Back to Journals » OncoTargets and Therapy » Volume 8
Disruption of NF-κB signaling by fluoxetine attenuates MGMT expression in glioma cells
Authors Song T, Li H, Tian Z, Xu C, Liu J, Guo Y
Received 2 April 2015
Accepted for publication 18 June 2015
Published 20 August 2015 Volume 2015:8 Pages 2199—2208
DOI https://doi.org/10.2147/OTT.S85948
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Faris Farassati
Tao Song,1 Hui Li,2 Zhiliang Tian,3 Chaojiu Xu,4 Jingfang Liu,1 Yong Guo1
1Department of Neurosurgery, Xiangya Hospital, Central South University, 2Department of Immunology and Microbiology, Medical School of Jishou University, 3Department of Neurosurgery, 4Department of Oncology, The Hospital of Xiangxi Autonomous Prefecture, Jishou, People’s Republic of China
Background: Resistance to temozolomide (TMZ) in glioma is modulated by the DNA repair protein O6-methylguanine-DNA methyltransferase (MGMT). This study aimed to examine the effects of fluoxetine (FLT) on MGMT expression in glioma cells and to investigate its underlying mechanisms.
Materials and methods: Expression of MGMT, GluR1, or IκB kinase β (IKKβ) was attenuated using short hairpin RNA-mediated gene knockdown. The 3-(4,5-dimethylthiazol -2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to evaluate the growth inhibition induced by FLT or TMZ. Terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick end labeling (TUNEL) was conducted to detect apoptotic cells. Western blotting was conducted to analyze the protein expression of MGMT, IKKβ, and NF-κB/p65 following FLT treatment. The murine subcutaneous xenograft model was used to evaluate the combinational effect of TMZ and FLT.
Results: FLT markedly reduced MGMT expression in glioma cells, which was independent of GluR1 receptor function. Further, FLT disrupted NF-κB/p65 signaling in glioma cells and consequently attenuated NF-κB/p65 activity in regulating MGMT expression. Importantly, FLT sensitized MGMT-expressing glioma cells to TMZ, as FLT enhanced TMZ’s ability to impair the in vitro tumorigenic potential and to induce apoptosis in glioma cells. Knockdown of MGMT or IKKβ expression abolished the synergistic effect of FLT with TMZ in glioma cells, which suggested that FLT might sensitize glioma cells to TMZ through down-regulation of MGMT expression. Consistently, TMZ combined with FLT markedly attenuated NF-κB/p65 activity, reduced MGMT expression, and suppressed in vivo tumor growth in the murine subcutaneous xenograft model.
Conclusion: Our findings demonstrated that FLT attenuated MGMT expression by disrupting NF-κB signaling and sensitized glioma cells to TMZ, which may warrant further investigation toward possible clinical application in MGMT-expressing glioma.
Keywords: glioma, fluoxetine, MGMT
Introduction
Glioma is the most common aggressive adult primary tumor of the central nervous system. Treatment includes surgery, radiotherapy, and adjuvant temozolomide (TMZ) chemotherapy. TMZ is an alkylating agent prodrug, delivering a methyl group to purine bases of DNA (O6-guanine, N7-guanine, and N3-adenine). The primary cytotoxic lesion, O6-methylguanine, can be removed by O6-methylguanine-DNA methyltransferase (MGMT) in tumors.1 Strategies devised to thwart resistance and enhance response to TMZ, including inhibition of MGMT which contributes to TMZ resistance, are under clinical evaluation. Attenuation of MGMT expression or protein function prior to alkylating agent chemotherapy prevents O6-methylguanine repair and consequently sensitizes tumors to TMZ.2
Antidepressants are commonly prescribed for cancer patients suffering from depressive disorders. Recent studies showed that tricyclic antidepressants reduce the cancer risk of gliomas and that a selective serotonin reuptake inhibitor (SSRI), used as an antidepressant, has an antiproliferative or a cytotoxic effect on certain cancers, due to its ability to pass through the blood and brain barrier and directly carry out its pharmaceutical effects in regions of the brain.3,4 With these characteristics, antidepressants are promising leads for glioma treatment.
