Back to Archived Journals » Journal of Neurorestoratology » Volume 5
Direct interaction of receptor tyrosine kinases, EphA4 and PDGFRβ, plays an important role in the proliferation of neural stem cells
Authors Chen QF, Sawada T, Sakaguchi K, Han F
Received 17 April 2017
Accepted for publication 9 May 2017
Published 6 July 2017 Volume 2017:5 Pages 133—141
DOI https://doi.org/10.2147/JN.S139820
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Hongyun Huang
Qingfa Chen,1 Takahiro Sawada,2 Kazushige Sakaguchi,2 Fabin Han1,3
1Centre for Stem Cells and Regenerative Medicine, The Institute for Tissue Engineering & Regenerative Medicine,The Liaocheng People’s Hospital/Liaocheng University, Liaocheng, People’s Republic of China; 2Department of Molecular Cell Biology and Molecular Medicine, Institute of Advanced Medicine, Wakayama Medical University, Wakayama, Japan; 3Centre for Stem Cells and Regenerative Medicine, The Institute of Translational Medicine, The Second Hospital of Shandong University, Jinan, Shandong, People’s Republic of China
Abstract: Receptor tyrosine kinases mediate the extracellular signals and transmit them into the cytoplasm by activating intracellular proteins through tyrosine phosphorylation. Both Ephs and platelet-derived growth factor (PDGF) receptors (PDGFRs) have been implicated in neurogenesis, but the functional interaction between these two pathways is poorly understood. Here, we demonstrated that EphA4 directly interacts with PDGFRβ and mutually activates each other when expressed in HEK293T cells. H9-derived neural stem cells express Ephs and PDGFRs, and their proliferation is stimulated by ephrin-A1 and PDGF-BB with further augmentation by their combined application. As both EphA4 and PDGFRβ play important roles in preventing neurodegeneration and promoting neuroprotection, their interaction and transactivation might transduce the signal through the EphA4/PDGFRβ complex and augment the proliferation of neural stem cells.
Keywords: EphA4, PDGFRβ, neural stem cell, transactivation, proliferation
Introduction
Receptor tyrosine kinases (RTKs) constitute a distinct family of transmembrane proteins present only in multicellular animals. These proteins transduce extracellular signals into the cytoplasm and are implicated in regulating cell growth, proliferation, migration, differentiation, and apoptosis.1–4 As the largest RTK subfamily, Eph receptors have type A and B subclasses according to their specificity to bind with their ligands, ephrins.5 In general, EphAs are bound with ephrin-As and EphBs are bound with ephrin-Bs; however, cross-specificity is also present. The ephrin-A class of ligands has a glycosyl phosphatidylinositol linkage to contact the cell membrane, whereas the ephrin-B class of ligands has a different structure containing a short cytoplasmic and transmembrane domain. Cell–cell contact-dependent signaling pathway from ephrins to Ephs (forward signaling) or from Ephs to ephrins (reverse signaling) regulates many physiological and developmental processes. Bidirectional signaling possesses many functions, including neural stem cell maintenance and plasticity regulation in the proliferation zone of adult brain,6,7 neuron migration,8 axon guidance,9 angiogenesis,10 bone homeostasis,11 embryonic patterning,12 tumorgenesis,13–18 insulin secretion,19 and so on. EphA4 expression is very high in the brain, and recently, EphA4 has been proposed to be implicated in Alzheimer’s disease (AD),20,21 Parkinson’s disease (PD),22,23 amyotrophic lateral sclerosis (ALS),24 and other neurodegenerative diseases. Hence, EphA4 may have functions for protecting neuron loss and reversing the aging cells. To further explore the EphA4-mediated signaling pathways and their biological functions in the brain, one important thing is to detect the signaling molecules interacting with EphA4.
