Back to Journals » Cancer Management and Research » Volume 12
Discovery of Ebselen as an Inhibitor of 6PGD for Suppressing Tumor Growth
Authors Feng Q, Li X, Sun W, Li Y, Yuan Y, Guan B, Zhang S
Received 25 March 2020
Accepted for publication 9 July 2020
Published 5 August 2020 Volume 2020:12 Pages 6921—6934
DOI https://doi.org/10.2147/CMAR.S254853
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Yong Teng
Qi Feng,1 Xiuru Li,1 Wenjing Sun,1 Yubo Li,2 Yu Yuan,2 Baozhang Guan,1 Shuai Zhang3
1The First Affiliated Hospital, Biomedical Translational Research Institute, Jinan University, Guangzhou, Guangdong 510632, People’s Republic of China; 2Tianjin State Key Laboratory of Modern Chinese Medicine, School of Chinese Materia Medica, Tianjin University of Traditional Chinese Medicine, Tianjin 301617, People’s Republic of China; 3School of Integrative Medicine, Tianjin University of Traditional Chinese Medicine, Tianjin 301617, People’s Republic of China
Correspondence: Shuai Zhang; Baozhang Guan Email [email protected]; [email protected]
Introduction: The 6-phosphogluconate dehydrogenase (6PGD) was upregulated in many solid cancers and plays an important role in tumorigenesis. In the present study, we want to discover an old drug as an inhibitor of 6PGD for suppressing tumor growth.
Methods: We determined the expression of 6PGD in cancer tissues using Gene Expression Omnibus (GEO) profiles and explored the importance of 6PGD expression in cancer progression by using Kaplan–Meier Plotter. We identified Ebselen as a 6PGD inhibitor by using 6PGD in vitro enzyme activity assay. Cell viability, cell proliferation, tumor growth and cell metabolism assay were used to explore the role of 6PGD and its inhibitor in cancer cells.
Results: We found that the expression of 6PGD was upregulated in different cancer tissues and it can promote tumorigenesis. Here, we analyzed our 6PGD inhibitor screening data again and found an old drug Ebselen, which blocks cancer cell proliferation and tumor growth by inhibiting 6PGD enzyme activity, while knocking down 6PGD would partially abolish the inhibition of Ebselen on cell proliferation and cell metabolism.
Conclusion: Our results suggested that the conventional drug Ebselen could serve as a novel inhibitor of 6PGD for suppressing cancer growth by inhibiting 6PGD enzyme activity.
Keywords: Ebselen, 6PGD, oxidative pentose phosphate pathway, cancer
Introduction
Mounting studies showed that the expression of 6-phosphogluconate dehydrogenase (6PGD) was upregulated in many solid cancers, including colorectal cancers,1 cervical intraepithelial neoplasia,2,3 liver cancer,4 cervical cancer,5 anaplastic thyroid carcinoma (ATC),6 breast cancer,7 ovarian cancer and lung cancer.8 Sukhatme reported that knockdown of 6PGD in lung cancer H1975 cells inhibits cell proliferation in vitro and tumor growth in vivo.9 Lin et al found that knocking down 6PGD inhibits lipogenesis and tumor growth in numerous cancer cells through Ru-5-P-dependent inhibition of LKB1–AMPK signaling.10 Several studies showed that 6PGD is also activated by post-translation modification in cancer cells.11–13 Liu et al found that 6PGD is activated by phosphorylating at tyrosine (Y) 481 by Src family kinase Fyn, leading to tumor growth and radiation resistance.11 Shan et al demonstrated that 6PGD is activated by lysine acetylation in human cancer,12 while Sheng et al found that N6-methyladenosin (m6A) mRNA modifications reader YTHDF2 directly binds to the m6A modification site of 6PGD and promotes 6PGD mRNA translation.13 Thus, 6PGD may be a promising anticancer target in clinical treatment. In the previous study, we developed 6PGD inhibitor Physcion, which effectively inhibits cancer cell proliferation and tumor growth by targeting 6PGD M15 site.10 Elf et al also demonstrated that applying the combination of the Physcion with anti-malarial agent dihydroartemisinin (DHA) would synergistically inhibit leukemia cell growth.14 Lastly, we found that targeting 6PGD by Physcion could sensitize cisplatin-resistant cancer cells to cisplatin treatment.8 Therefore, we reasoned that combining Physcion with chemotherapy drugs may improve the efficacy of single-agent chemotherapy treatment and overcome resistance in human cancer. Later, several studies validated our hypothesis; Chen et al study showed that targeting 6PGD by Physcion inhibits hepatocellular carcinoma cell growth and sensitizes hepatocellular carcinoma to chemotherapeutic agent treatment.4 Guo et al reported that the inhibition of 6PGD by Physcion enhances chemosensitivity in cervical cancer.5 Ma et al study demonstrated that inhibiting 6PGD reverses doxorubicin resistance in anaplastic thyroid cancer.6 Yang et al study suggested that the inhibition of 6PGD by Physcion augments chemotherapy efficacy in breast cancer.7 Bhanot et al study uncovered that knockdown of 6PGD inhibits Acute myeloid leukemia (AML) cells growth and reverses chemotherapeutics daunorubicin and cytarabine resistance.15
Researches into old drugs for a range of human diseases have seen a revival in recent years. Metformin, which is well commonly used for type 2 diabetes, has extended its function and become a potential anticancer agent. The objectives of the present study were to develop old drugs as 6PGD inhibitor for suppressing tumor growth. Thus, we analyzed our previous 6PGD inhibitor screening database10 and discovered an old drug Ebselen (2‐phenyl‐1,2‐benzisoselenazol-3(2H)-one) from 2000 FDA-approved drugs. Ebselen is an organoselenium compound, with anti-inflammatory, anti-oxidant and cytoprotective activity.16 Mounting studies showed that Ebselen presents a potential chemopreventive activity to protect against carcinogenesis.17–20 In particular, Nakamura’s finding strongly suggested that Ebselen is a potential chemopreventive agent in inflammation-associated carcinogenesis.17
In the present study, we found that Ebselen inhibits cancer cell proliferation and tumor growth by targeting 6PGD in vivo. Previous studies showed that Ebselen targets multiple pathways' proteins, such as glutamate dehydrogenase [57], lactate dehydrogenase [58]. Here, we first reported that Ebselen can also inhibit 6PGD. These results suggested that Ebselen as a novel inhibitor of 6PGD, may represent a promising approach for selectively killing cancer cells and will be used for cancer treatment in future.
