Back to Journals » Infection and Drug Resistance » Volume 15
Disseminated Nocardiosis with Pulmonary Fungus and Secondary Epilepsy: A Case Report
Received 22 April 2022
Accepted for publication 5 July 2022
Published 23 July 2022 Volume 2022:15 Pages 3919—3925
DOI https://doi.org/10.2147/IDR.S371903
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Wu Yang, Tingting Liu
Department of Critical Care Medicine, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, People’s Republic of China
Correspondence: Tingting Liu, Department of Critical Care Medicine, The First Affiliated Hospital, Zhejiang University School of Medicine, No. 79, Qingchun Road, Shangcheng District, Hangzhou, Zhejiang, People’s Republic of China, Tel +86-13588785095, Fax +86 571-87236838, Email [email protected]
Abstract: Disseminated nocardiosis is a rare, life-threatening disease that usually found in immunocompromised patients, and Nocardia farcinica is one of the most common causative pathogens. The difficulty in identifying the bacterium and the delay in initiating appropriate therapy often influence the prognosis of patients with disseminated nocardiosis. Here, we present a rare case of disseminated nocardiosis in a 61-year-old female with pulmonary fungus and secondary epilepsy. She received targeted antibiotic therapy and showed a great recovery in clinical symptoms and radiological signs. Disseminated nocardiosis can be easily overlooked due to the absence of characteristic symptoms and limitations of clinical examinations. Given the variability in antibiotic susceptibility patterns, the management of disseminated nocardiosis must be individualized. Therefore, early diagnosis and targeted antibiotic treatment are critical for the prognosis of disseminated nocardiosis.
Keywords: disseminated nocardiosis, Nocardia farcinica, pulmonary fungus, secondary epilepsy
Introduction
Nocardia farcinica is an opportunistic pathogen with weak acid resistance, primarily infecting immunocompromised patients such as those with AIDS, organ transplantation, long-term hormone administration, malignant tumors, or diabetes.1,2 So far, nearly 80 species of Nocardia have been identified, with approximately 33% of them capable of infecting humans.3,4 Nocardia farcinica accounts for 24.5% of nocardiosis.5 It primarily invades the body through the respiratory tract and results in disseminated nocardiosis.3 Disseminated nocardiosis is an uncommon, life-threatening infection with high mortality rate owing to difficulties in early diagnosis and prompt treatment.6–8 Herein, we report a case of disseminated nocardiosis caused by Nocardia farcinica in a 61-year-old female patient with a history of long-term hormone administration and symptoms of chills, high fever, cough, white mucous sputum, and epileptic seizure. With the fast and highly sensitive diagnosis approach, the patient received prompt treatment and eventually recovered.
Case Report
A 61-year-old female with chills and fever was admitted to the emergency department of the first affiliated hospital of Zhejiang university school of medicine on December 27, 2021. She developed a sudden fever of 39.4°C with no apparent cause, accompanied by chills, malaise, cough, and a small amount of white mucous sputum. She had a history of rheumatoid arthritis for more than 15 years and had taken methylprednisolone (2 mg, twice a day) since then. She was diagnosed with allergic vasculitis about one year ago and the dosage of methylprednisolone was adjusted to 16 mg, twice a day. She had skin ulcers on her lower extremities one month before admission, and after regular treatment, the skin ulcers seemed healed upon admission to our emergency department.
Laboratory findings upon admission to the emergency department were shown as follows: white blood cell, 21.52×109/L; neutrophils, 91.1%; lymphocytes, 5.5%; red blood cell, 4.33×1012/L; hemoglobin, 118 g/L; platelet, 172×109/L; C-reactive protein, 129.97 mg/L; and procalcitonin, 2.45 ug/L. Other laboratory results were within the normal range. The chest computed tomography (CT) demonstrated multiple nodular foci with partial cavitations in the lower lobes of both lungs (Figure 1A).