Platelet-derived growth factor (PDGF) family of RTKs represents another signaling pathway. PDGF growth factors include four distinct subclasses (A–D) that bind to their receptors, PDGFRα and PDGFRβ, after dimerization.25–27 PDGF, as a novel factor for neuron protection and neuron growth, plays a key role in regulating neurogenesis and hence is the mutation target of neurodegenerative diseases.28–31 Bush et al reported that PDGF-BB is implicated in playing key roles in neural stem/progenitor cell (NPC) proliferation and neurogenesis under the condition of HIV-associated neurological disorders. They observed that PDGF-BB could restore the hippocampal NPC proliferation through cognate receptors of HIV Tat-cocaine. PDGF-BB also regulates NPC proliferation and neurogenesis through miR-9/MCPIP1 axis.29,32 Zachrisson et al33 found that PDGF-BB is effective in counteracting histological, behavioral, and biochemical changes in the experimental rat model of PD. Treatment with PDGF-BB normalized the rotational behavior, and the effect lasted for 10 weeks. Paul et al34 found that intracerebroventricular (ICV) injection in PD individuals was tolerated well at all doses tested, supporting PDGF-BB as a proper candidate for further treatment of PD patients.
We have previously reported that ephrin-A1 injection reverses neuronal regeneration and alleviates the symptoms in a 6-OHDA-lesioned PD rat model,23 and that the interaction of EphA4 and FGFRs promotes mouse embryonic NPC proliferation and neurogenesis via FRS2α and ERK1/2 downstream of the FGF/FGFR signaling.35,36 Here, we found that EphA4 and PDGFRβ have a direct interaction and can transactivate each other when coexpressed in cells. PDGF-BB and ephrin-A1 appear to enhance proliferation of neural stem cells, suggesting that these ligands might be good candidates for curing neurological diseases such as AD and related disorders in human.
Materials and methods
Reagents
Recombinant human PDGF-BB (Cat. #220-BB), recombinant human ephrin-A1-Fc (Cat. #6417-A1), and recombinant human IgG(Fc) were purchased from R&D Systems, Inc. (Minneapolis, MN, USA). In this study, we used clustered ephrin-A1-Fc in which 1 mg ephrin-A1 was oligomerized via incubation with 2.4 mg of recombinant human IgG(Fc) for >1 h according to the manufacturer’s instructions. PDGFR inhibitor STI571 was purchased from Selleck (Munich, Germany). The following primary antibodies were used in this study: mouse anti-HA, rat anti-HA (Hoffmann-La Roche Ltd., Basel, Switzerland; 1:4,000); mouse anti-FLAG M2 (Sigma-Aldrich, St Louis, MO, USA; 1:4,000); rabbit anti-EphA4, rabbit anti-PDGFRβ, and mouse anti-GFP (Santa Cruz Biotechnology Inc., Dallas, TX, USA; 1:2,000); and mouse anti-phosphotyrosine (EMD Millipore, Billerica, MA, USA; 1:4,000).
Cell culture
Mammalian HEK293T cells (Clontech, Mountain View, CA, USA) were cultured as previously reported.37 Neural stem cells derived from H9 human embryonic stem cells (H9-NSCs) were obtained from Thermo Fisher Scientific (Waltham, MA, USA) and cultured following the manufacturer’s protocols.38 Cells were passaged to generation 3 for RNA extraction, cell proliferation assay, or immunoprecipitation and immunoblotting.
Reverse transcription (RT)-polymerase chain reaction (PCR)
H9-NSCs were rinsed with PBS after 3-day culture. The cells were homogenized using TRI Reagent (Sigma-Aldrich; Cat. #T9424), and total RNA was then extracted using standard methods. RT and subsequent PCR were performed using the conditions as previously reported.36 The product sizes and the forward and reverse primer sequences are presented in Table 1.
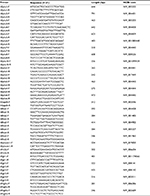  | Table 1 Primers used in this research |
Plasmid transfection
EphA4 and PDGFRβ eukaryotic expression vectors were constructed as previously reported.37,39 Mutants of EphA4 and PDGFRβ plasmids were constructed using QuikChange Lightning Site-Directed Mutagenesis Kit (Stratagene, Santa Clara, CA, USA) following standard instructions. Plasmid transient transfection was performed using PerFectin (Genlantis, San Diego, CA, USA) into HEK293T cells. Before ligand stimulation, HEK293T cells or H9-NSCs were starved in serum-free medium containing 0.5% (m/v) bovine serum albumin (Sigma) for 5 h.
