Back to Journals » OncoTargets and Therapy » Volume 13
Discovering Biomarkers in Peritoneal Metastasis of Gastric Cancer by Metabolomics
Authors Pan G , Ma Y, Suo J, Li W, Zhang Y, Qin S , Jiao Y , Zhang S, Li S, Kong Y, Du Y, Gao S, Wang D
Received 11 January 2020
Accepted for publication 19 June 2020
Published 27 July 2020 Volume 2020:13 Pages 7199—7211
DOI https://doi.org/10.2147/OTT.S245663
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr XuYu Yang
Guoqiang Pan,1,* Yuehan Ma,1,* Jian Suo,1,* Wei Li,1 Yang Zhang,1 Shanshan Qin,2 Yan Jiao,3 Shaopeng Zhang,1 Shuang Li,1 Yuan Kong,1 Yu Du,4 Shengnan Gao,4 Daguang Wang1
1Department of Gastrointestinal Surgery, First Hospital of Jilin University, Changchun, Jilin Province 130000, People’s Republic of China; 2Department of Radiology, Affiliated Hospital of Qingdao, Qingdao 266000, People’s Republic of China; 3Department of Hepatobiliary and Pancreatic Surgery, First Hospital of Jilin University, Changchun, Jilin Province 130000, People’s Republic of China; 4Department of First Operation Room, First Hospital of Jilin University, Changchun, Jilin Province 130000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Daguang Wang
Department of Gastrointestinal Surgery, First Hospital of Jilin University, Changchun, Jilin Province 130000, People’s Republic of China
Tel/ Fax +86 17808068189
Email [email protected]
Background and Objective: Metabolomics has recently been applied in the field of oncology. In this study, we aimed to use metabolomics to explore biomarkers in peritoneal metastasis of gastric cancer.
Methods: Peritoneal lavage fluid (PLF) of 65 gastric cancer patients and related clinical data were collected from the First Hospital of Jilin University. The metabolic components were identified by liquid chromatography-mass spectrometry (LC-MS). Total ion current (TIC) spectra, principal component analysis (PCA), and the Student’s t-test were used to identify differential metabolites in PLF. A support vector machine (SVM) was used to screen the differential metabolites in PLF with a weight of 100%. Cluster analysis was used to evaluate the similarity between samples. Receiver operating characteristic (ROC) curve analysis was used to assess the diagnostic ability of the metabolites. Univariate and multivariate logistic regression analyses were used to identify potential risk factors for peritoneal metastasis of gastric cancer.
Results: We found the differential levels of PLF metabolites by LC-MS, TIC spectra, PCA and the t-test. Cluster analysis showed the co-occurrence of metabolites in the peritoneal metastasis group (p< 0.05). ROC analysis showed the diagnostic ability of metabolites (p< 0.05). Univariate and multivariate logistic regression analyses showed the potential independent risk factors for peritoneal metastasis in gastric cancer patients (p< 0.05).
Conclusion: Through the statistical analysis of metabolomics, we found that TG (54:2), G3P, α-aminobutyric acid, α-CEHC, dodecanol, glutamyl alanine, 3-methylalanine, sulfite, CL (63:4), PE-NMe (40:5), TG (53:4), retinol, 3-hydroxysterol, tetradecanoic acid, MG (21:0/0:0/0:0), tridecanoic acid, myristate glycine and octacosanoic acid may be biomarkers for peritoneal metastasis of gastric cancer.
Keywords: gastric cancer, metabolomics, peritoneal metastasis, diagnosis
Introduction
Gastric cancer, one of the most common malignant tumours after lung cancer and liver cancer, occurs in the upper digestive tract.1,2 Approximately 20% of gastric cancer patients are diagnosed with peritoneal metastasis before surgery.3 More than half of advanced gastric cancer patients have peritoneal metastasis after surgery, which leads to poor prognosis.3 The 5-year survival rate of patients with positive peritoneal lavage cytology is approximately 12%, and the median survival time of patients with peritoneal metastasis is approximately 6–7 months.4,5 However, the sensitivity of gastric cancer peritoneal metastasis imaging and tumour marker detection is low. Therefore, the need to find sensitive diagnostic markers of gastric cancer with peritoneal metastasis is urgent.
Metabolomics can accurately discover the basic characteristics and material basis of life activities.6–10 It can enlarge small changes in the genome and proteome, reflecting the endpoint of gene functional activities and changes in the biochemical phenotype of organisms, and is also directly related to the final effect of these activities.11 Therefore, metabolomics is considered the final direction of omics research.12 Yue et al13 found 43 arginine metabolites helpful for the accurate diagnosis of small cell lung cancer by LC-MS. Zhang et al14 found that the levels of 9 metabolites, such as glutamic acid and glutamine, were significantly different in the cancerous tissues and normal tissues of 40 patients with oesophageal squamous cell carcinoma by LC-MS and that this difference was closely related to the pathological characteristics of lymph node metastasis and postoperative survival time. However, the application of metabolomics to peritoneal metastasis of gastric cancer is still unclear.