Materials and Methods
Reagents and Antibodies
Antibody against 6PGD (1:1000 times dilution) (catalog number: 13389) and β-actin (1:1000 times dilution) (catalog number: 3700) were from Cell signaling. 6PGD shRNA was purchased from TranSheepBio (TRCN0000028631, 5`CCGGCCAGTTTGATGGTGATAAGAACTCGAGTT CTTATCACCATCAAACTGGTTTTT3`). Lipofectamine RNAiMAX transfection reagent was purchased from Thermo Fisher Scientific (catalog number: 13778150). Ebselen was purchased from Sigma-Aldrich (catalog number: No. E3520). RPMI 1640 medium (with glutamine) was purchased from Sigma-Aldrich (catalog number: No. E3520) Dulbecco Modified Eagle Medium (DMEM, with glutamine) Cell culture. The H1299, K562 and H226 cells were gifts from Dr. Zhi Shi (Jinan University, Guangdong, China). The HDF and PIG1 cells were gifts from Dr. Jing Chen (Emory University, USA). The human breast cancer cell lines MDA-MB-231, SUM159, MCF-7, BT474, T47D, HCC-1954 and HCC-202 were gifts from Dr. Yuanzhao Lu (Jinan University, Guangdong, China). The liver normal cell QSS7701, liver cancer cells Huh7, Hep-3b, Hep-G2, the lung cancer cells H460, H1944, H157 were obtained from Guangzhou Jenniobio Biotechnology Co., Ltd. (Guangzhou, China). The human normal epithelial lung cell line BEAS-2B was a gift from Dr. Chenglai Xia (Guangzhou Medical University, Guangdong, China). The H1299, K562 and H226 cells were cultured in an RPMI 1640 (with L-Glutamine) medium with 10% fetal bovine serum (FBS, ExCell Bio) at 37°C and 5% CO2. HDF cells were cultured in FibroLife medium with 10% FBS. PIG1 cells were cultured in Medium 254 with 10% FBS. The MDA-MB-231, SUM159, MCF-7, BT474, T47D, HCC-1954, HCC-202, Huh7, Hep-3b, Hep-G2,QSS7701, H460, H1944, H157 and BEAS-2B cells were cultured in Dulbecco Modified Eagle Medium (DMEM, with L-Glutamine) with 10% FBS.
Generate stable knockdown cell lines
Stable knockdown of endogenous 6PGD was achieved using lentiviral vector harboring shRNA construct (TranSheepBio; 5ʹ-CCGGGTGGATGATTTCATCGAGAAACTCGAGTTTCTCGATGAAATCATCCACTTTTT −3ʹ).
Western Blotting
Cells were lysed with lysis buffer (150 mM NaCl, 10 mM HEPES[pH=7.0], 1%NP40, 5 mM Na4P2O7, 5 mM NaF, 2 mM Na3VO4) containing protease inhibitor (complete ULTRA Tablets, Min, EDTA-free, EASYpack, Roche) on ice for 30 mins and then centrifuged at 12,000 rpm for 15 mins at 4°C. We loaded whole-cell protein extracts (30 μg/lane) into 12% SDS-PAGE; then, they were separated by running at 90-volt voltage for 30 mins and running at 150-volt voltage for 90 mins, and transferred onto PVDF membranes (Millipore). The membranes were blocked with 5% non-fat milk for 2 h and then incubated overnight at 4°C, or at room temperature for 2 h with the primary antibody and for 1 h at room temperature with the secondary antibody. Signals were detected using Luminol substrate solution.