Fluoxetine (FLT, also known by the trade name Prozac) is an antidepressant of the SSRI class. FLT is often used for the treatment of major depressive disorder (including pediatric depression), obsessive compulsive disorder (in both adults and children), bulimia nervosa, panic disorder, and premenstrual dysphoric disorder.5 Therapeutic doses of FLT for depression patients are in the range of 20–60 mg/day and brain concentration of FLT can reach up to approximately 30 μM.6,7 Importantly, the high concentration of FLT achieved in brain makes it an ideal drug to treat glioma. Although recent study indicated that FLT suppresses glioma cells by evoking α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR)-mediated calcium-dependent apoptosis, the underlying mechanism still remains poorly understood.8
In this study, we attempted to explore the potential for antidepressants to be used to treat MGMT-expressing glioma. We demonstrated that FLT attenuated MGMT expression by disrupting Nuclear Factor Of Kappa Light Polypeptide Gene Enhancer In B-Cells (NF-κB) signaling and sensitized glioma cells to TMZ, which may warrant further investigation in developing therapeutic strategy against glioma.
Methods and materials
Reagents and cell lines
FLT, TMZ, and MTT were purchased from Sigma-Aldrich (St Louis, MO, USA). Antibodies to NF-κB/p65 and p-NF-κB/p65 (S536) were purchased from Cell Signaling Technology (Cambridge, MA, USA). MGMT promoter luciferase reporter plasmid pLightSwitch/MGMT was obtained from SwitchGear Genomics Inc (Carlsbad, CA, USA). IκB kinase β (IKKβ) inhibitor (SC-514), monoclonal antibody to MGMT, β-actin, and His-tag were obtained from Santa Cruz Biotechnology (Dallas, TX, USA). Human glioma cell lines with high MGMT expression (T98G, U138, SF767, and U251) were obtained from American Type Culture Collection (ATCC, VA, USA) and cultured according to the ATCC protocol. The experimental protocol regarding the use of these human glioma cell lines was approved by the Institutional Research Ethics Committee of Xiangya hospital, Central South University.
Lentiviral construction and transduction
Lentiviruses encoding shRNAs (MGMT, GluR1, and IKKβ) and lentiviral open reading frame (ORF) expression clones (MGMT, NF-κB/p65) were constructed by GeneCopoeia Inc. (Rockville, MD, USA). The production of lentiviral particles and in vitro infection of cells with the lentiviruses were described previously.9
Cytotoxicity assay
A standard MTT assay was performed to measure the cytotoxicity of drugs. Cells (3×103 cells per well) were cultured in 96-well plates for 24 hours, then incubated with different concentrations of TMZ (1.0 mM–10 μM) for another 72 hours. Relative absorbance of MTT was measured to calculate cell growth inhibition. Cell viability in the vehicle control (dimethyl sulfoxide) was considered as to be 100%. The mean drug concentration required to inhibit cell proliferation by 50% (IC50) was calculated using Calcusyn Version 2.0 software (Biosoft, Cambridge, UK).
Colony formation assay
Cells cultured in six-well plates were treated with FLT or TMZ, and continuously cultured for another 10–14 days to allow for colony formation. Cells were washed with cold phosphate-buffered saline (PBS), fixed with 75% methanol, and stained with 0.5% crystal violet in methanol. The colonies consisting of >50 cells were counted under a dissecting microscope.
Western blot analysis
Cell lysates were prepared using cell lysis buffer containing 1% Nonidet P-40 (NP-40), 0.5% sodium deoxycholate, 0.1% SDS, and protease inhibitor cocktail. After standard sodium dodecyl sulfate polyacrylamide gel electropheresis (SDS-PAGE) and Western blotting procedures, proteins were visualized using the enhanced chemiluminescence (ECL) system (Amersham Biosciences, NJ, USA).
TUNEL assay
The level of apoptosis in glioma cells was assessed using the terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick end labeling (TUNEL) method. The percentage of TUNEL-labeled cells in each section was determined at a magnification of 400× by counting 500 cells in five randomly selected fields.
Real-time PCR
Total RNA was extracted from glioma cells with the RNeasy Mini kit (Qiagen, Valencia, CA, USA), according to the manufacturer’s instructions. Real-time Polymerase Chain Reaction (RT-PCR) was performed with SuperScript III One-Step RT-PCR System with Platinum Taq DNA Polymerase (Thermo Fisher Scientific, Waltham, MA, USA), according to the manufacturer’s instructions. Real-time PCR primers for MGMT were synthesized as described previously.10
MGMT promoter activity assay
MGMT promoter activity assay was conducted as described previously.11 Briefly, at 24 hours after pLightSwitch/MGMT transfection, the cells were lysed for 15 minutes on ice with 200 μL of luciferase lysis buffer (Promega, Madison, WI, USA). Luciferase assays were carried out using a Promega assay kit and a luminometer (Berthold, Bad Wildbad, Germany).