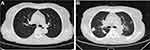 |
Figure 1 Chest CT images of the patient. (A) Chest CT on December 27, 2021. (B) Chest CT imaging on December 29, 2021. |
Given the possibility that the above findings were caused by infection, the patient was then transferred to our respiratory ward for further treatment. Her vital signs were shown as follows: temperature, 36.2°C; blood pressure, 112/60 mmHg; pulse rate, 84 beats/min; and respiration rate, 20 breaths/min. She was conscious with no systemic symptoms. The chest CT demonstrated small nodules and pulmonary bullae in the right lower lung (Figure 1B). The abdominal enhanced CT showed a low-density focus in the right abdominal wall, small stones in the left kidney, a small amount of pelvic fluid, and low-density nodules next to the upper pole of the right kidney. The result of blood next-generation sequencing (NGS) from the emergency room revealed Nocardia farcinica (Table 1). Staphylococcus aureus and Nocardia farcinica were found in sputum culture. Antibiotic susceptibility testing revealed that the cultured bacterium was sensitive to trimethoprim-sulfamethoxazole (TMP-SMX), amikacin, linezolid, and imipenem (Table 2). Cryptococcus neofermans capsule antigen, fungal D-glucan, T-cell and gene Xpert for tuberculosis, autoantibodies, and rheumatoid factor tests were all negative. To counteract the infection, the patient was treated with TMP-SMX, linezolid, and imipenem based on the antibiotic susceptibility test. Given the patient’s occult blood in the stool, the hormone was suspended on the advice of gastroenterology, rheumatology, and immunology consultants. On December 31, 2021, the patient experienced a sudden generalized seizure with loss of consciousness for approximately one minute. Her airway was immediately opened, and midazolam was used for anti-epilepsy. The brain-enhanced magnetic resonance imaging (MRI) revealed nodular and patchy abnormal signal lesions with clear boundaries were found in the left frontal lobe and right basal ganglia, and that the lesions appeared gyri-form, ring-like, and inhomogeneous after enhancement. The largest lesion was approximately 4.7×3.5 cm with a low signal on T1WI, a high signal on T2WI, and an inhomogeneous high signal on FLAIR (Figure 2A).
 |
Table 1 Pathogenic Microorganism Identified in a Blood Sample |
 |
Table 2 Nocardia Susceptibility Results by the Kirby-Bauer Test |
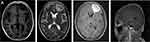 |
Figure 2 Brain MRI images of the patient. (A) Brain MRI on December 30, 2021. |
The patient was diagnosed with disseminated nocardiosis and then transferred to the intensive care unit (ICU). In the ICU, she was performed with ventilator-assisted ventilation (BIPAP mode: FiO2, 25%; peak airway pressure, 20 cmH2O; PEEP, 5 cmH2O). The cerebrospinal fluid (CSF) test showed: pressure, 250 mmH2O; colorless and clear; Pan’s test negative; erythrocytes, 40/L; nucleated cells, 2/L; chlorine, 122 mM; glucose, 3.2 mM; and protein, 0.36 g/L. The Cryptococcus neoformans capsular antigen and tubercle Bacillus tests in both CSF and blood were negative. Nocardia farcinica was also found positive in bronchoalveolar lavage fluid (BALF). Mycobacterium tuberculosis was negative in the sputum and the BALF. The patient was continuously given TMP-SMX, imipenem, and amikacin. Simultaneously, she was administered levetiracetam and topiramate for counteracting epilepsy, and mannitol for reducing intracranial pressure. During her 10-day stay in the ICU (from December 31, 2021 to January 10, 2022), the patient had no seizures but had a persistent fever (the highest body temperature was 39.4°C) and a large amount of yellow purulent sputum. Auscultation of both lungs revealed sporadic moist rales. The chest CT on January 10, 2022 revealed multiple nodules with partial cavities and lung vasculitis in both lungs (Figure 3A). In multi-repeated sputum cultures, Nocardia farcinica, Aspergillus flavus, and Burkholderia neocepacia were discovered (Table 3). Considering the progression of vasculitis and exudative lesions in lungs, a tracheotomy was performed and the ventilator parameters were adjusted (BIPAP mode: FiO2, 45%; peak airway pressure, 26 cmH2O; PEEP, 8 cmH2O). Amikacin was then suspended, and voriconazole was added for antifungal therapy. Since the patient had a history of allergic vasculitis, she was given methylprednisolone (40 mg, intravenously every 12 h) and mannitol (20 g, intravenously every 12h). Until January 17, 2022, the patient had no fever or seizures, and showed a great improvement in pulmonary ventilation function. The brain and chest CT on January 17, 2022 revealed a low-density lesion in the left frontal lobe of the brain and multiple infectious lesions accompanied by local cavities in both lungs (Figure 3B). Compared with the chest CT images on January 10, 2022 (Figure 1A), those on January 17, 2022 indicated a great alleviation of pulmonary infection in this patient (Figure 3B). The antibiotic regimen was then adjusted to TMP-SMX, linezolid, voriconazole, and methylprednisolone without mannitol. The patient was performed with high flow-assisted ventilation (oxygen concentration, 30%; flow rate, 50 L/min) after a spontaneous breathing trial, and the tracheostomy cannula was removed on January 21, 2022.