Cell Proliferation and Viability Assays
Cell numbers were counted by trypan blue exclusion under a microscope (x40) at indicated times to indicate cell proliferation. Cell viability was quantified by Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.). In brief, cell proliferation assays were performed by seeding 5 × 104 cells in 6-well plates. Relative cell proliferation was determined by cell counting at 4 days after being seeded and the percentage cell proliferation of the control. Cell growth was determined by cell numbers recorded at 0, 1, 2, 3 and 4 days after being seeded. To determine cell viability, cells were seeded in 96-well plates and treated with different concentrations of Ebselen. Cell viability was quantified by Cell Counting Kit-8 as described previously.8
Oxidative PPP Flux Assay
Oxidative PPP flux assay was determined by using 14CO2 release as described previously.10 In brief, we seeded cells on a 6-cm dish, then placed it in a 10-cm dish with 2 sealed pinholes on the top. We treated the cells on the 6-cm dish with [1–14C]- or [6–14C]-glucose, respectively, at a final specific activity of 10 μCi/mL glucose at 37°C for 3 h. Then, the oxidative PPP flux was stopped by injecting 0.3 mL of 50% TCA through one of the holes on the top, and at the same time 14CO2 released was trapped by injecting 0.3 mL of Hyamine hydroxide into a small cup placed on the 10-cm dish through the second hole. We sealed each dish with parafilm and placed the dish at room temperature overnight. Hyamine hydroxide in the small cup was dissolved into 60% methanol and directly subjected to scintillation counting.
Cellular Metabolite Extraction and Measurement
Cellular metabolites were extracted and spectrophotometrically measured by measuring the intracellular concentrations of 6-PG and Ru-5-P as described previously.10 In brief, to determine the cellular concentration of 6-PG and Ru-5-P, we homogenized 200 μL of packed cell pellets in 0.6 mL of hypotonic lysis buffer. Then, the supernatants were harvested by centrifuging in a cold room at 4°C for 10 mins at maximum speed. The supernatants were applied to Amicon Ultra tubes with 10 KDa cut off filter (Millipore, USA). The flow-through containing the metabolites was used for the measurement.
We determined the 6-PG and Ru-5-P concentration according to the 6PGD and D-ribulose 5-phosphate 3-epimerase enzyme activity, respectively.
NADPH/NADP+ Ratio Assay
NADPH/NADP+ ratio was measured by a Colorimetric Assay Kit (Sigma-Aldrich) as described previously.10 In brief, we lysed the harvested cells (2 x 106) with 200 mL of NADP+ (or NADPH) extraction buffer. Then, we incubated the lysed cells at 60°C for 5 mins, and the added 20 mL of assay buffer and 200 mL of the counter NADPH (or NADP+) extraction buffer were added to neutralize the extracts. The extracts were centrifuged at 12,000 rpm for 5 mins, and the supernatants were used to check the NADPH/NADP+ ratio according to the manufacturer’s protocol. The absorbance at 565 nm from the reaction mixture was measured by a plate reader at 0 min and 30 mins.
Lactate Production Assay
Cellular lactate production was measured by a colorimetric-based lactate assay kit (MBL) as described previously.10 In brief, the cellular lactate production was measured under normoxia with a fluorescence-based lactate assay kit (MBL). Phenol red-free RPMI medium without FBS was added to a 6-well plate of subconfluent cells, and the plate was incubated for 1 h at 37°C. After incubation, 1 mL of media from each well was assessed using the lactate assay kit. Cell numbers were counted by a microscope (x40).
Intracellular ATP Assay
Intracellular ATP was measured by an ATP Colorimetric/Fluorometric Assay Kit (Sigma-Aldrich) as previously described.10 In brief, the intracellular ATP concentration was measured by an ATP bioluminescent somatic cell assay kit (Sigma). 1 x 106 cells were trypsinized and resuspended in ultrapure water. Luminescence was measured with a spectrofluorometer (SpectraMax Gemini; Molecular Devices) immediately after the addition of ATP enzyme mixing to the cell suspension.
Intracellular Reactive Oxygen Species (ROS) Production
The amount of intracellular ROS was measured by detecting dichlorodihydrofluorescein, which is the cleavage product of carboxy-H2DCFDA (Invitrogen) by ROS as described previously.10 In brief, we seeded 2 x 105 cells in a 6-well plate, and then we washed cells with PBS and added 5μM carboxy-H2DCFDA into cells and incubated them for 30 mins. Lastly, the cells were harvested, resuspended in PBS and analyzed using a FACS (BD Biosciences; excitation and emission at 490 and 530 nm, respectively).
6PGD Enzyme Activity Assay
6PGD enzyme activity was determined by the NADPH production rate assay as described previously.10 In brief, 6PGD enzyme activity was determined by the NADPH production rate in assay buffer (0.1mM NADP+, 0.2 mM 6-phosphogluconate, 1mM MgCl2 and 50mM Tris, pH 8.1). Ten μg of protein from cell lysate was added and the reaction was then initiated by adding NADP+. The increase in 340 nm absorbance (OD340) which is used as a measure of NADPH production was obtained every 30 s for 25 mins on a spectrofluorometer (SpectraMax Gemini; Molecular Device).