Immunoprecipitation and immunoblotting
Cells were harvested in lysis A buffer with 50 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid buffer, 1% Triton X-100, 5 mM ethylene diamine tetraacetic acid, 50 mM sodium chloride, protease inhibitors (1 µM pepstatin A, 1 mM phenylmethylsulfonyl fluoride, 1 µM leupeptin, and 1 µM aprotinin), and phosphatase inhibitors (50 mM sodium fluoride, 10 mM sodium pyrophosphate, and 1 mM sodium orthovanadate). Immunoprecipitation was performed with indicated antibodies overnight at 4°C; after a wash for three times in washing buffer, immunoblotting was performed with diluted primary antibodies following the manufacturer’s instructions using the standard procedure.37 To confirm reproducibility, experiments were performed more than once.
Cell proliferation assay
Cell proliferation was measured using a CellTiter96 Aqueous One Solution Cell Proliferation Assay (MTS) kit (Promega, Madison, WI, USA) according to the manufacturer’s protocol. In brief, cells (1 × 103) were starved overnight and then seeded on 96-well plastic plates in a normal medium with growth factors. The indicated ligands (PDGF-BB, 20 ng/mL; ephrin-A1, 0.5 µg/mL) were added into the culture wells in a medium free of growth factors. After cultured for 3 days in the new media at 37°C, the cells were further incubated with CellTiter96 Aqueous One Solution Reagent for 1 h. The absorbance was recorded at 490 nm wavelength using a 96-well plate reader (iMarkTM; Bio-Rad Laboratories Inc., Hercules, CA, USA).
Statistical analysis
Data are analyzed using Graphpad Prism 6 by two-way ANOVA followed by Dunnett’s multiple comparison tests. A value of p<0.0001 was considered as statistically significant difference. All the values were expressed as mean±SD.
Results
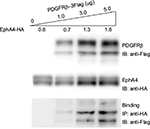
Interaction between EphA4 and PDGFRβ
To evaluate the interaction between PDGFRβ and EphA4, both molecules were overexpressed in HEK293T cells, and their binding was examined using the immunoprecipitation and immunoblotting method. pcDNA3.1 plasmid was used to equalize the total amount of DNA in each transfection. As shown in Figure 1, the endogenous PDGFRβ was detected difficultly by immunoblotting with a specific antibody, while PDGFRβ expression level increases strongly under the elevated amount of exogenous PDGFRβ, and a complex formation of EphA4 and PDGFRβ was detected by immunoblotting followed by immunoprecipitation using the antibodies shown. The result also demonstrated that their direct interaction is in their protein dose-dependent fashion.
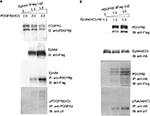
Transphosporylation between EphA4 and PDGFRβ
To investigate the functional consequences of ectopically expressing PDGFRβ and EphA4 and their subsequent complex formation, we next analyzed the transphosphorylation of EphA4 and PDGFRβ when transiently coexpressed in mammalian cells. We overexpressed EphA4 expression vector in HEK293T cells together with expression vector for PDGFRβ(KD), a kinase-inactive mutant of PDGFRβ in which an Ala residue was substituted for Tyr-634 (Figure 2A).40 Immunoblotting with an antiphosphotyrosine antibody followed by immunoprecipitation with a specific antibody of the kinase-inactive mutant of PDGFRβ in cells coexpressing EphA4(WT) has verified that EphA4 self-phosphorylated by overexpression in HEK293T cells causes the activation of kinase-inactive PDGFRβ mutant through tyrosine phosphorylation. Meanwhile, expression vector for PDGFRβ(WT) was ectopically transfected in HEK293T cells together with the expression vector for EphA4(KD), a kinase-inactive mutant of EphA4 in which a Met residue was substituted for Val-653. The experiment also shows that PDGFRβ in HEK293T cells activated by exogenous transfection induces the kinase-inactive EphA4 mutant tyrosine phosphorylation (Figure 2B).