 |
Table 3 Pathogenic Microorganism Identified in BALF |
 |
Figure 3 Chest CT images of the patient. (A) Chest CT on January 10, 2022. (B) Chest CT on January 17, 2022. (C) Chest CT on February 6, 2022. (D) Chest CT on April 16, 2022. |
The patient was then transferred to the general ward on January 21, 2022 as her condition became stable On January 27, 2022, the administration of linezolid was suspended. TMP-SMX, voriconazole, and methylprednisolone were orally administered to the patient for anti-infection and anti-allergic vasculitis. On January 29, 2022, Nocardia farcinica and fungi were not discovered in her BALF. However, the cytomegalovirus antigen (PP65) was detected positive in blood. We then supplemented the treatment regimen with ganciclovir (500mg, orally tid) for anti-virus. She had no fever within one week (February 1–7, 2022), and her chest CT imaging signs were greatly recovered on February 6, 2022 (Figure 3C).
The patient was discharged on February 6, 2022. She continued taking TMP-SMX, ganciclovir, methylprednisolone, and voriconazole and had no obvious clinical symptoms including fever, cough, and seizure within the 2-month follow-up. The chest CT on April 16, 2022 showed several bronchial cavities in the lungs (Figure 3D). Her head MRI was gradually improving (Figure 4A and B). The treatment regimen was then changed to a combination of TMP-SMX and triamcinolone.
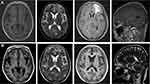 |
Figure 4 Brain MRI images of the patient. (A) Brain MRI on January 25, 2022. (B) Brain MRI on April 16, 2022. |
Discussion
Disseminated nocardiosis is a rare, life-threatening disease that usually affects immunocompromised patients, and Nocardia farcinica is one of the most common causative pathogens.2,8–11 Its early diagnosis appears difficult and its overall prognosis remains unsatisfactory, partly owing to the variability of clinical manifestations and limitations of laboratory tests.1–2,12 Meanwhile, co-infection with Nocardia farcinica and Aspergillus flavus in the lung is rare, severe and even fatal.13–15 Furthermore, physicians have relatively insufficient experience in its treatment.10 Thus, early diagnosis and appropriate antibiotic therapy are necessary for a favorable prognosis.
This elderly female patient has rheumatoid arthritis and allergic vasculitis, and has been on corticosteroids for over 15 years. A weakened immune system increases the risk of nocardiosis. This patient had an acute onset of high fever and cough with yellow purulent sputum when admitted to our hospital, which appears inconsistent with the typical nocardiosis course. However, one month prior to admission, the patient had skin ulcers on both lower limbs, and the skin ulcers seemed healed after regular treatment in the dermatology department. Since the dermatological treatment of skin ulcers could reduce the detection rate of bacteria, we could not rule out the possibility of Nocardia farcinica invading the lungs and central nervous system (CNS) through the ulcerated skin despite the negative results of repeated bacterial cultures. The diagnosis of nocardiosis is primarily based on etiological diagnosis, which means that Nocardia farcinica can be isolated and identified from clinical specimens including sputum, BALF and tissues, among which, sputum specimen can be used to detect more than 70% of the cases. Clinical high-throughput sequencing is one of the most reliable and accurate methods for identifying microorganisms. It can facilitate clinical treatment and reduce fatality rates of severe infections.7,16 In this case, high-throughput sequencing of blood quickly identified Nocardia farcinica the next day after admission to our hospital. Subsequent high-throughput sequencing of BALF further supported Nocardia farcinica as the pathogen. The application of high-throughput sequencing could save our time for precise diagnosis and subsequent treatment, reduce the rate of misdiagnosis, and improve the survival rate of patients with nocardiosis. Consistent with the results from high-throughput sequencing, repeated sputum cultures in January, 2022 detected a small amount of Nocardia farcinica, further suggesting the diagnosis of pulmonary nocardiosis.
This patient experienced seizures after admitted to our hospital, and the brain MRI showed nodular and patchy ring-enhanced lesions in the left frontal lobe and right basal ganglia. However, the results from general bacterial culture and high-throughput sequencing of CSF were negative, which is likely due to the relatively insufficient time of bacterial culture in this case. The longest culture time for positive detection of Nocardia previously reported was 22 days,17 while the culture time in this case was 7 days, which may explain the negative result of our bacterial culture. Moreover, a report on Nocardia species in China showed that the detection rate of Nocardia in the CSF of patients with CNS nocardiosis was only 3.8%.18 In this case, the patient refused a repeat CSF collection. Since the CSF sample was collected only once, the positive detection rate was further reduced.9 Although the bacterial culture and high-throughput sequencing of CSF were negative in this case, the clinical signs and imaging findings support the diagnosis of CNS nocardiosis and secondary epilepsy.