Tumor Formation in Nude Mice
Nude mice (nu/nu, male 4–6-week-old) were subcutaneously injected with 20 x 106 H1299 cells harboring empty vector on the left flank, and cells with stable knockdown of endogenous 6PGD on the right flank, seven mice were included in each experiment and were performed according to the institutional ethical guidelines for animal experiments described in our previous study.
For drug evaluation of Ebselen, every xenograft mice and nude mice (nu/nu, male 4–6-week-old) were subcutaneously injected with 20 x 106 H1299 cells on the right flank. After subcutaneous injection of 20 x 106 H1299 cells for 20 days, the drug was administered by daily i.p. injection at a dose of 20 mg/kg (8 mice were randomized to each group) for 9 days according to Hanavan’s study.21
Tumor growth was recorded by measurement of two perpendicular diameters using the formula 4π/3 x (width/2)2 x (length/2). The tumors were harvested and weighed at the experimental endpoint, and the masses of tumors (g) treated with vehicle control (DMSO or PLKO.1) and S3 (6PGD shRNA) were compared by a two-tailed unpaired Student’s t-test.
Molecular Docking Study
The study was performed based on the crystal structure of 6PGD (PDB code: 5UQ9). Ebselen was initially positioned into the NADP+ binding site and then docked by CDOCKER in BIOVIA Discovery Studio 2017 R2 (DS) software. Docking results showed that there are van der Waals interaction, electrostatic interaction, and hydrogen bond interaction when the ligand binds to the receptor.
In vitro Thermal Shift Assay
We incubated recombinant protein 6PGD (r6PGD) and Ebselen in the PCR tubes on ice for 30 mins (compare with DMSO), then transferred PCR tubes to the PCR machine and heated them at a temperature from 34.9°C to 73°C for 3 mins, and followed cooled them for 3 mins at RT. We centrifuged the heated recombinant protein at 14,000 rpm for 20 mins at 4°C in order to separate soluble fractions from precipitates. Lastly, we transferred the supernatants to new microtubes and analyzed them by SDS-PAGE followed by Western blot analysis.
Fluorescence-Based Thermal Shift (FTS) Assay
We performed FTS assay as described previously.22,23 Firstly, we diluted Ebselen in DMSO and performed all assay experiments in a 50 μl volume system with 5 μM protein and fresh SYPRO orange solution (SYPRO orange: 5000X concentration in DMSO; Life Technologies S-6650). Secondly, after we added protein and compounds into the well of PCR plates, we shook and centrifuged the PCR plates, which were sealed with optical seal. Next, we performed Thermal scanning (10 to 95 μC at 1.5 μC/min) by using a real-time PCR setup (CFX384-Biorad Laboratories) and fluorescence intensity was monitored at every 10 s. Lastly, we analyzed the Curve fitting, melting temperature calculation and report generation on the raw FTS data by using software (Data was calculated by Bio-Rad CFX manager software and Excel).
Primary Tissue Samples from Patients with Leukemia and Healthy Donors
Approval of the use of human specimens was given by the Institutional Review Board of First Affiliated Hospital, Jinan University. All clinical samples were obtained under informed consent and approval of the Jinan University Review Board. Clinical information of the patients was obtained from the pathologic files at First Affiliated Hospital, Jinan University under the guidelines and approval from the Institutional Review Board of First Affiliated Hospital, Jinan University according to the Health Insurance Portability and Accountability Act. Mononuclear cells were isolated from peripheral blood and bone marrow samples from patients with leukemia or from healthy donors using lymphocyte separation medium (Cellgro). Cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum and penicillin/streptomycin and incubated with increasing concentrations of Ebselen for up to 72 h.
Ethics Statements
This study was carried out in accordance with the recommendations of Requirements of the Ethical Review System of Biomedical Research Involving Human by National Health and Family Planning Commission of China, Jinan University Ethics Committee with written informed consent from all subjects. All subjects were given written informed consent in accordance with the Declaration of Helsinki.
All animal experiments were approved by the Laboratory Animal Ethics Committee Jinan University and Use Committee and conducted in accordance with the regulations of the Service of Consumables and Veterinary Affairs-Division of Animal Protection (SCAV-EXPANIM).
Bioinformatic Analysis
The public Gene Expression Omnibus datasets (GSE19804 and GSE76427) were used for bioinformatic analysis. Kaplan–Meier Plotter (http://kmplot.com/analysis/) was used for overall survival.
Statistical Analysis
Statistical analyses were performed using Student’s t-test between two groups. One-way ANOVA was used for multiple group comparison. All data were obtained from three independent experiments performed in triplicate and were presented as the mean ± standard error. P<0.05 was considered to indicate a statistically significant difference.