In this study, we collected the PLF of 65 gastric cancer patients and related clinical data from the First Hospital of Jilin University. The metabolic components of the PLF were determined by LC-MS. TIC spectra, PCA, and the t-test were used to identify differential metabolites in PLF samples. An SVM was used to screen the differential metabolites with a weight of 100%. Cluster analysis was used to evaluate the similarity between samples. ROC analysis was used to assess the diagnostic ability of the metabolites. Univariate and multivariate logistic regression analyses were used to identify potential risk factors for peritoneal metastasis in gastric cancer patients. We found the differential levels of PLF metabolites by LC-MS, TIC spectra, PCA and the t-test. Cluster analysis showed the co-occurrence of metabolites in the peritoneal metastasis group. ROC analysis showed the diagnostic ability of metabolites. Univariate and multivariate logistic regression analyses showed the potential independent risk factors for peritoneal metastasis in gastric cancer patients. In the end, we found that TG (54:2), G3P, α-aminobutyric acid, α-CEHC, dodecanol, glutamyl alanine, 3-methylalanine, sulfite, CL (63:4), PE-NMe (40:5), TG (53:4), retinol, 3-hydroxysterol, tetradecanoic acid, MG (21:0/0:0/0:0), tridecanoic acid, myristate glycine and octacosanoic acid may be biomarkers for peritoneal metastasis of gastric cancer.
Patients and Methods
Patient Source and Sample Collection
From August 2018 to December 2018, 62 patients with gastric cancer (45 males and 17 females) underwent laparoscopic exploration or laparoscopic radical gastrectomy. Informed consent was obtained from patients and their families before surgery, and the study was approved by the Ethics Committee of The First Hospital of Jilin University, together with confirmation of patient written informed consent, and compliance with the Declaration of Helsinki. The inclusion criteria for patients with gastric cancer were as follows: samples obtained from the First Hospital of Jilin University; a pathological diagnosis of gastric cancer; an age of no more than 75 years; the presence of primary tumours; good liver function, heart function, renal function and bone marrow function; and no other serious immunosuppressive diseases or simultaneous malignant tumours. The exclusion criteria for patients with gastric cancer were as follows: congenital diseases; poor general condition; severe organic diseases; previous radical or palliative surgery, radiotherapy, chemotherapy or biotherapy; complications of gastrointestinal haemorrhage; perforation; and serious infection.
Two hundred millilitres of lavage fluid were collected from the subphrenic space, subhepatic space and Douglas fossa of 62 patients with gastric cancer.
Exfoliative Cytology
After centrifugation, the supernatants of PLF samples from 62 gastric cancer patients were discarded, and the precipitates were retained. After smearing, the exfoliative cytology was detected by pasteurization.
qRT-PCR
The total RNA was extracted from the PLF samples by TRIzol™ reagent (Invitrogen Thermo Science) and then reverse transcribed to produce cDNA. Finally, the cDNA was amplified by PCR. Table 1 lists the sequences of the primers used. We used TransStart TIP Green qPCR SuperMix (cat. No. AQ131, TransGen Biotech Co., Ltd., Beijing, China) for RT qPCR analysis. The analysis mixture contained 0.2 g of DNA, 0.2 M forward primers, 0.2 M reverse primers, and 10 μL of qPCR SuperMix in a total volume of 20 µL. The conditions were as follows: 94.0°C for 30 seconds, followed by 40 cycles of 94.0°C for 5 seconds and 60.0°C for 30 seconds. Three replicates of each sample were analysed in a CFX 96 Touch Real-time Polymerase Chain Reaction Detection System (Bio-Rad Laboratory Ltd.). The relative expression of actin and CEA in the different experimental groups was calculated by the 2-ΔΔCq method.
 |
Table 1 CEA mRNA Results of Peritoneal Lavage |
LC-MS
Four microliters of each sample were chromatographed onto a C18 reverse-phase column (2.1 × 100 mm, 1.8 μm, Waters, Milford, MA) using an Agilent 1290 Infinity liquid chromatography system (Agilent, Santa Clara, CA). During chromatographic separation, the column was maintained at 40°C. Elution was performed at a flow rate of 400 μL/min, with 5% acetonitrile in water for the first 2 min, a linear gradient of 5% to 95% acetonitrile over the next 15 min, and 95% acetonitrile in water for the last 2 min. Both acetonitrile and water contained 0.1% formic acid.