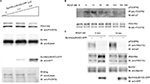
Inhibition of the interaction between EphA4 and PDGFRβ by an EphA4 dominant-negative mutant, EphA4 (ΔJM,KD)
EphA4(ΔJM,KD), in which 591–602 amino acids were deleted and a Met residue was substituted for Val-653, was used to inhibit the interaction between EphA4 and FGFR to prove that EphA4 transphosphorylates FGFR.36,41 Here, we examined whether EphA4(ΔJM,KD) could also inhibit binding of EphA4 to PDGFRβ. Fixed amounts of PDGFRβ (2 µg per 6 cm plate) and EphA4(WT) (1 µg per 6 cm plate) were coexpressed with increasing amounts of EphA4(ΔJM,KD) in HEK293T cells, and the binding of EphA4 to PDGFRβ was analyzed. We found that EphA4(ΔJM,KD) inhibited the interaction between EphA4 and PDGFRβ in a dose-dependent fashion (Figure 3A). Results show that EphA4(ΔJM,KD) is also a molecule that inhibits the signaling pathway through the Eph–PDGFRβ complex.
The next step was to analyze whether there is a dominant-negative effect; the expression vector for EphA4(ΔJM,KD) was transfected into HEK293T cells together with the expression vectors for EphA4 and PDGFRβ. Time course study shows that the peak of PDGFRβ tyrosine phosphorylation was at 15 min under PDGF-BB (100 ng/mL) stimulation (Figure 3B). When cotransfected fixed amounts of EphA4(WT) and PDGFRβ(WT) in HEK293T cells, as shown in Figure 3C, EphA4(ΔJM,KD) significantly suppressed PDGF-BB-mediated tyrosine phosphorylation of PDGFRβ(WT) at either 0 or 15 min (the peak of ligand stimulation). Results show that the binding of EphA4 to PDGFRβ is important for both EphA4 and PDGFRβ signaling pathways.
Interaction of EphA4 and PDGFRβ in the proliferation of embryonic stem cells deriving neural stem cells
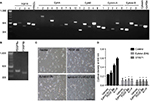
We investigated the expression patterns of EphAs and PDGFRs in the proliferative regulation of H9-NSCs. Almost all EphAs (EphA2, EphA4, EphA6, EphA7, EphA8, and EphA10) and all PDGFR family members (PDGFRα and PDGFRβ) were detected in the NSCs by RT-PCR (Figure 4A and B).
H9-NSCs show an increase in their cell number under PDGF-BB (20 ng/mL) and/or ephrin-A1 (0.5 µg/mL) stimulation after a 3-day culture when seeded on a matrigel-coated plate (Figure 4C). We also evaluated the role of these ligands on cell proliferation of H9-NSCs using MTS assay (Figure 4D). Dunnett’s multiple comparison test followed by two-way ANOVA depicted that the optical density increased significantly under the stimulation with ephrin-A1-Fc (p<0.05) and PDGF-BB (p<0.0001), respectively, compared with nonstimulation. These results suggest that activation of the cells with endogenous Ephs or PDGFRs promotes proliferation of H9-NSCs. Furthermore, the optical density showed further increase (p<0.0001) under the stimulation with both ephrin-A1-Fc and PDGF-BB, suggesting enhanced proliferation of H9-NSCs by simultaneous stimulation with two ligands. Expression of the dominant-negative EphA4 or administration of STI571 (inhibitor of PDGFRs) strongly inhibited the proliferation of H9-NSCs under both ligands.
Discussion
In this report, we found that EphA4 and PDGFRβ bind to each other in a dose-dependent manner, and EphA4 and PDGFRβ transphosphorylate each other when transiently coexpressed in the same cells. A dominant-negative molecule of EphA4 can inhibit the interaction of EphA4 with PDGFRβ. Stimulation with PDGF-BB and ephrin-A1-Fc enhanced neural stem cell proliferation, and the receptor complex involving EphA4 and PDGFRβ might mediate the signaling pathway.