Results
The expression of 6PGD is elevated in lung cancer and is important for tumor growth
To determine the role of 6PGD in cancer, we checked the expression of 6PGD and analyzed the correlation between its expression levels and the outcomes of lung cancer patients. Firstly, we determined the expression of 6PGD in lung cancer tissues using Gene Expression Omnibus (GEO) profiles (GSE19804), and we found that the expression of 6PGD was higher in cancer tissues compared with non-tumors tissues (Figure 1A). To further explore the importance of 6PGD expression in lung cancer progression, we used available Kaplan–Meier Plotter (http://kmplot.com/analysis/) (6PGD: accession number 201118_at) database to analyze the lung cancer patient overall survival based on the expression of 6PGD. Higher levels of 6PGD (red) are significantly correlated with reduced overall survival compared to low 6PGD levels (black) (Figure 1B). The same results were obtained from liver cancer (Figure 1C and D).
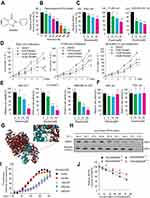
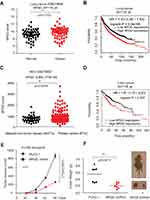 |
Figure 1 6PGD expression is evaluated in cancer and is important for tumor growth. (A) Expression of 6PGD in lung cancer tissues and matched noncancerous, calculated from GEO profiles (GSE19804). (B) Kaplan–Meier curves of overall survival in lung cancer patients with high and low expression of 6PGD, calculated from (http://kmplot.com/analysis). (C) Expression of 6PGD in liver cancer tissues and matched noncancerous, calculated from GEO profiles (GSE76427). (D) Kaplan–Meier curves of overall survival in liver cancer patients with high and low expression of 6PGD, calculated from (http://kmplot.com/analysis). (E) Tumor growth was compared between xenograft nude mice injected with 6PGD knockdown H1299 cells and control vector cells. The values were given as mean ± SD. (F) Left: Tumor mass in xenograft nude mice injected with 6PGD knockdown H1299 cells compared to mice (n=7) injected with the control vector cells. Right: Dissected tumors in a representative nude mouse are shown. The data represent mean values ± SD from three independent experiments. |
To address the functional consequence of higher 6PGD expression in lung cancer, we determined the effect of 6PGD on tumor growth. We established 6PGD stable knockdown H1299 cell line and found that knocking down 6PGD significantly inhibits tumor growth (Figure 1E and F). Collectively, our results suggested that 6PGD promotes lung cancer cell growth, and highly correlates with patient overall survival, which suggests that 6PGD is a potential anti-cancer target.
Discovery and Development of Ebselen as a 6PGD Inhibitor
We analyzed our screening results for 6PGD inhibitor10 and identified Ebselen as a 6PGD inhibitor from a library of 2000 FDA-approved small-molecule compounds (Figure 2A). To validate this finding, we performed in vitro 6PGD activity assay with increasing concentrations of Ebselen (from 0 to 150 nM) using recombination 6PGD protein. The 6PGD enzyme activity decreased with increasing concentrations of Ebselen (Figure 2B). Moreover, Ebselen also inhibited 6PGD activity in H1299, K562 and MDA-MB-231cells (Figure 2C), leading to decreased H1299, K562 and MDA-MB-231cell proliferation (Figure 2D) and cell viability (Figure 2E), whereas 6PGD knockdown cells were partially resistant to Ebselen treatment (Supplementary Figure 1A). However, Ebselen did not significantly affect cell viability of the control proliferating cells including human dermal fibroblasts (HDFs) and immortalized human melanocyte PIG1 cells (Figure 2F).
The molecular docking study based on the crystal structure of 6PGD (PDB code: 5UQ9) suggested that Ebselen fits in a pocket near the binding site of NADP+ surrounded by residues including Lys 76, Asn 103, Lys 184 and Lys 261 of 6PGD (Figure 2G). To examine whether Ebselen binds to and inhibits 6PGD, we performed a thermal melt shift assay to examine the interaction of protein (6PGD) and Ebselen.
Incubation of increasing concentrations of Ebselen raises 6PGD melting temperature (Tm) in a dose-dependent manner, suggesting that Ebselen directly binds to the protein (Figure 2H and I). Furthermore, we found that the inhibition of Ebselen on 6PGD enzyme activity was reversed by the NADPH (Figure 2J). Collectively, these results suggested that Ebselen inhibits 6PGD activity by competing with cofactor NADP+.
To explore whether the antitumor role is universal, we determined the effect of Ebselen on cell viability in several cancer cells. Firstly, we found that Ebselen treatment also resulted in decreased cell viability of liver cancer cells including, HepG2, Hep-3B and HuH7, not normal liver cells QSS7701 (Supplementary Figure 1B). Secondly, Ebselen treatment resulted in decreased cell viability of lung cancer cells including, A549, H1944 and H466, not normal lung BEAS-2B cell (Supplementary Figure 1C). Finally, Ebselen treatment resulted in decreased cell viability of breast cancer cells including, T47D, BT474, MCF-7, SUM159, HCC-202 and HCC-1954 cells (Supplementary Figure 1D). Collectively, these results suggested that Ebselen can act as a universal anit-cancer inhibitor partially through targeting 6PGD.