Statistical Analysis
The t-test was carried out to analyse the positive ion mode and negative ion mode data, and differential metabolites were screened by p<0.05. An SVM was used to precisely identify different metabolites. The BRB array tool was used for cluster analysis to uncover the distributions of poorly metabolised foreign bodies in patients with gastric cancer.15 ROC curves were drawn with SPSS based on a series of different binary classifications (demarcation value or determination threshold) as well as the true-positive rate (sensitivity), the ordinate and the false-positive rate (1-specificity) according to the abscissa. Univariate and multivariate logistic regression analyses were used in SPSS to identify risk factors for peritoneal metastasis of gastric cancer.
Results
Different Levels of Metabolites in the PLF of Gastric Cancer Patients
According to the pathological data, the patients were divided into two groups. Patients in group A had serous invasion, and those in group B did not have serous invasion. According to the results of exfoliative cytology of the PLF, findings during surgery and pathological data, a group positive for peritoneal metastasis (group C) and a group negative for peritoneal metastasis of gastric cancer (group D) were found. According to qRT-PCR analysis of CEA mRNA in PLF, patients were divided into a CEA-positive group (group E) and a CEA-negative group (group F; Table 1).
Mass spectral data of the metabolites were obtained by LC-MS. Moreover, we found a difference in the expression of metabolites between groups A and B, groups C and D, and groups E and F by the TIC spectra (Figure 1). Differences in the levels of metabolites between groups A and B, groups C and group D, and groups E and group F were further verified by PCA (Figure 2).
 |
Figure 1 TIC spectra of the different groups. TIC analysis of groups A and B (A and B), groups C and D (C and D), and groups E and F (E and F). |
 |
Figure 2 PCA of the different groups. PCA of groups A and B (A and B), groups C and D (C and D), and groups E and F (E and F). |
The results of a t-test to analyse data in positive and negative ion mode found 213 differential metabolites in positive ion mode (supplemental material Table 1) and 174 differential metabolites in negative ion mode between groups A and B (supplemental material Table 2). In addition, 190 differential metabolites (supplemental material Table 3) between groups C and D were screened under cation mode, and 115 differential metabolites (supplemental material Table 4) were screened in negative ion mode. Screening of groups E and F revealed 501 differential metabolites in positive ion mode (supplemental material Table 5) and 246 differential metabolites in negative ion mode (supplemental material Table 6).
Differential Metabolites in the PLF of Patients with Peritoneal Metastasis Screened with a Weight of 100%
We used an SVM to carry out discrimination analysis to distinguish the patients in each group and further screened differential metabolites with a weight of 100%. Four differential metabolites in positive ion mode and 4 differential metabolites in negative ion mode were identified between groups A and B (Table 2). Two differential metabolites in positive ion mode and 2 differential metabolites in negative ion mode were identified between groups C and D (Table 3). Ten differential metabolites in positive ion mode and 4 differential metabolites in negative ion mode were identified between groups E and F (Table 4). The mass to charge ratios (M/Z) were used to screen the human metabolome database (HMDB) to find the corresponding substances. The differential metabolites between groups A and B were sulfite, TG (54:2), G3P, α-aminobutyric acid, α-CEHC, dodecanol, alanine glutamyl, and 3-methylpropionic acid (Table 2). The differential metabolites between groups C and D were sulfite, G3P, Cl (63:4), and PE-NMe (40:5) (Table 3). The differential metabolites between groups E and F were sulfite, G3P, TG (54:2), α-aminobutyric acid, α-CEHC, glutamyl alanine, retinol, 3-hydroxysterol, tetradecanoic acid, MG (21:0/0:0/0:0), tridecanoic acid, myristate glycine, octadecanoic acid, and TG (53:4) (Table 4).
 |
Table 2 Groups A and B Screened Out Different Metabolites |
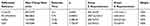 |
Table 3 Groups C and D Screened Out Different Metabolites |
 |
Table 4 Groups E and F Screened Out Different Metabolites |
Cluster analysis showed the co-occurrence of metabolites in groups A, C and E. As shown in Figure 3, the levels of TG (54:2), sulfite, G3P, α-aminobutyric acid, α-CEHC, glutamyl alanine and 3-methylpropionic acid in group A were similar. In addition, the levels of CL (63:1), PE-NMe (10:5), sulfite and G3P in group C were similar. The levels of sulfite, TG (54:2), G3P, α-aminobutyric acid, pyrite, TG (53:4), retinal, α-CEHC, 3-hydroxysterol, tetradecanoic acid, Mg (21:0/0:0/0:0), tridecanoic acid, and octadecanoic acid in group E were similar.