As reported previously, we found that the cytoplasmic domains of EphA4 and FGFRs interact with each other, and the protein complex can transphosphorylate each other when overexpressed or stimulated with their ligands, which reinforces downstream signaling through activation of FRS2α and ERK1/2. The receptor complex promotes NSC proliferation in response to combined simulation with ephrin-A1 and FGF2.35–37 In this study, we have demonstrated that PDGFRβ also binds to and phosphorylates EphA4. The signal through the PDGFRβ/EphA4 complex augments NSC proliferation under the stimulation by PDGF-BB and ephrin-A1.
PDGF-BB is a member of the PDGF family comprising other four ligands (PDGF-AA, -CC, -DD, and -AB) that interact with two RTKs, PDGFRα and PDGFRβ.25–27 When PDGFRs are activated with ligands, they interact with and phosphorylate many downstream proteins, including the docking proteins, FRS2α, which coordinately activates multiple signaling pathways through the protein complex formation.42 PDGF-BB plays key roles in the in vitro proliferation30 and neuronal differentiation of neural stem cells derived from the embryonic hippocampus.32 Furthermore, PDGF signaling prevents the cerebral hemisphere from cryogenic injury in adulthood mice.43 Conditional disruption of PDGFRβ shows deficits in fear conditioning, prepulse inhibition, spatial memory, social interaction, and forced swimming.44 EphA4 signaling mediates axon guidance, neuronal boundary formation, cell growth, angiogenesis, and cell migration.45 Deletion of EphA4 in mice shows deficiency such as hindlimb locomotion, neuron differentiation, and migration during corticogenesis, midline axon guidance, and small body size.35,46–48 Among the abnormalities during the development, resembling defects in dendritic spine density, impairment in hippocampus-dependent memory formation, and long-term potentiation are caused by deletion of either PDGFRβ or EphA4, suggesting neuron development and maturation require the presence of both PDGFRβ and EphA4.21,49,50 These findings suggest that the PDGFRβ/EphA4 receptor complex mediates a variety of signals.
Miao et al51 reported that ephrin-A1 attenuated ERK activation through PDGF signaling and exerted the antimitogenic functions in a cell-type-specific manner. This is in contrast to our findings. The reason for this difference might be 1) we used clustered ephrin-A1 by pretreating soluble ephrin-A1-Fc with anti-IgG(Fc), and 2) we used neural stem cells in our study, while Miao et al used prostatic epithelial cells and endothelial cells.
Recent reports in animal studies showed that ICV injection of either PDGF-BB for 2 weeks or clustered ephrin-A1-Fc for 1 week could restore production of dopaminergic neurons and achieve functional improvement in several PD animal models.23,34 Our current findings might provide molecular evidence for curing PD with PDGF-BB and ephrin-A1. Coinjection of ephrin-A1 and PDGF-BB would be more effective in increasing dopaminergic neurons in the substantia nigra for functional recovery of Parkinsonian rat models. Stem cells may also offer a powerful new approach to model and study PD and AD.52,53 We studied the effect of transplantation of the induced pluripotent stem cells and the human umbilical blood-derived stem cells to PD or AD animal models in our laboratory and found a significant symptomatic recovery.54,55 In future, we would also like to combine the transplantation of stem cells and coinjection of ephrin-A1 and PDGF-BB to cure the neurodegenerative disease in PD and AD animal models.
Conclusion
PDGFRβ and EphA4 can mutually bind to and trans-phosphorylate in a dose-dependent manner when cotransfected in HEK293T cells. NSCs express PDGFRs and almost all the Ephs and ephrins. Direct interaction and transphosphorylation of EphA4 and PDGFRβ may play an important role in the proliferation of H9-derived NSCs. These NSCs appear to integrate the cell contact-dependent ephrin/Eph receptor signal with the humoral signals transduced by PDGF/PDGFRβ.
Acknowledgments
All the participants in the study are appreciated. The authors are grateful to Dr Paul Lu from the University of California at San Diego for his help in preparing this manuscript and Dr Carl-Henrik Heldin from Ludwig Institute for Cancer Research for using their pcDNA3.1/PDGFRβ and pcDNA3.1/PDGFRβ(KD) plasmids. The work was supported by National Scientific Foundation of China (NSFC 81571241, to FH), a Research Grant on Priority Areas from the Wakayama Medical University (to KS and TS), and a Research Initiative Grant for Doctor from Liaocheng People’s Hospital (to QC).