Ebselen Regulates Cancer Cell Metabolism
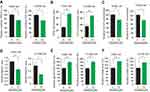
Lin et al studies showed that knockdown or inhibition of 6PGD would block oxidative PPP flux.10 Thus, in the present study, we also found that Ebselen treatment resulted in decreased oxidative PPP flux, leading to increasing intracellular 6-PG levels and decreasing Ru-5-P levels and NADPH/NADP+ ratio. While the lactate production and ROS level were upward in the K562 and H1299 cells (Figure 3A–F).
In the normal lung BEAS-2B cells, Ebselen treatment also resulted in decreased NADPH/NADP+ ratio but did not have an effect on lactate level (Supplementary Figure 2A and B), while the effect of Ebselen on ROS level was partially abolished by knocking down 6PGD in H1299 and BEAS-2B cells (Supplementary Figure 2C–E). The elimination of 6PGD is not complete by shRNA and this also could explain the effects of Ebselen on ROS are very moderate. These results suggested that Ebselen regulates cell metabolism by targeting 6PGD.
Ebselen Inhibits Leukemia Patient Cell Survival
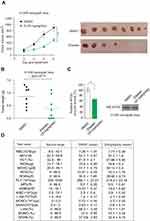
Moreover, Ebselen also inhibited 6PGD activity in human primary leukemia cells (Figure 4A) and peripheral blood cells isolated from healthy donors (Supplementary Figure 3A), not G6PD enzyme activity (Supplementary Figure 3B). Next, we tested the inhibition of Ebselen on cell proliferation and found that Ebselen treatment leads to decreased cell viability and cell proliferation in human primary leukaemia cells (Figure 4B and C and Supplementary Figure 3C and D). In contrast, Ebselen treatment did not affect the cell viability of peripheral blood cells isolated from healthy donors (Figure 4D). Collectively, our data suggested that Ebselen can be a promising potential anticancer agent without obvious toxicity to human blood cells.
Ebselen Regulates Leukemia Patient Cell Metabolism
We also observed that Ebselen treatment increased 6-PG accumulation, inhibited Ru-5-P production, elevated ROS levels, decreased NADPH/NADP+ ratio, and increased lactate production in human primary leukemia cells (Figure 5). These results were the same as Ebselen treatment cancer cell lines.
Ebselen targeting 6PGD represents a promising anti-cancer drug in vivo. Nude mice were subcutaneously injected with H1299 cells; then, Ebselen (20 mg/kg/day, intraperitoneally [i.p.]) treatment resulted in significantly decreased tumor growth and masses compared with mice receiving dimethylsulphoxide (DMSO; Figure 6A and B). We sacrificed the mice and got the tumor tissue and determined the 6PGD activity, we found that the 6PGD activity was decreased in Ebselen treatment group compared with DMSO treatment group (Figure 6C). Additionally, chronic treatment with Ebselen had no effect on the complete blood counts in the Ebselent treatment nude mice compared with DMSO treatment nude mice (Figure 6D). These results suggested that the inhibition of 6PGD by Ebselen in vivo confers a specific inhibitory effect on tumor growth.
Discussion
Mounting evidence demonstrated that cancer cells with higher oxidative PPP flux ratio support cell growth, survival, and drug resistance.8,10–12,24,25 6PGD is an important enzyme in the oxidative PPP that catalyzes the decarboxylating reduction of 6-phosphogluconate (6-PG) to ribulose 5-phosphate (Ru-5-P) in the presence of cofactor NADP+.8,12 Many studies have revealed that 6PGD has multi-faceted roles in tumorigenesis, such as cell proliferation, survival, metastasis and drug resistance. Thus, inhibition of 6PGD enzyme activity or suppression of 6PGD expression in tumor has the potential to decrease the growth and invasion of tumor and reverse drug resistance. Here we found that the expression of 6PGD was elevated in cancer and established that 6PGD promotes tumor growth in vivo, which strengthens the connection between elevated 6PGD expression and tumor aggression. These data support our and other groups’ studies, which demonstrates a pro-tumorigenic role for 6PGD.
The enzymatic activity of 6PGD is crucial for its roles in tumor growth, chemoresistance, and drug resistance.8,11,12 The inhibitor Physcion and its derivative S3 were developed to effectively inhibit the enzyme activity of 6PGD, reduce the growth of tumor cells and reverse cisplatin resistance,8,10 supporting the idea that directly targeting 6PGD enzyme activity has anti-proliferation properties. Thus, small-molecule inhibitors of 6PGD may have therapeutic efficacy for cancer treatment.