Animal studies
Immunocompromised nude mice were obtained from the breeding facility at the animal center of Central South University. All animal studies were performed in accordance with institutional ethical guidelines for experimental animal care. We engrafted U138 cells (2×106) subcutaneously into the right thigh of nude (nu/nu) 6- to 8-week-old mice. When the tumor volume reached approximately 50 mm3, mice were randomly assigned to each of four groups (Control, TMZ, FLT, and TMZ+FLT; n=5). Mice were treated by intraperitoneal injection with TMZ (10 mg/kg/day) or FLT (10 mg/kg/day) or TMZ+FLT 5 days per week until the end of the experiment. PBS was used as negative control. In order to determine tumor volume by external caliper, the greatest longitudinal diameter (a) and the greatest transverse diameter (b) were determined. Tumor volume based on caliper measurements was calculated by the modified ellipsoidal formula: tumor volume (mm3) = a×b2/2. After drug treatment for 3 weeks, Mice from each group were euthanized for histopathology assessment of in vivo tumor response. The subcutaneous xenografts embedded in the Optimal Cutting Temperature (OCT) compound were stored in liquid nitrogen overnight, and then sectioned at 10 μm thickness on a MicromHM200 cryotome (Eryostar). Immunohistochemical staining (p-NF-κB/p65, MGMT, and Ki-67) was applied on tumor sections as described previously.9
Statistical analysis
All data were presented as mean ± standard deviation. Statistical significance (P<0.05) was determined by Student’s t-test.
Results
FLT attenuates MGMT expression in glioma cells
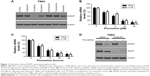
We evaluated the in vitro effect of six antidepressants on MGMT protein expression in T98G cells. Interestingly, highly reduced MGMT expression was observed in T98G cells following FLT treatment (Figure 1A). Real-time PCR analysis showed that MGMT mRNA expression declined following FLT treatment in a dose- and time-dependent manner in T98G and U138 glioma cells (Figure 1B and C), which suggested that FLT might affect MGMT gene transcription. Recent report showed that FLT can bind directly to GluR1 and trigger transmembrane Ca2+ influx and apoptosis, which led to our hypothesis that the effect of FLT on MGMT is mediated by GluR1.8 However, we did not observe any difference in MGMT expression between GluR1-expressing and GluR1-depleted T98G cells following FLT treatment (Figure 1D). Therefore, the effect of FLT on MGMT expression might not be associated with GluR1 function.
FLT disrupts NF-κB signaling to reduce MGMT expression in glioma cells
Several signaling pathways (extracellular regulated protein kinases [ERK1/2], c-Jun N-terminal kinase [JNK1/2], NF-κB/p65, mammalian target of rapamycin [mTOR], signal transducers and activators of transcription 3 [STAT3], and V-Akt murine thymoma viral oncogene homolog 1 [V-Akt murine thymoma viral oncogene homolog 1]) have been suggested to be involved in the transcription regulation of MGMT.10–15 Western blotting analysis was performed to monitor the signaling activities of these pathways after FLT treatment (10 μM, 24 hours). Considerably, reduced p-NF-κB/p65 (S536) and MGMT protein levels were observed in FLT-treated T98G and U138 cells (Figure 2A and B). FLT attenuated NF-κB/p65 S536 phosphorylation, reduced MGMT expression, and increased NF-κB inhibitor IκBα in a time-dependent manner in T98G and U138 glioma cells (Figure 2C). FLT also reduced p-NF-κB/p65 and MGMT protein levels in SF767 and U251 cells (Figure 2D). Previous study identified two NF-κB-binding sites in the promoter of MGMT: one is at -766 (MGMT-κB-1) and the other is at -90 (MGMT-κB-2).11 We co-transfected luciferase reporter plasmid containing MGMT gene promoter and NF-κB/p65 plasmid (wild-type, phospho-null S536A, and phospho-mimetic S536D) into T98G cells, and treated these cells with FLT or NF-κB kinase IKKβ inhibitor SC-514 (Figure 3A). FLT or IKKβ inhibitor SC-514 markedly attenuated wild-type NF-κB/p65-induced MGMT promoter activity (P<0.05) (Figure 3B). However, neither drug worked in cells expressing inactive NF-κB/p65 (S536A) or constitutively active (S536D) mutants (Figure 3B). These results suggested that FLT might reduce MGMT expression by inactivating NF-κB/p65 activity in glioma cells.