 |
Figure 3 Cluster analysis of the different groups. Cluster analysis of groups A and B (A), groups C and D (B), and groups E and F (C). |
Differential Metabolites Have Good Diagnostic Ability for Peritoneal Metastasis in Gastric Cancer Patients
ROC analysis showed that sulfite, TG (54:2), G3P, α-aminobutyric acid, α-CEHC, dodecanol, glutamyl alanine and 3-methylalanine had good diagnostic ability in groups A and B (Table 5; Figure 4). In groups C and D, sulfite, G3P, Cl (63:4), and PE-NMe (40:5) had good diagnostic ability (Table 6; Figure 4). In groups E and F, sulfite, G3P, TG (54:2), α-aminobutyric acid, TG (53:4), α-CEHC, glutamyl alanine, retinol, 3-hydroxysterol, tetradecanoic acid, MG (21:0/0:0/0:0), tridecanoic acid, myristate glycine and octacosanoic acid had good diagnostic ability (Table 7; Figure 4).
 |
Table 5 Area Under ROC Curve of Differential Metabolites in Groups A and B |
 |
Table 6 Area Under ROC Curve of Differential Metabolites in Groups C and D |
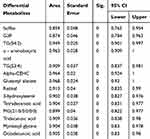 |
Table 7 Area Under ROC Curve of Differential Metabolites in Groups E and F |
 |
Figure 4 ROC curve analysis of metabolites in the different groups. ROC curve analysis of differential metabolites between groups A and B (A), groups C and D (B), and groups E and F (C). |
Metabolites are Independent Risk Factors for Peritoneal Metastasis in Gastric Cancer Patients
Univariate regression analysis showed that sulfite, TG (54:2), G3P, α-aminobutyric acid, α-CEHC, dodecanol, glutamyl alanine and 3-methylpropionic acid were risk factors for peritoneal metastasis of gastric cancer in groups A and B (Table 8). Sulfite, G3P, Cl (63:4), and PE-NMe (40:5) were risk factors for peritoneal metastasis of gastric cancer in groups C and D (Table 9). Sulfite, glyceraldehyde 3-phosphate, TG (54:2), α-aminobutyric acid, TG (53:4), α-CEHC, glutamyl alanine, retinol, 3-hydroxysterol, tetradecanoic acid, Mg (21:0/0:0/0:0), tridecanoic acid, myristate glycine and octacosanoic acid were risk factors for peritoneal metastasis of gastric cancer in groups E and F (Table 10).
 |
Table 8 Univariate Logistic Regression for Group A and Group B |
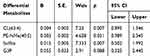 |
Table 9 Univariate Logistic Regression for Group C and Group D |
 |
Table 10 Univariate Logistic Regression for Group E and Group F |
Multivariate regression analysis showed that sulfite, TG (54:2), G3P, α-aminobutyric acid, α-CEHC, dodecanol, glutamyl alanine and 3-methylalanine were independent risk factors for peritoneal metastasis of gastric cancer in groups A and B (Table 11). Sulfite, CL (63:4), and PE-NMe (40:5) were independent risk factors for peritoneal metastasis of gastric cancer in groups C and D (Table 12). Sulfite, G3P, TG (54:2), α-aminobutyric acid, TG (53:4), α-CEHC, glutamyl alanine, retinol, 3-hydroxysterol, tetradecanoic acid, MG (21:0/0:0/0:0), tridecanoic acid, myristate glycine and octacosanoic acid were independent risk factors for peritoneal metastasis of gastric cancer in groups E and F (Table 13).
 |
Table 11 Multivariate Logistic Regression of Group A and Group B |
 |
Table 12 Multivariate Logistic Regression of Group C and Group D |
 |
Table 13 Multivariate Logistic Regression of Group E and Group F |
Discussion
Gastric cancer, which has a high incidence and poor prognosis, seriously threatens human health.2,16,17 Peritoneal metastasis is an important factor in the death of gastric cancer patients. Most patients diagnosed with peritoneal metastasis of gastric cancer have already had cancerous ascites and metastasis and lost the opportunity for treatment.18,19 However, there are no obvious symptoms or signs of the early stage of peritoneal metastasis of gastric cancer, and conventional ultrasound, CT and other detection methods cannot diagnose peritoneal metastasis precisely. Therefore, the need to find more sensitive diagnostic markers for peritoneal metastasis of gastric cancer is urgent. Compared with some single molecular markers, metabolic diagnostic markers are more comprehensive and accurate.20–33 Metabolomics plays an important role in the screening of tumour biomarkers. A large number of studies have found potential biomarkers of gastric cancer, colorectal cancer, oesophageal cancer, liver cancer, ovarian cancer and other malignant tumours in blood, urine, tissue and other samples through metabolomics.34–37 In this study, we found that TG (54:2), G3P, α-aminobutyric acid, α-CEHC, dodecanol, glutamyl alanine, 3-methylalanine, sulfite, CL (63:4), PE-NMe (40:5), TG (53:4), retinol, 3-hydroxysterol, tetradecanoic acid, MG (21:0/0:0/0:0), tridecanoic acid, myristate glycine and octacosanoic acid have good diagnostic ability for gastric cancer metastasis and are potential independent risk factors for gastric cancer patients with peritoneal metastasis.