In a high throughput screen of 2000 FDA-approved small-molecule compounds library utilizing recombinant 6PGD, we first identified Physcion as a specific inhibitor of 6PGD enzyme activity.10 Later, we analyzed the high throughput screen data, we further identified Ebselen as an inhibitor of 6PGD enzyme activity. To validate our data, we performed 6PGD enzyme activity in vitro by using recombinant 6PGD and found that Ebselen inhibits 6PGD enzyme activity in a dose-dependent manner. In addition, we also found that Ebselen treatment of tumor cell lines resulted in significantly decreased cell proliferation and tumor growth compared to DMSO vehicle-treated cells. But different cancer cell lines responded to Ebselen with differential sensitivity, maybe due to different cancer cell lines have a different background or different 6PGD expression. Lastly, Ebselen treatment of human primary leukemia cells increased 6-PG accumulation, inhibited Ru-5-P production, elevated ROS levels, decreased NADPH/NADP+ ratio, and increased lactate production, while the incubation of Ebselen with normal blood cells and non-malignant fibroblasts did not result in toxicity. These results are consistent with the cells expressing 6PGD-specific shRNAs or treated with Physcion.10 Importantly, in 6PGD-knockdown cells, the effect of Ebselen treatment was partially blocked. These data supported that Ebselen by targeting 6PGD enzyme activity demonstrates an anti-tumor role. In the future, we can apply Ebselen to reverse drug resistance by targeting 6PGD as Physcion reverses cisplatin resistance by targeting 6PGD. There are some drugs (DHEA) by targeting G6PD to reverse drug resistance.26 However, there is benefit in inhibiting 6PGD over G6PDH to inhibit cancer cell proliferation, that is because suppression of expression or inhibition of enzyme activity of G6PD could not affect cancer cell proliferation in some cancer cell lines.27,28
We were initially concerned that the inhibition of Ebselen on 6PGD enzyme activity might make Ebselen appear to inhibit 6PGD spuriously through its glutathione peroxidase-like activity on 6PGD cysteine-reactive. The molecular docking study suggested that Ebselen fits in a pocket near the binding site of NADP+ of 6PGD and the 6PGD enzyme activity assay confirmed that the decreased 6PGD enzyme activity was rescued by increasing the concentration of co-factor NADP+.
Conclusion
In summary, this study uncovers a crucial central role of Ebselen in anticancer by targeting 6PGD, which leads to inhibiting tumor growth. Our study demonstrated that focusing on targeting 6PGD could remarkably inhibit cancer growth. These findings provide a crucial role of Ebselen in anticancer progression and underscore the potential of targeting 6PGD as a novel strategy for cancer prevention and treatment.
Abbreviations
6PGD, 6-phosphogluconate dehydrogenase; DHA, dihydroartemisinin; AML, Acute myeloid leukemia; GEO, Gene Expression Omnibus.
Data Sharing Statement
The analyzed data sets generated during the study are available from the corresponding authors on reasonable request.
Acknowledgments
The authors wish to thank Dr. Jun Fan (School of medicine, Jinan University), who provided kind assistance with the drug screening. We thank Dr. Shannon Elf (The University of Chicago) for the critical review and edit of the manuscript.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Bravard A, Luccioni C, Muleris M, Lefrancois D, Dutrillaux B. Relationships between UMPK and PGD activities and deletions of chromosome 1p in colorectal cancers. Cancer Genet Cytogenet. 1991;56:45–56. doi:10.1016/0165-4608(91)90361-W
2. Basu J, Duttagupta C, Vermund SH, et al. Alterations in erythrocyte glutathione metabolism associated with cervical dysplasias and carcinoma in situ. Cancer Invest. 1993;11:652–659. doi:10.3109/07357909309046937
3. Jonas SK, Benedetto C, Flatman A, et al. Increased activity of 6-phosphogluconate dehydrogenase and glucose-6-phosphate dehydrogenase in purified cell suspensions and single cells from the uterine cervix in cervical intraepithelial neoplasia. Br J Cancer. 1992;66:185–191. doi:10.1038/bjc.1992.240
4. Chen H, Wu D, Bao L, et al. 6PGD inhibition sensitizes hepatocellular carcinoma to chemotherapy via AMPK activation and metabolic reprogramming. Biomed Pharmacother. 2019;111:1353–1358. doi:10.1016/j.biopha.2019.01.028
5. Guo H, Xiang Z, Zhang Y, Sun D. Inhibiting 6-phosphogluconate dehydrogenase enhances chemotherapy efficacy in cervical cancer via AMPK-independent inhibition of RhoA and Rac1. Clin Transl Oncol. 2019;21:404–411. doi:10.1007/s12094-018-1937-x
6. Ma L, Cheng Q. Inhibiting 6-phosphogluconate dehydrogenase reverses doxorubicin resistance in anaplastic thyroid cancer via inhibiting NADPH-dependent metabolic reprogramming. Biochem Biophys Res Commun. 2018;498:912–917. doi:10.1016/j.bbrc.2018.03.079