Imaging features varies in pulmonary nocardiosis, including exudation, consolidation, nodular shadow, cavity, pleural effusion, interstitial lesions, and other nonspecific pulmonary features. Multiple imaging features can co-occur when the pulmonary nocardiosis progresses rapidly, making it easy to be missed or misdiagnosed as tuberculosis, fungal, or tumor infection.2,7 In this case, tuberculosis and tumor screening were both negative during the hospitalization. As the disease progressed, repeated sputum cultures revealed multiple pulmonary infections by bacteria, fungi, and viruses. The pulmonary infections gradually dissipate and transform into an image dominated by cavities and ground-glass opacity following antibiotic therapy. The general fatality rate of nocardiosis is 20%-55%, but if Nocardia spreads into the brain, the fatality rate dramatically increases.9,18 TMP-SMX remains the first-line drug for treating nocardiosis.19 TMP-SMX combined with amikacin and imipenem can be used to treat severe nocardiosis. However, antibiotic sensitivity varies among Nocardia species. Imipenem, linezolid, and TMP-SMX were found to be effective against Nocardia farcinica based on the antibiotic susceptibility report of this case. Except for imipenem, the other two antibiotics can easily penetrate the blood-brain barrier and have high blood concentrations after systemic administration.2,9,19,20 A combination use of drugs is recommended for patients with CNS nocardiosis, and drugs that can penetrate the blood-brain barrier should be intravenously administered for six weeks. In this case, the patient was treated with intravenous antibiotics for six weeks, and then replaced with oral antibiotics until her clinical symptoms and imaging signs were recovered.8 Due to recurrence of nocardiosis, antibiotic therapy in immunocompromised patients should be extended to 6 to 12 months.9,19 In this case, the duration of antibiotic therapy lasted for over 5 months. The initial treatment regimen consisted of TMP-SMX (0.96g), linezolid, and imipenem, and as the disease progressed, the treatment regimen was changed to TMP-SMX (1.44g), amikacin, imipenem, and linezolid. Due to pulmonary aspergillosis and increased alveolar hemorrhage and exudation, we adjusted treatment regimen to TMP-SMX (1.44g), voriconazole, imipenem, linezolid, methylprednisolone. The clinical symptoms and the imaging results had greatly improved after treatment. Fungi, Mycobacterium tuberculosis, and Nocardia farcinica were no longer found in sputum culture or BALF macro gene screening. Only respiratory syncytial virus and cytomegalovirus were detected, and ganciclovir was then used for anti-virus therapy. TMP-SMX, ganciclovir, methylprednisolone, and voriconazole were continued after discharge.
It is worth noting that patients with disseminated nocardiosis may have a history of hormone administration, and it is unclear whether abrupt hormone withdrawal will aggravate the condition. In this case, the patient had orally administered hormones for a long term. Considering the positive occult blood in the stool, the hormone administration was suspended, but unexpectedly, the condition of the patient rapidly deteriorated after that. The symptoms gradually improved after re-using the hormones. The abrupt reduction or withdrawal of corticosteroids may aggravate allergic vasculitis or rheumatoid arthritis and then affect disseminated nocardiosis. Hence, appropriate corticosteroids remain clinically essential for controlling the progress of disseminated nocardiosis in patients with a history of long-term hormone administration.
Conclusion
Disseminated Nocardia has a poor prognosis, especially those involving the central nervous system, drug-resistant bacterial infection and delayed diagnosis, with a high mortality rate. Elder, immunocompromised, organ transplantation, and long-term administration of hormones are at increased risk for disseminated nocardiosis. Early diagnosis and appropriate antibiotic therapy could help reduce the morbidity of immunocompromised patients suffering from disseminated nocardiosis. The application of high-throughput detection and mass spectrometry has greatly improved Nocardia detection rates.