Metabonomics is a hot topic in recent years. It has been reported that glucose metabolism plays a key role in the growth of gastric cancer.38 However, we found that some lipid metabolites play a key role in peritoneal metastasis of gastric cancer, which may be caused by different pathological processes of gastric cancer. Sulfite is mainly produced from the metabolism of sulfur-containing amino acids (cysteine, methionine) in the human body.39 Current research shows that the level of homocysteine in the sera of patients with oesophageal cancer, gastric cancer, colorectal cancer and other malignant tumours is significantly increased. Sulfite was found to have an antitumour effect by affecting cell cycle arrest, apoptosis, invasion and colony formation in SH-SY5Y tumour cells.40 Xu et al41 used Mendel randomization to analyse 27 case–control studies on the relationship between the level of blood homocysteine and the risk of gastric cancer and proved that the level of blood homocysteine had a significant impact on the risk of gastric cancer. An increase in sulfite content in peritoneal lavage fluid may indicate an increase in homocysteine levels, which is consistent with previous research results.
G3P is an important metabolite of glycolysis and the pentose phosphate pathway.42 Glycolysis is the main energy source of tumour cells.43,44 The pentose phosphate pathway not only provides 5-ribonucleic acid for the rapid proliferation of tumour cells; in addition, the p53 protein has been reported to inhibit the pentose phosphate pathway by binding glucose-6-phosphate dehydrogenase. In tumour cells, p53 is mutated, enhancing the pentose phosphate pathway.45 Studies have shown that G3P plays an important role in tumour cell survival, tumour angiogenesis, tumour cell gene expression regulation and mRNA posttranscriptional regulation.46
Lipid metabolism plays an important role in cancer.47 TG (54:2), PE-NMe, Cl (63:4), and TG (53:4) are triglycerides. MG (21:0/0:0/0:0:0:0) belongs to the glycerol monoester family. Myristate glycine, tridecanoic acid, octadecanoic acid, 3-methylpropionic acid and tetradecanoic acid are fatty acids. Dodecanol is a fatty alcohol in body fluids. It has been proven that the consumption of lipids and the levels of lipid metabolites are increased in gastric cancer, while the plasma levels of lipids are decreased in gastric cancer.48,49 Fatty acids can be used as a diagnostic marker of gastric cancer.50 In this study, we found that an increase in these lipid metabolites may indicate that lipid metabolism in the peritoneal environment in gastric cancer with peritoneal metastasis has changed significantly.
Amino acid metabolism and cholesterol metabolism play an important role in the occurrence and development of cancer. Glutamyl alanine is a naturally occurring dipeptide composed of glutamate and alanine. α-Aminobutyric acid is a nonessential amino acid mainly in the cytoplasm that is mainly produced from the catabolism of methionine, threonine and serine. 3-Hydroxysterol is the intermediate of cholesterol biosynthesis. Some studies showed that the levels of serum cholesterol and low-density lipoprotein were lower in patients with gastric cancer metastasis than in normal controls.51 Retinal, also known as vitamin A aldehyde, is a derivative of retinol after its oxidation. Retinol can be irreversibly oxidized to retinoic acid, which is involved in the regulation of some cellular functions, such as cell growth, proliferation and differentiation. The relationship between retinol intake and blood retinol concentration and the risk of gastric cancer were shown to be controversial in past case-control and cohort studies. Some studies have shown that retinol can reduce the risk of gastric cancer, while others have not found this relationship. A meta-analysis52 of 31 studies showed a slight negative correlation between retinol intake (with RR = 0.94, 95% CI: 0.87–1.03) or blood retinol level (with RR = 0.87, 95% CI: 0.73–1.05) and the risk of gastric cancer by comparing the highest and lowest intervals of the blood retinol level. Subgroup analysis showed a slight negative correlation between serum retinol level and gastric cancer risk in only Western countries.
To the best of our knowledge, this is the first study to identify the diagnostic role of metabolites in gastric cancer metastasis. Through our work, we can better help in the search for new methods to detect gastric cancer metastasis. However, the in-depth molecular mechanism has not been fully explored. In the future, we will continue to explore the molecular mechanism of metabolites in vitro and in vivo.