7. Yang X, Peng X, Huang J. Inhibiting 6-phosphogluconate dehydrogenase selectively targets breast cancer through AMPK activation. Clin Transl Oncol. 2018;20:1145–1152. doi:10.1007/s12094-018-1833-4
8. Zheng W, Feng Q, Liu J, et al. Inhibition of 6-phosphogluconate dehydrogenase reverses cisplatin resistance in ovarian and lung cancer. Front Pharmacol. 2017;8:421. doi:10.3389/fphar.2017.00421
9. Sukhatme VP, Chan B. Glycolytic cancer cells lacking 6-phosphogluconate dehydrogenase metabolize glucose to induce senescence. FEBS Lett. 2012;586:2389–2395. doi:10.1016/j.febslet.2012.05.052
10. Lin R, Elf S, Shan C, et al. 6-Phosphogluconate dehydrogenase links oxidative PPP, lipogenesis and tumour growth by inhibiting LKB1-AMPK signalling. Nat Cell Biol. 2015;17:1484–1496. doi:10.1038/ncb3255
11. Liu R, Li W, Tao B, et al. Tyrosine phosphorylation activates 6-phosphogluconate dehydrogenase and promotes tumor growth and radiation resistance. Nat Commun. 2019;10:991. doi:10.1038/s41467-019-08921-8
12. Shan C, Elf S, Ji Q, et al. Lysine acetylation activates 6-phosphogluconate dehydrogenase to promote tumor growth. Mol Cell. 2014;55:552–565. doi:10.1016/j.molcel.2014.06.020
13. Sheng H, Li Z, Su S, et al. YTH domain family 2 promotes lung cancer cell growth by facilitating 6-phosphogluconate dehydrogenase mRNA translation. Carcinogenesis. 2019. doi:10.1093/carcin/bgz152
14. Elf S, Lin R, Xia S, et al. Targeting 6-phosphogluconate dehydrogenase in the oxidative PPP sensitizes leukemia cells to antimalarial agent dihydroartemisinin. Oncogene. 2017;36:254–262. doi:10.1038/onc.2016.196
15. Bhanot H, Weisberg EL, Reddy MM, et al. Acute myeloid leukemia cells require 6-phosphogluconate dehydrogenase for cell growth and NADPH-dependent metabolic reprogramming. Oncotarget. 2017;8:67639–67650. doi:10.18632/oncotarget.18797
16. Sakurai T, Kanayama M, Shibata T, et al. Ebselen, a seleno-organic antioxidant, as an electrophile. Chem Res Toxicol. 2006;19:1196–1204. doi:10.1021/tx0601105
17. Nakamura Y, Feng Q, Kumagai T, et al. Ebselen, a glutathione peroxidase mimetic seleno-organic compound, as a multifunctional antioxidant. Implication for inflammation-associated carcinogenesis. J Biol Chem. 2002;277:2687–2694. doi:10.1074/jbc.M109641200
18. Sharma V, Tewari R, Sk UH, Joseph C, Sen E. Ebselen sensitizes glioblastoma cells to Tumor Necrosis Factor (TNFalpha)-induced apoptosis through two distinct pathways involving NF-kappaB downregulation and Fas-mediated formation of death inducing signaling complex. Int J Cancer. 2008;123:2204–2212. doi:10.1002/ijc.23771
19. Sinha R, El-Bayoumy K. Apoptosis is a critical cellular event in cancer chemoprevention and chemotherapy by selenium compounds. Curr Cancer Drug Targets. 2004;4:13–28. doi:10.2174/1568009043481614
20. Tewari R, Sharma V, Koul N, et al. Ebselen abrogates TNFalpha induced pro-inflammatory response in glioblastoma. Mol Oncol. 2009;3:77–83. doi:10.1016/j.molonc.2008.10.004
21. Hanavan PD, Borges CR, Katchman BA, et al. Ebselen inhibits QSOX1 enzymatic activity and suppresses invasion of pancreatic and renal cancer cell lines. Oncotarget. 2015;6:18418–18428. doi:10.18632/oncotarget.4099
22. Fedorov O, Niesen FH, Knapp S. Kinase inhibitor selectivity profiling using differential scanning fluorimetry. Methods Mol Biol. 2012;795:109–118. doi:10.1007/978-1-61779-337-0_7
23. Niesen FH, Berglund H, Vedadi M. The use of differential scanning fluorimetry to detect ligand interactions that promote protein stability. Nat Protoc. 2007;2:2212–2221. doi:10.1038/nprot.2007.321
24. Yao P, Sun H, Xu C, et al. Evidence for a direct cross-talk between malic enzyme and the pentose phosphate pathway via structural interactions. J Biol Chem. 2017;292:17113–17120. doi:10.1074/jbc.M117.810309
25. Feng Q, Li X, Sun W, et al. Targeting G6PD reverses paclitaxel resistance in ovarian cancer by suppressing GSTP1. Biochem Pharmacol. 2020;178:114092. doi:10.1016/j.bcp.2020.114092
26. Ramos-Montoya A, Lee W-NP, Bassilian S, et al. Pentose phosphate cycle oxidative and nonoxidative balance: a new vulnerable target for overcoming drug resistance in cancer. Int J Cancer. 2006;119:2733–2741. doi:10.1002/ijc.22227
27. Ghergurovich JM, García-Cañaveras JC, Wang J, et al. A small molecule G6PD inhibitor reveals immune dependence on pentose phosphate pathway. Nat Chem Biol. 2020;16(7):731–739. doi:10.1038/s41589-020-0533-x
28. Gao X, Zhao L, Liu S, et al. Gamma-6-phosphogluconolactone, a byproduct of the oxidative pentose phosphate pathway, contributes to AMPK activation through inhibition of PP2A. Mol Cell. 2019;76:857–871 e859. doi:10.1016/j.molcel.2019.09.007
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.