Disclosure
The authors report no conflicts of interest in this work.
References
Schlessinger J, Lemmon MA. SH2 and PTB domains in tyrosine kinase signaling. Sci STKE. 2003;2003(191):RE12. | ||
Porter AC, Vaillancourt RR. Tyrosine kinase receptor-activated signal transduction pathways which lead to oncogenesis. Oncogene. 1998;17(11 Reviews):1343–1352. | ||
Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2(2):127–137. | ||
Kaushansky A, Gordus A, Chang B, Rush J, MacBeath G. A quantitative study of the recruitment potential of all intracellular tyrosine residues on EGFR, FGFR1 and IGF1R. Mol Biosyst. 2008;4(6):643–653. | ||
Kullander K, Klein R. Mechanisms and functions of Eph and ephrin signalling. Nat Rev Mol Cell Biol. 2002;3(7):475–486. | ||
Nomura T, Goritz C, Catchpole T, Henkemeyer M, Frisen J. EphB signaling controls lineage plasticity of adult neural stem cell niche cells. Cell Stem Cell. 2010;7(6):730–743. | ||
Khodosevich K, Watanabe Y, Monyer H. EphA4 preserves postnatal and adult neural stem cells in an undifferentiated state in vivo. J Cell Sci. 2011;124(pt 8):1268–1279. | ||
Hu Y, Li S, Jiang H, Li MT, Zhou JW. Ephrin-B2/EphA4 forward signaling is required for regulation of radial migration of cortical neurons in the mouse. Neurosci Bull. 2014;30(3):425–432. | ||
Takeuchi S, Katoh H, Negishi M. Eph/ephrin reverse signalling induces axonal retraction through RhoA/ROCK pathway. J Biochem. 2015;158(3):245–252. | ||
Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93(5):741–753. | ||
Zhao C, Irie N, Takada Y, et al. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab. 2006;4(2):111–121. | ||
Xu Q, Mellitzer G, Robinson V, Wilkinson DG. In vivo cell sorting in complementary segmental domains mediated by Eph receptors and ephrins. Nature. 1999;399(6733):267–271. | ||
Fukai J, Yokote H, Yamanaka R, Arao T, Nishio K, Itakura T. EphA4 promotes cell proliferation and migration through a novel EphA4-FGFR1 signaling pathway in the human glioma U251 cell line. Mol Cancer Ther. 2008;7(9):2768–2778. | ||
Liu Q, Guo X, Que S, et al. LncRNA RSU1P2 contributes to tumorigenesis by acting as a ceRNA against let-7a in cervical cancer cells. Oncotarget. Epub 2016 Jul 26. | ||
Wang Y, Liu Z, Yao B, et al. Long non-coding RNA TUSC7 acts a molecular sponge for miR-10a and suppresses EMT in hepatocellular carcinoma. Tumour Biol. 2016;37(8):11429–11441. | ||
de Marcondes PG, Bastos LG, de-Freitas-Junior JC, Rocha MR, Morgado-Diaz JA. EphA4-mediated signaling regulates the aggressive phenotype of irradiation survivor colorectal cancer cells. Tumour Biol. 2016;37(9):12411–12422. | ||
de Marcondes PG, Morgado-Diaz JA. The role of EphA4 signaling in radiation-induced EMT-like phenotype in colorectal cancer cells. J Cell Biochem. 2016;118(3):442–445. | ||
Jing X, Sonoki T, Miyajima M, et al. EphA4-deleted microenvironment regulates cancer development and leukemoid reaction of the isografted 4T1 murine breast cancer via reduction of an IGF1 signal. Cancer Med. 2016;5(6):1214–1227. | ||
Konstantinova I, Nikolova G, Ohara-Imaizumi M, et al. EphA-Ephrin-A-mediated beta cell communication regulates insulin secretion from pancreatic islets. Cell. 2007;129(2):359–370. | ||
Rosenberger AF, Rozemuller AJ, van der Flier WM, Scheltens P, van der Vies SM, Hoozemans JJ. Altered distribution of the EphA4 kinase in hippocampal brain tissue of patients with Alzheimer’s disease correlates with pathology. Acta Neuropathol Commun. 2014;2:79. | ||