Conclusion
In this study, we discovered the role of metabolites in peritoneal metastasis of gastric cancer. TG (54:2), G3P, α-aminobutyric acid, α-CEHC, dodecanol, glutamyl alanine, 3-methylalanine, sulfite, CL (63:4), PE-NMe (40:5), TG (54:2), TG (53:4), retinol, 3-hydroxysterol, tetradecanoic acid, MG (21:0/0:0/0:0), tridecanoic acid, myristate glycine and octacosanoic acid have good diagnostic ability and are potential markers of peritoneal metastasis in gastric cancer. In the future, we will continue to explore the specific molecular mechanism of these metabolites in peritoneal metastasis of gastric cancer.
Abbreviations
PLF, peritoneal lavage fluid; LC-MS, liquid chromatograph-mass spectrometry; TIC, total ion current; SVM, support vector machine; ROC, receiver operating characteristic; G3P, glyceraldehyde-3-phosphate; M/Z, mass to charge ratio; CI, confidence interval; PCA, principal component analysis; TG, triglyceride; MG, monoglyceride; α-EHEC, (S) −3,4-dihydro-6-hydroxy-2,5,7,8-tetramethyl-2H-1-benzopyran-2-propanoic acid.
Ethics Statement
The patient sample comes from the First Affiliated Hospital of Jilin University. All patients have the right of written informed consent, and compliance with the declaration of Helsinki.
Disclosure
The authors declare that they have no conflict of interest.
References
1. Carioli G, Bertuccio P, Malvezzi M, et al. Cancer mortality predictions for 2019 in Latin America. Int J Cancer. 2019.
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi:10.3322/caac.21551
3. Kinami S, Funaki H, Fujita H, Nakano Y, Ueda N, Kosaka T. Local resection of the stomach for gastric cancer. Surg Today. 2017;47(6):651–659. doi:10.1007/s00595-016-1371-z
4. Galata C, Blank S, Weiss C, Ronellenfitsch U, Reissfelder C, Hardt J. Role of postoperative complications in overall survival after radical resection for gastric cancer: a retrospective single-center analysis of 1107 patients. Cancers. 2019;11(12):1890. doi:10.3390/cancers11121890
5. Feng F, Liu J, Wang F, et al. Prognostic value of differentiation status in gastric cancer. BMC Cancer. 2018;18(1):865. doi:10.1186/s12885-018-4780-0
6. Kaushik AK, DeBerardinis RJ. Applications of metabolomics to study cancer metabolism. Biochim Biophys Acta Rev Cancer. 2018;1870(1):2–14. doi:10.1016/j.bbcan.2018.04.009
7. Jamali L, Tofigh R, Tutunchi S, et al. Circulating microRNAs as diagnostic and therapeutic biomarkers in gastric and esophageal cancers. J Cell Physiol. 2018;233(11):8538–8550. doi:10.1002/jcp.26850
8. Simonian M, Mosallayi M, Mirzaei H. Circulating miR-21 as novel biomarker in gastric cancer: diagnostic and prognostic biomarker. J Cancer Res Ther. 2018;14(2):475.
9. Faghihloo E, Araei Y, Mohammadi M, Mirzaei H, Mohammadi HR, Mokhtari-Azad T. The effect of oxamflatin on the E-cadherin expression in gastric cancer cell line. Cancer Gene Ther. 2016;23(11):396–399. doi:10.1038/cgt.2016.52
10. Mirzaei H, Khataminfar S, Mohammadparast S, et al. Circulating microRNAs as potential diagnostic biomarkers and therapeutic targets in gastric cancer: current status and future perspectives. Curr Med Chem. 2016;23(36):4135–4150. doi:10.2174/0929867323666160818093854