FLT sensitizes MGMT-expressing glioma cells to TMZ
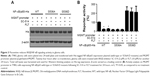
Down-regulation of MGMT expression by FLT might be a feasible strategy to overcome TMZ-resistance. Treatment with FLT alone or combined with TMZ blocked NF-κB signaling and reduced MGMT expression in T98G and U138 cells (Figure 4A). TMZ combined with FLT exhibited significantly lower IC50 than TMZ alone in T98G and U138 cells (Figure 4B, P<0.05). FLT also sensitized SF767 and U251 cells to TMZ-induced growth inhibition (Figure S1). We next assessed the in vitro tumorigenic potential of glioma cells using the colony formation assay. TMZ alone exhibited a mild inhibitory effect on colony formation in T98G and U138 glioma cells. However, TMZ combined with FLT led to a significant reduction in colony formation compared with either drug alone (P<0.05, Figure 4C). Consistently, TUNEL analysis showed that TMZ plus FLT significantly increased the TUNEL-labeled apoptotic cells, as compared to either drug alone (P<0.05, Figure 4D).
FLT sensitizes glioma cells to TMZ by disrupting NF-κB/MGMT pathway
To confirm the hypothesis that FLT sensitizes glioma cells to TMZ by regulating the NF-κB/MGMT pathway, we compared the combinational effect of TMZ plus FLT in non-targeting control, MGMT-depleted or IKKβ-depleted T98G cells. We did not observe the enhanced cytotoxicity in TMZ plus FLT treatment compared to TMZ alone in T98G cells with MGMT or IKKβ depletion (Figure 5A and B). We also over-expressed a CMV-driven MGMT gene construct in T98G cells (Figure 5C). Cell viability assay showed that FLT did not enhance the cytotoxicity of TMZ in T98G cells constitutively expressing MGMT, as compared to T98G cells expressing empty vector (Figure 5D). These results suggested that FLT might sensitize glioma cells to TMZ by down-regulating the NF-κB-MGMT pathway.
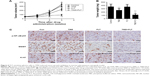
FLT sensitizes glioma cells to TMZ-induced tumor suppression in the murine xenograft model
In order to confirm that the effect of TMZ could be enhanced by FLT, we decided to test the efficacy of combined treatment in the murine subcutaneous xenograft model. As shown in Figure 6A, at 2 and 3 weeks after drug treatment, TMZ monotherapy or TMZ+FLT treatment slowed down tumor growth compared with PBS-treated controls (P<0.05). Impressively, TMZ plus FLT treatment showed dramatically suppressed tumor growth through the end of the experimental period compared to TMZ monotherapy (P<0.05). Quantification of tumor mass at week 3 showed that TMZ plus FLT treatment led to significantly less growth than PBS control or TMZ alone (P<0.05) (Figure 6B). These results collectively indicate that FLT enhances TMZ-induced tumor suppression in vivo. Immunohistochemical analysis of in vivo tumors revealed that, similar to in vitro results, FLT effectively disrupted NF-κB/p65 S536 phosphorylation and reduced MGMT expression (Figure 6C). Moreover, combinational therapy significantly decreased the proliferative parker Ki-67 positivity, as compared to PBS control or either drug alone (P<0.05). Collectively, the results presented here highlight the effectiveness of the concomitant use of TMZ and FLT, which may have relevant therapeutic implications.
Discussion
Resistance to TMZ-based chemotherapy is complex and not yet clarified. Some mechanisms have been revealed, principally the rapid repair of DNA damage which is induced by TMZ. TMZ resistance in glioblastoma multiforme (GBM) patients is mediated predominantly by the MGMT protein. Inhibitor of MGMT activity, such as 6-O-benzylguanine may block MGMT-mediated DNA repair and significantly restore the TMZ sensitivity in patients with TMZ-resistant GBM.2 Recent strategies aiming at down-regulating MGMT expression in cancer cells have shown promising results in order to combat resistance to TMZ.16–19 Further studies on drugs, which have been demonstrated to be well tolerated and with low systematic toxicity by long-term clinical practice have potential in modulating MGMT expression, are warranted to see whether they can decrease MGMT activity and sensitize glioma cells to TMZ chemotherapy with fewer side effects.