Fu AK, Hung KW, Huang H, et al. Blockade of EphA4 signaling ameliorates hippocampal synaptic dysfunctions in mouse models of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2014;111(27):9959–9964. | ||
Shi M, Movius J, Dator R, et al. Cerebrospinal fluid peptides as potential Parkinson disease biomarkers: a staged pipeline for discovery and validation. Mol Cell Proteomics. 2015;14(3):544–555. | ||
Jing X, Miwa H, Sawada T, et al. Ephrin-A1-mediated dopaminergic neurogenesis and angiogenesis in a rat model of Parkinson’s disease. PLoS One. 2012;7(2):e32019. | ||
Van Hoecke A, Schoonaert L, Lemmens R, et al. EPHA4 is a disease modifier of amyotrophic lateral sclerosis in animal models and in humans. Nat Med. 2012;18(9):1418–1422. | ||
Li X, Ponten A, Aase K, et al. PDGF-C is a new protease-activated ligand for the PDGF alpha-receptor. Nat Cell Biol. 2000;2(5):302–309. | ||
LaRochelle WJ, Jeffers M, McDonald WF, et al. PDGF-D, a new protease-activated growth factor. Nat Cell Biol. 2001;3(5):517–521. | ||
Heldin CH, Eriksson U, Ostman A. New members of the platelet-derived growth factor family of mitogens. Arch Biochem Biophys. 2002;398(2):284–290. | ||
Mohapel P, Frielingsdorf H, Haggblad J, Zachrisson O, Brundin P. Platelet-derived growth factor (PDGF-BB) and brain-derived neurotrophic factor (BDNF) induce striatal neurogenesis in adult rats with 6-hydroxydopamine lesions. Neuroscience. 2005;132(3):767–776. | ||
Yang L, Chen X, Hu G, Cai Y, Liao K, Buch S. Mechanisms of platelet-derived growth factor-BB in restoring HIV Tat-cocaine-mediated impairment of neuronal differentiation. Mol Neurobiol. 2016;53(9):6377–6387. | ||
Yao H, Duan M, Yang L, Buch S. Platelet-derived growth factor-BB restores human immunodeficiency virus Tat-cocaine-mediated impairment of neurogenesis: role of TRPC1 channels. J Neurosci. 2012;32(29):9835–9847. | ||
Yang CM, Hsieh HL, Yu PH, Lin CC, Liu SW. IL-1beta induces MMP-9-dependent brain astrocytic migration via transactivation of PDGF receptor/NADPH oxidase 2-derived reactive oxygen species signals. Mol Neurobiol. 2015;52(1):303–317. | ||
Yang L, Chao J, Kook YH, Gao Y, Yao H, Buch SJ. Involvement of miR-9/MCPIP1 axis in PDGF-BB-mediated neurogenesis in neuronal progenitor cells. Cell Death Dis. 2013;4:e960. | ||
Zachrisson O, Zhao M, Andersson A, et al. Restorative effects of platelet derived growth factor-BB in rodent models of Parkinson’s disease. J Parkinsons Dis. 2011;1(1):49–63. | ||
Paul G, Zachrisson O, Varrone A, et al. Safety and tolerability of intracerebroventricular PDGF-BB in Parkinson’s disease patients. J Clin Invest. 2015;125(3):1339–1346. | ||
Chen Q, Arai D, Kawakami K, et al. EphA4 regulates the balance between self-renewal and differentiation of radial glial cells and intermediate neuronal precursors in cooperation with FGF signaling. PLoS One. 2015;10(5):e0126942. | ||
Sawada T, Arai D, Jing X, et al. Trans-activation between EphA and FGFR regulates self-renewal and differentiation of mouse embryonic neural stem/progenitor cells via differential activation of FRS2alpha. PLoS One. 2015;10(5):e0128826. | ||
Yokote H, Fujita K, Jing X, et al. Trans-activation of EphA4 and FGF receptors mediated by direct interactions between their cytoplasmic domains. Proc Natl Acad Sci U S A. 2005;102(52):18866–18871. | ||