11. Armitage EG, Ciborowski M. Applications of Metabolomics in Cancer Studies. Adv Exp Med Biol. 2017;965:209–234.
12. Zhang WT, Zhang ZW, Guo YD, et al. Discovering biomarkers in bladder cancer by metabolomics. Biomark Med. 2018;12(12):1347–1359. doi:10.2217/bmm-2018-0229
13. Yue X, He J, Zhang R, et al. Biotransformation-based metabolomics profiling method for determining and quantitating cancer-related metabolites. J Chromatogr A. 2018;1580:80–89. doi:10.1016/j.chroma.2018.10.034
14. Zhang H, Wang L, Hou Z, et al. Metabolomic profiling reveals potential biomarkers in esophageal cancer progression using liquid chromatography-mass spectrometry platform. Biochem Biophys Res Commun. 2017;491(1):119–125. doi:10.1016/j.bbrc.2017.07.060
15. Smith CA, O’Maille G, Want EJ, et al. METLIN: a metabolite mass spectral database. Ther Drug Monit. 2005;27(6):747–751. doi:10.1097/01.ftd.0000179845.53213.39
16. Coimbra FJF, de Jesus VHF, Franco CP, et al. Predicting overall and major postoperative morbidity in gastric cancer patients. J Surg Oncol. 2019;120(8):1371–1378. doi:10.1002/jso.25743
17. Naeli P, Pourhanifeh MH, Karimzadeh MR, et al. Circular RNAs and gastrointestinal cancers: epigenetic regulators with a prognostic and therapeutic role. Crit Rev Oncol Hematol. 2020;145:102854. doi:10.1016/j.critrevonc.2019.102854
18. Zhang WH, Song XH, Chen XZ, et al. Characteristics and survival outcomes related to the infra-pyloric lymph node status of gastric cancer patients. World J Surg Oncol. 2018;16(1):116. doi:10.1186/s12957-018-1412-8
19. Jin JJ, Wang W, Dai FX, et al. Marital status and survival in patients with gastric cancer. Cancer Med. 2016;5(8):1821–1829. doi:10.1002/cam4.758
20. Cai H, Jiao Y, Li Y, Yang Z, He M, Liu Y. Low CYP24A1 mRNA expression and its role in prognosis of breast cancer. Sci Rep. 2019;9(1):13714. doi:10.1038/s41598-019-50214-z
21. Hou L, Zhang X, Jiao Y, et al. ATP binding cassette subfamily B member 9 (ABCB9) is a prognostic indicator of overall survival in ovarian cancer. Medicine. 2019;98(19):e15698. doi:10.1097/MD.0000000000015698
22. Jiao Y, Fu Z, Li Y, Meng L, Liu Y. High EIF2B5 mRNA expression and its prognostic significance in liver cancer: a study based on the TCGA and GEO database. Cancer Manag Res. 2018;10:6003–6014. doi:10.2147/CMAR.S185459
23. Jiao Y, Li Y, Fu Z, et al. OGDHL expression as a prognostic biomarker for liver cancer patients. Dis Markers. 2019;2019:9037131. doi:10.1155/2019/9037131
24. Jiao Y, Li Y, Jiang P, Han W, Liu Y. PGM5: a novel diagnostic and prognostic biomarker for liver cancer. PeerJ. 2019;7:e7070. doi:10.7717/peerj.7070
25. Jiao Y, Li Y, Liu S, Chen Q, Liu Y. ITGA3 serves as a diagnostic and prognostic biomarker for pancreatic cancer. Onco Targets Ther. 2019;12:4141–4152. doi:10.2147/OTT.S201675
26. Jiao Y, Li Y, Lu Z, Liu Y. High trophinin-associated protein expression is an independent predictor of poor survival in liver cancer. Dig Dis Sci. 2019;64(1):137–143. doi:10.1007/s10620-018-5315-x
27. Li Y, Jiao Y, Fu Z, Luo Z, Su J, Li Y. High miR-454-3p expression predicts poor prognosis in hepatocellular carcinoma. Cancer Manag Res. 2019;11:2795–2802. doi:10.2147/CMAR.S196655
28. Li Y, Jiao Y, Li Y, Liu Y. Expression of la ribonucleoprotein domain family member 4B (LARP4B) in liver cancer and their clinical and prognostic significance. Dis Markers. 2019;2019:1569049. doi:10.1155/2019/1569049
29. Li Y, Jiao Y, Luo Z, Li Y, Liu Y. High peroxidasin-like expression is a potential and independent prognostic biomarker in breast cancer. Medicine. 2019;98(44):e17703. doi:10.1097/MD.0000000000017703
30. Bunevicius A. The association of digit ratio (2D: 4D) with cancer: a systematic review and meta-analysis. Dis Markers. 2018;2018:7698193. doi:10.1155/2018/7698193
31. Fu B, Yan P, Zhang S, et al. Cell-free circulating methylated SEPT9 for noninvasive diagnosis and monitoring of colorectal cancer. Dis Markers. 2018;2018:6437104. doi:10.1155/2018/6437104
32. Ieni A, Angelico G, Barresi V, et al. Human epidermal growth factor receptor 2 status in gastric carcinomas with distinctive prevalent cribriform component. Dis Markers. 2018;2018:1505428. doi:10.1155/2018/1505428