Consent Statement
The patient provided written informed consent to allow the case details and any accompanying images to be published. No specific ethics committee approval was required for this study.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mehta HH, Shamoo Y. Pathogenic Nocardia: a diverse genus of emerging pathogens or just poorly recognized? PLoS Pathog. 2020;16:e1008280. doi:10.1371/journal.ppat.1008280
2. Wilson JW. Nocardiosis: updates and clinical overview. Mayo Clin Proc. 2012;87:403–407. doi:10.1016/j.mayocp.2011.11.016
3. Conville PS, Brown-Elliott BA, Smith T, Adrian M. The complexities of nocardia taxonomy and identification. J Clin Microbiol. 2018;56:1–10. doi:10.1128/JCM.01419-17
4. Wu J, Li X, Zhang T, Lin X, Chen YC. Disseminated Nocardia farcinica involves the spinal cord: a case report and review of the literature. BMC Infect Dis. 2021;21:1224. doi:10.1186/s12879-021-06905-y
5. Valdezate S, Garrido N, Carrasco G, et al. Epidemiology and susceptibility to antimicrobial agents of the main Nocardia species in Spain. J Antimicrob Chemother. 2017;72:754–761. doi:10.1093/jac/dkw489
6. Song J, Dong L, Ding Y, Zhou J. A case report of brain abscess caused by Nocardia farcinica. Eur J Med Res. 2021;26:83. doi:10.1186/s40001-021-00562-2
7. Gao S, Liu Q, Zhou X, Dai X, He H. Primary cutaneous nocardiosis due to nocardia farcinica: a case report of an often overlooked infection. Infect Drug Resist. 2021;14:1435–1440. doi:10.2147/IDR.S306161
8. Corsini Campioli C, Castillo Almeida NE, O’Horo JC, et al. Clinical presentation, management, and outcomes of patients with brain abscess due to Nocardia species. Open Forum Infect Dis. 2021;8:ofab067. doi:10.1093/ofid/ofab067
9. Kumar VA, Augustine D, Panikar D, et al. Nocardia farcinica brain abscess: epidemiology, pathophysiology, and literature review. Surg Infect. 2014;15:640–646. doi:10.1089/sur.2012.205
10. You Y, Chen W, Zhong B, Song Z, Yang X. Disseminated nocardiosis caused by Nocardia elegans: a case report and review of the literature. Infection. 2018;46:705–710. doi:10.1007/s15010-018-1144-2
11. Lee EK, Kim J, Park DH, et al. Disseminated nocardiosis caused by Nocardia farcinica in a patient with colon cancer: a case report and literature review. Medicine. 2021;100:e26682. doi:10.1097/MD.0000000000026682
12. Wang S, Jiang B, Li Y, Shang Y, Liu Z, Zhang Y. A case report of disseminated nocardiosis with ocular involvement in a myasthenia gravis patient and literature review. BMC Neurol. 2019;19:243. doi:10.1186/s12883-019-1482-4
13. Gomes F, La Feria P, Costa C, Texeira H. Nocardia cyriacigeorgica and aspergillus co-infection in a patient with giant-cell arteritis. Eur J Case Rep Intern Med. 2019;6:000997. doi:10.12890/2019_000997
14. Meena DS, Kumar D, Bohra GK, et al. Pulmonary Nocardiosis with Aspergillosis in a patient with COPD: a rare co-infection. ID Cases. 2020;20:e00766. doi:10.1016/j.idcr.2020.e00766
15. Misra DP, Parida JR, Chowdhury AC, Agarwal V. Pulmonary co-infection with Nocardia and Aspergillus in a patient with adult-onset still’s disease receiving steroids and tacrolimus. BMJ Case Rep. 2014;2014:bcr2014207335–bcr2014207335. doi:10.1136/bcr-2014-207335
16. Weng SS, Zhang HY, Ai JW, et al. Rapid detection of nocardia by next-generation sequencing. Front Cell Infect Microbiol. 2020;10:13. doi:10.3389/fcimb.2020.00013
17. Poonyagariyagorn HK, Gershman A, Avery R, et al. Challenges in the diagnosis and management of Nocardia infections in lung transplant recipients. Transpl Infect Dis. 2008;10(6):403–408. doi:10.1111/j.1399-3062.2008.00338.x
18. Zintgraff J, Prieto M, Pena M, et al. When reporting Nocardia spp is not enough. Brain abscess caused by Nocardia farcinica. Access Microbiol. 2020;2:acmi000091. doi:10.1099/acmi.0.000091
19. Margalit I, Lebeaux D, Tishler O, et al. How do I manage nocardiosis? Clin Microbiol Infect. 2021;27:550–558. doi:10.1016/j.cmi.2020.12.019
20. Bae JN, MacFall JR, Krishnan KR, Payne ME, Steffens DC, Taylor WD. Dorsolateral prefrontal cortex and anterior cingulate cortex white matter alterations in late-life depression. Biol Psychiatry. 2006;60:1356–1363. doi:10.1016/j.biopsych.2006.03.052
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