FLT, a specific SSRI, is one of the most popular antidepressants. The mechanism of how FLT treats depression has been ascribed to its inhibitory action on serotonin transporters. Abdul et al showed that FLT inhibited the growth of human prostate cancer cells.20 Levkovitz et al indicated that FLT activated caspase-3 and induced apoptosis in rat glioma cell lines.21 Liu et al proposed that FLT suppresses glioma by evoking AMPAR-mediated calcium-dependent apoptosis.8 Since Food and Drug Administration-approved medications is more safety and cost-effective than new agents, the novel use of old drugs for cancer therapy became a more acceptable strategy in the pharmaceutical study.22,23 In this study, we aimed to examine the effect of FLT in MGMT-expressing glioma cells. We found that FLT markedly reduced MGMT expression in glioma cells, which was not associated with GluR1 receptor function. Importantly, FLT sensitized glioma cells to TMZ-induced tumor suppression both in vitro and in vivo. Knockdown of MGMT expression abolished the synergistic effect of FLT with TMZ in glioma cells, which suggested that FLT might sensitize glioma cells to TMZ through down-regulation of MGMT expression. Our findings might suggest a novel combination strategy for TMZ-based chemotherapy, which could be further investigated in pre-clinical studies.
To date, several signaling pathways including ERK1/2, JNK1/2, NF-κB, mTOR, STAT3, and AKT have been suggested to be involved in the transcription regulation of MGMT.10–15 A previous study showed that transient transfection of human embryo kidney HEK293 cells with the NF-κB subunit p65 would induce a 55-fold increase in MGMT expression, whereas addition of the NF-κB inhibitor δNIκB (N-terminal domain of IκBα) wholly abrogates the augmented expression of MGMT.11 Therefore, down-regulation of the NF-κB-MGMT pathway is a feasible strategy to overcome TMZ resistance. Our findings showed that FLT attenuated MGMT expression through disruption of NF-κB signaling activation. Given that FLT is an effective inhibitor of NF-κB signaling activation, the competence of FLT to suppress the NF-κB–MGMT pathway and to overcome TMZ-resistance might be of clinical significance.24 In addition, the critical role of the FLT-suppressed NF-κB–MGMT pathway was demonstrated in cytotoxicity study, as TMZ plus FLT significantly reduced NF-κB S536 phosphorylation and MGMT expression, accompanied with impaired glioma cell growth in T98G and U138 glioma cells. Furthermore, knockdown of NF-κB upstream kinase IKKβ mostly abrogated the synergistic effect of FLT with TMZ, confirming that FLT enhanced the TMZ effect on glioma cells through disruption of NF-κB–MGMT signaling activity.
Conclusion
Our findings showed that FLT and TMZ combinational treatment could effectively reverse TMZ-resistance of glioma cells. This bioactivity might be carried out by inhibiting the NF-κB–MGMT pathway. Although Shapovalov et al showed that FLT modulates breast cancer metastasis to the brain in the murine model,25 these results might reflect the complexity and diversity of tumor cells or cancer types (glioma and breast cancer). Further investigations into possible mechanisms of interaction among FLT, tumor cells, and the brain microenvironment might be necessary before we expand its use as a therapeutic agent against glioma.
Acknowledgment
This study was supported by National Natural Science Foundation of People’s Republic of China (Nos 81200644 and 81460244).
Disclosure
The authors report no conflicts of interest in this work.