33. Kang W, Zheng Q, Lei J, Chen C, Yu C. Prognostic value of long noncoding RNAs in patients with gastrointestinal cancer: a systematic review and meta-analysis. Dis Markers. 2018;2018:5340894. doi:10.1155/2018/5340894
34. Zhang A, Sun H, Yan G, Wang P, Han Y, Wang X. Metabolomics in diagnosis and biomarker discovery of colorectal cancer. Cancer Lett. 2014;345(1):17–20. doi:10.1016/j.canlet.2013.11.011
35. Chen X, Yu D. Metabolomics study of oral cancers. Metabolomics. 2019;15(2):22. doi:10.1007/s11306-019-1483-8
36. McCartney A, Vignoli A, Biganzoli L, et al. Metabolomics in breast cancer: a decade in review. Cancer Treat Rev. 2018;67:88–96. doi:10.1016/j.ctrv.2018.04.012
37. Xiao S, Zhou L. Gastric cancer: metabolic and metabolomics perspectives (review). Int J Oncol. 2017;51(1):5–17. doi:10.3892/ijo.2017.4000
38. Yuan LW, Yamashita H, Seto Y. Glucose metabolism in gastric cancer: the cutting-edge. World J Gastroenterol. 2016;22(6):2046–2059. doi:10.3748/wjg.v22.i6.2046
39. Chen L, Tang M, Chen C, et al. Efficient bacterial inactivation by transition metal catalyzed auto-oxidation of sulfite. Environ Sci Technol. 2017;51(21):12663–12671. doi:10.1021/acs.est.7b03705
40. Dodurga Y, Secme M, Eroglu C, et al. Investigation of the effects of a sulfite molecule on human neuroblastoma cells via a novel oncogene URG4/URGCP. Life Sci. 2015;143:27–34. doi:10.1016/j.lfs.2015.10.005
41. Xu W, Cheng Y, Zhu H. Evaluation of an association of blood homocysteine levels with gastric cancer risk from 27 case-control studies. Medicine. 2016;95(20):e3700. doi:10.1097/MD.0000000000003700
42. Tastensen JB, Schonheit P. Two distinct glyceraldehyde-3-phosphate dehydrogenases in glycolysis and gluconeogenesis in the archaeon haloferax volcanii. FEBS Lett. 2018;592(9):1524–1534. doi:10.1002/1873-3468.13037
43. Ganapathy-Kanniappan S. Molecular intricacies of aerobic glycolysis in cancer: current insights into the classic metabolic phenotype. Crit Rev Biochem Mol Biol. 2018;53(6):667–682. doi:10.1080/10409238.2018.1556578
44. Oppermann H, Birkemeyer C, Meixensberger J, Gaunitz F. Non-enzymatic reaction of carnosine and glyceraldehyde-3-phosphate accompanies metabolic changes of the pentose phosphate pathway. Cell Prolif. 2019;e12702.
45. Munemoto M, Mukaisho KI, Miyashita T, et al. Roles of the hexosamine biosynthetic pathway and pentose phosphate pathway in bile acid-induced cancer development. Cancer Sci. 2019;110(8):2408–2420. doi:10.1111/cas.14105
46. Sirover MA. Pleiotropic effects of moonlighting glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in cancer progression, invasiveness, and metastases. Cancer Metastasis Rev. 2018;37(4):665–676. doi:10.1007/s10555-018-9764-7
47. Ramos-Bueno RP, Gonzalez-Fernandez MJ, Guil-Guerrero JL. Various acylglycerols from common oils exert different antitumor activities on colorectal cancer cells. Nutr Cancer. 2016;68(3):518–529. doi:10.1080/01635581.2016.1152382
48. Enjoji M, Kohjima M, Ohtsu K, et al. Intracellular mechanisms underlying lipid accumulation (white opaque substance) in gastric epithelial neoplasms: a pilot study of expression profiles of lipid-metabolism-associated genes. J Gastroenterol Hepatol. 2016;31(4):776–781. doi:10.1111/jgh.13216
49. Xu L, Guo T, Qu X, et al. Beta-elemene increases the sensitivity of gastric cancer cells to TRAIL by promoting the formation of DISC in lipid rafts. Cell Biol Int. 2018;42(10):1377–1385. doi:10.1002/cbin.11023
50. Jiang Z, Shen H, Tang B, Yu Q, Ji X, Wang L. Quantitative proteomic analysis reveals that proteins required for fatty acid metabolism may serve as diagnostic markers for gastric cancer. Clin Chim Acta. 2017;464:148–154. doi:10.1016/j.cca.2016.11.032
51. Hu D, Peng F, Lin X, et al. Prediction of three lipid derivatives for postoperative gastric cancer mortality: the Fujian prospective investigation of cancer (FIESTA) study. BMC Cancer. 2018;18(1):785. doi:10.1186/s12885-018-4596-y
52. Wu Y, Ye Y, Shi Y, et al. Association between vitamin A, retinol intake and blood retinol level and gastric cancer risk: a meta-analysis. Clin Nutr. 2015;34(4):620–626. doi:10.1016/j.clnu.2014.06.007
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
