bloodstream infection with renal insufficiency are more difficult to cure, have a longer course of treatment, and require prolonged use of antimicrobial drugs.
Data Sharing Statement
The datasets generated and analyzed during the current study are available from the corresponding authors on reasonable requests.
Ethics Approval and Consent to Participate
The study protocol was approved by the Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology. Informed consent was obtained from all the study subjects before enrollment.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No funding was received for this study.
Disclosure
The authors declare that they have no competing interests.
References
1. Thorndike J, Kollef MH. Culture-negative sepsis. Curr Opin Crit Care. 2020;26(5):473–477. doi:10.1097/MCC.0000000000000751
2. Aghazadeh A, Lin AY, Sheikh MA, et al. Universal microbial diagnostics using random DNA probes. Sci Adv. 2016;2(9):e1600025. doi:10.1126/sciadv.1600025
3. Yang WH, Heithoff DM, Aziz PV, et al. Accelerated aging and clearance of host anti-inflammatory enzymes by discrete pathogens fuels sepsis. Cell Host Microbe. 2018;24(4):500–513. doi:10.1016/j.chom.2018.09.011
4. Laupland KB, Pasquill K, Dagasso G, et al. Population-based risk factors for community-onset bloodstream infections. Eur J Clin Microbiol Infect Dis. 2020;39(4):753–758. doi:10.1007/s10096-019-03777-8
5. Krautzig S. Clarification of renal insufficiency in geriatric patients. Z Gerontol Geriatr. 2021;54:197–204. doi:10.1007/s00391-021-01877-9
6. Dagasso G, Conley J, Steele L, et al. Risk of bloodstream infection in patients with renal dysfunction: a population-based cohort study. Epidemiol Infect. 2020;148:e105. doi:10.1017/S0950268820001041
7. Goodyear MD, Krleza-Jeric K, Lemmens T. The declaration of Helsinki. BMJ. 2007;335:624–625. doi:10.1136/bmj.39339.610000.BE
8. Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):762–774. doi:10.1001/jama.2016.0288
9. Santella B, Folliero V, Pirofalo GM, et al. Sepsis-A retrospective cohort study of bloodstream infections. Antibiotics. 2020;9:12. doi:10.3390/antibiotics9120851
10. Kassim A, Omuse G, Premji Z, et al. Comparison of Clinical laboratory standards Institute And European committee on antimicrobial susceptibility testing guidelines for the interpretation of antibiotic susceptibility at a University teaching hospital in Nairobi, Kenya: a cross-sectional study. Ann Clin Microbiol Antimicrob. 2016;15(1):1–7. doi:10.1186/s12941-016-0135-3
11. Bleakley G, Cole M. Recognition and management of sepsis: the nurse’s role. Br J Nurs. 2020;29(21):1248–1251. doi:10.12968/bjon.2020.29.21.1248
12. Arensman K, Shields M, Beganovic M, et al. Fluoroquinolone versus beta-lactam oral step-down therapy for uncomplicated streptococcal bloodstream infections. Antimicrob Agents Chemother. 2020;64(11):e01515–01520. doi:10.1128/AAC.01515-20
13. Koelling EE, Jazaeri O. An overview of renal insufficiency, race, and glomerular filtration rate calculation for the vascular surgeon. J Vasc Surg. 2022;75(1):3–4. doi:10.1016/j.jvs.2021.10.023
14. Rojas L, Muñoz P, Kestler M, et al. Bloodstream infections in patients with kidney disease: risk factors for poor outcome and mortality. J Hospital Infect. 2013;85(3):196–205. doi:10.1016/j.jhin.2013.07.009
15. Lin T, Chang P, Chen I, et al. Risk factors and mortality associated with multi-drug-resistant Gram-negative bacterial infection in adult patients following abdominal surgery. J Hospital Infect. 2022;119:22–32. doi:10.1016/j.jhin.2021.09.021
16. Caballero RG, Noriega RAL, Negrete DCC, et al. (2021)“risk factors associated with infections by resistant multi-drug bacteria in critical patients with kidney substitution therapy”. Aditum J Clin Biomed Res. 2021;3(4):15.
17. Paul M, Bhatia M, Rekha US, et al. Microbiological profile of blood stream infections in febrile neutropenic patients at a tertiary care teaching Hospital in Rishikesh, Uttarakhand. J Lab Physicians. 2020;12(02):147–153. doi:10.1055/s-0040-1716661
18. Ballot DE, Nana T, Sriruttan C, et al. Bacterial bloodstream infections in neonates in a developing country. Int Sch Res Notice. 2012;2012. doi:10.5402/2012/508512
19. Robledo IE, Aquino EE, Vázquez G. Detection of the KPC gene in Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter baumannii during a PCR-based nosocomial surveillance study in Puerto Rico. Antimicrob Agents Chemother. 2011;55(6):2968–2970. doi:10.1128/AAC.01633-10
20. Sikarwar AS, Batra H. Prevalence of antimicrobial drug resistance of Klebsiella pneumoniae in India. International Journal of Bioscience. Biochem Bioinforma. 2011;1(3):211.
21. Lee H, Yong D, Yum JH, et al. Dissemination of 16S rRNA methylase-mediated highly amikacin-resistant isolates of Klebsiella pneumoniae and Acinetobacter baumannii in Korea. Diagn Microbiol Infect Dis. 2006;56(3):305–312. doi:10.1016/j.diagmicrobio.2006.05.002
22. Viehman JA, Nguyen MH. Treatment options for carbapenem-resistant and extensively drug-resistant Acinetobacter baumannii infections. Drugs. 2014;74(12):1315–1333. doi:10.1007/s40265-014-0267-8
23. Hsueh P-R, Hoban DJ, Carmeli Y, et al. Consensus review of the epidemiology and appropriate antimicrobial therapy of complicated urinary tract infections in Asia-Pacific region. J Infect. 2011;63(2):114–123. doi:10.1016/j.jinf.2011.05.015
24. Bereket W, Hemalatha K, Getenet B, et al. Update on bacterial nosocomial infections. Eur Rev Med Pharmacol Sci. 2012;16(8):1039–1044.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.