Kadoya K, Lu P, Nguyen K, et al. Spinal cord reconstitution with homologous neural grafts enables robust corticospinal regeneration. Nat Med. 2016;22(5):479–487. | ||
Vanlandewijck M, Lebouvier T, Andaloussi Mae M, et al. Functional characterization of germline mutations in PDGFB and PDGFRB in primary familial brain calcification. PLoS One. 2015;10(11): e0143407. | ||
Westermark B, Siegbahn A, Heldin CH, Claesson-Welsh L. B-type receptor for platelet-derived growth factor mediates a chemotactic response by means of ligand-induced activation of the receptor protein-tyrosine kinase. Proc Natl Acad Sci U S A. 1990;87(1):128–132. | ||
Sawada T, Jing X, Zhang Y, et al. Ternary complex formation of EphA4, FGFR and FRS2alpha plays an important role in the proliferation of embryonic neural stem/progenitor cells. Genes Cells. 2010;15(3):297–311. | ||
Chen PY, Simons M, Friesel R. FRS2 via fibroblast growth factor receptor 1 is required for platelet-derived growth factor receptor beta-mediated regulation of vascular smooth muscle marker gene expression. J Biol Chem. 2009;284(23):15980–15992. | ||
Ishii Y, Oya T, Zheng L, et al. Mouse brains deficient in neuronal PDGF receptor-beta develop normally but are vulnerable to injury. J Neurochem. 2006;98(2):588–600. | ||
Nguyen PT, Nakamura T, Hori E, et al. Cognitive and socio-emotional deficits in platelet-derived growth factor receptor-beta gene knockout mice. PLoS One. 2011;6(3):e18004. | ||
Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133(1):38–52. | ||
Dottori M, Hartley L, Galea M, et al. EphA4 (Sek1) receptor tyrosine kinase is required for the development of the corticospinal tract. Proc Natl Acad Sci U S A. 1998;95(22):13248–13253. | ||
Kramer ER, Knott L, Su F, et al. Cooperation between GDNF/Ret and ephrinA/EphA4 signals for motor-axon pathway selection in the limb. Neuron. 2006;50(1):35–47. | ||
Jing X, Miyajima M, Sawada T, et al. Crosstalk of humoral and cell-cell contact-mediated signals in postnatal body growth. Cell Rep. 2012;2(3):652–665. | ||
Shioda N, Moriguchi S, Oya T, et al. Aberrant hippocampal spine morphology and impaired memory formation in neuronal platelet-derived growth factor beta-receptor lacking mice. Hippocampus. 2012;22(6):1371–1378. | ||
Chen W, Baylink DJ, Brier-Jones J, et al. PDGFB-based stem cell gene therapy increases bone strength in the mouse. Proc Natl Acad Sci U S A. 2015;112(29):E3893–E3900. | ||
Miao H, Wei BR, Peehl DM, et al. Activation of EphA receptor tyrosine kinase inhibits the Ras/MAPK pathway. Nat Cell Biol. 2001;3(5):527–530. | ||
Han F, Baremberg D, Gao J, et al. Development of stem cell-based therapy for Parkinson’s disease. Transl Neurodegener. 2015;4:16. | ||
Han FW, Wang W, Chen C. Research progress in animal models and stem cell therapy for Alzheimer’s disease. J Neurorestoratol. 2014;3:11–22. | ||
Han F, Wang W, Chen B, et al. Human induced pluripotent stem cell-derived neurons improve motor asymmetry in a 6-hydroxydopamine-induced rat model of Parkinson’s disease. Cytotherapy. 2015;17(5):665–679. | ||
Chen C, Duan J, Shen A, Wang W, et al. Transplantation of human umbilical cord blood-derived mononuclear cells induces recovery of motor dysfunction in a rat model of Parkinson’s disease. J Neurorestoratol. 2016;4:23–33. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.