References
Johannessen TC, Bjerkvig R. Molecular mechanisms of temozolomide resistance in glioblastoma multiforme. Expert Rev Anticancer Ther. 2012;12(5):635–642. | ||
Fan CH, Liu WL, Cao H, Wen C, Chen L, Jiang G. O6-methylguanine DNA methyltransferase as a promising target for the treatment of temozolomide-resistant gliomas. Cell Death Dis. 2013;4:e876. | ||
Park DM, Sathornsumetee S, Rich JN. Medical oncology: treatment and management of malignant gliomas. Nat Rev Clin Oncol. 2010;7(2):75–77. | ||
Siegal T. Which drug or drug delivery system can change clinical practice for brain tumor therapy? Neuro Oncol. 2013;15(6):656–669. | ||
Ketter TA. Acute and maintenance treatments for bipolar depression. J Clin Psychiatry. 2014;75(4):e10. | ||
Henry ME, Schmidt ME, Hennen J, et al. A comparison of brain and serum pharmacokinetics of R-fluoxetine and racemic fluoxetine: a 19-F MRS study. Neuropsychopharmacology. 2005;30(8):1576–1583. | ||
Mukherjee J, Das MK, Yang ZY, Lew R. Evaluation of the binding of the radiolabeled antidepressant drug, 18F-fluoxetine in the rodent brain: an in vitro and in vivo study. Nucl Med Biol. 1998;25(7):605–610. | ||
Liu KH, Yang ST, Lin YK, et al. Fluoxetine, an antidepressant, suppresses glioblastoma by evoking AMPAR-mediated calcium-dependent apoptosis. Oncotarget. 2015;6(7):5088–5101. | ||
Fu J, Yang QY, Sai K, et al. TGM2 inhibition attenuates ID1 expression in CD44-high glioma-initiating cells. Neuro Oncol. 2013;15(10):1353–1365. | ||
Okada M, Sato A, Shibuya K, et al. JNK contributes to temozolomide resistance of stem-like glioblastoma cells via regulation of MGMT expression. Int J Oncol. 2014;44(2):591–599. | ||
Lavon I, Fuchs D, Zrihan D, et al. Novel mechanism whereby nuclear factor kappaB mediates DNA damage repair through regulation of O(6)-methylguanine-DNA-methyltransferase. Cancer Res. 2007;67(18):8952–8959. | ||
Sato A, Sunayama J, Matsuda K, et al. MEK-ERK signaling dictates DNA-repair gene MGMT expression and temozolomide resistance of stem-like glioblastoma cells via the MDM2-p53 axis. Stem Cells. 2011;29(12):1942–1951. | ||
Weiler M, Blaes J, Pusch S, et al. mTOR target NDRG1 confers MGMT-dependent resistance to alkylating chemotherapy. Proc Natl Acad Sci U S A. 2014;111(1):409–414. | ||
Grogan PT, Sarkaria JN, Timmermann BN, Cohen MS. Oxidative cytotoxic agent withaferin A resensitizes temozolomide-resistant glioblastomas via MGMT depletion and induces apoptosis through AKT/mTOR pathway inhibitory modulation. Invest New Drugs. 2014;32(4):604–617. | ||
Kohsaka S, Wang L, Yachi K, et al. STAT3 inhibition overcomes temozolomide resistance in glioblastoma by downregulating MGMT expression. Mol Cancer Ther. 2012;11(6):1289–1299. | ||
Huang H, Lin H, Zhang X, Li J. Resveratrol reverses temozolomide resistance by downregulation of MGMT in T98G glioblastoma cells by the NF-κB-dependent pathway. Oncol Rep. 2012;27(6):2050–2056. | ||
Ryu CH, Yoon WS, Park KY, et al. Valproic acid downregulates the expression of MGMT and sensitizes temozolomide-resistant glioma cells. J Biomed Biotechnol. 2012;2012:987495. | ||
Kushwaha D, Ramakrishnan V, Ng K, et al. A genome-wide miRNA screen revealed miR-603 as a MGMT-regulating miRNA in glioblastomas. Oncotarget. 2014;5(12):4026–4039. | ||
Quintavalle C, Mangani D, Roscigno G, et al. MiR-221/222 target the DNA methyltransferase MGMT in glioma cells. PLoS One. 2013; 8(9):e74466. | ||
Abdul M, Logothetis CJ, Hoosein NM. Growth-inhibitory effects of serotonin uptake inhibitors on human prostate carcinoma cell lines. J Urol. 1995;154(1):247–250. | ||
Levkovitz Y, Gil-Ad I, Zeldich E, Dayag M, Weizman A. Differential induction of apoptosis by antidepressants in glioma and neuroblastoma cell lines: evidence for p-c-Jun, cytochrome c, and caspase-3 involvement. J Mol Neurosci. 2005;27(1):29–42. | ||
Blagosklonny MV. A new science-business paradigm in anticancer drug development. Trends Biotechnol. 2003;21(3):103–106. | ||
Triscott J, Lee C, Hu K, et al. Disulfiram, a drug widely used to control alcoholism, suppresses the self-renewal of glioblastoma and over-rides resistance to temozolomide. Oncotarget. 2012;3(10):1112–1123. | ||
Yang JM, Rui BB, Chen C, et al. Acetylsalicylic acid enhances the anti-inflammatory effect of fluoxetine through inhibition of NF-κB, p38-MAPK and ERK1/2 activation in lipopolysaccharide-induced BV-2 microglia cells. Neuroscience. 2014;275:296–304. | ||
Shapovalov Y, Zettel M, Spielman SC, et al. Fluoxetine modulates breast cancer metastasis to the brain in a murine model. BMC Cancer. 2014;14:598. |
Supplementary material
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.